Back to Journals » Neuropsychiatric Disease and Treatment » Volume 19
The Relationship Between Neuron-Specific Enolase and Clinical Outcomes in Patients Undergoing Mechanical Thrombectomy
Authors Peng Q, Chen W, E Y, Deng Y , Xu Z , Wang S , Fu X, Wei B, Wang M, Hou J, Zhang Y, Duan R
Received 9 December 2022
Accepted for publication 30 March 2023
Published 4 April 2023 Volume 2023:19 Pages 709—719
DOI https://doi.org/10.2147/NDT.S400925
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Richard J Porter
Qiang Peng,1,* Wenxiu Chen,2,* Yan E,1,* Yang Deng,3 Zhaohan Xu,1 Siyu Wang,1 Xinxin Fu,3 Bin Wei,1 Meng Wang,1 Jiankang Hou,1 Yingdong Zhang,1 Rui Duan1
1Department of Neurology, Nanjing First Hospital, Nanjing Medical University, Nanjing, Jiangsu, 210006, People’s Republic of China; 2Department of Critical Care Medicine, Nanjing First Hospital, Nanjing Medical University, Nanjing, Jiangsu, 210006, People’s Republic of China; 3School of Basic Medicine and Clinical Pharmacy, China Pharmaceutical University, Nanjing, Jiangsu, 210000, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yingdong Zhang; Rui Duan, Department of Neurology, Nanjing First Hospital, Nanjing Medical University, No. 68 Changle Road, Nanjing, 210006, People’s Republic of China, Email [email protected]; [email protected]
Purpose: Neuron-specific enolase (NSE) is considered a biomarker for the severity of nervous system diseases. We sought to explore whether serum NSE concentration in ischemic stroke patients undergoing mechanical thrombectomy (MT) is related to 3-month functional outcome and symptomatic intracranial hemorrhage (sICH).
Patients and Methods: We retrospectively collected the data of acute ischemic stroke patients with anterior circulation infarction receiving MT within 6 h in our stroke center. Favorable outcome and poor outcome at 3 months were defined as modified Rankin Scale (mRS) score 0– 2 and 3– 6, respectively. sICH was defined according to the Heidelberg bleeding classification. We used multivariate logistic regression model and receiver operating characteristic curves to investigate the correlation between NSE and clinical outcomes.
Results: Among the 426 patients enrolled, 40 (9.4%) patients developed sICH. Three-month favorable outcome in 160 (37.6%) and poor outcome in 266 (62.4%) patients were observed. Serum NSE levels was significantly correlated with 3-month mRS score (R = 0.473, P < 0.001). A cutoff value of 15.29 and 23.12 ng/mL for serum NSE was detected in discriminating 3-month poor outcome (area under the curve, 0.724) and sICH (area under the curve, 0.716), respectively. Multivariate analysis showed that high serum NSE levels were independently associated with 3-month poor outcome (odds ratio [OR] 5.049, 95% confidence interval [CI] 2.933– 8.689, P< 0.001) and sICH (OR 5.111, 95% CI 2.210– 11.820, P < 0.001).
Conclusion: Our study demonstrated that high serum NSE levels after receiving MT were independently associated with 3-month poor outcome and sICH in acute ischemic stroke patients. Serum NSE levels could be a good predictor of clinical outcomes for patients receiving MT.
Keywords: neuron-specific enolase, ischemic stroke, mechanical thrombectomy, symptomatic intracranial hemorrhage, prognosis
Introduction
Stroke serves as one of the main causes of mortality and long-term morbidity for adults worldwide.1 Several randomized controlled trials suggested the benefit of mechanical thrombectomy (MT) in abating death and disability in patients with acute ischemic stroke due to large vessel occlusion in the anterior circulation,2,3 However, some patients may develop a series of complications such as symptomatic intracranial hemorrhage (sICH) after receiving MT, which is potentially associated with poor prognosis.4 More than half of the ischemic stroke patients may not achieve favorable functional outcome after 3 months even if they had been treated with MT, according to previous studies.5,6 Therefore, early clinical evaluation is crucial to improve the prognosis of these patients, and serum biomarkers could be useful.
Enolase is a glycolytic enzyme catalyzing the biosynthesis of phosphoenolpyruvate with 2-posphoglycerate. It is dimer with three immunologically distinct subunits: α, β and γ. The γ-enolase is also known as neuron-specific enolase (NSE), which is highly localized in neurons and neuroendocrine cells, but present only in inappreciable amounts in peripheral blood under physiological conditions.7,8 After brain injury, NSE could be released from the damaged neurons into the blood through the impaired blood–brain barrier (BBB).9 Therefore, NSE is considered as a biomarker that reflects neuronal damage and BBB disruption, providing reference for the diagnosis and prognosis of neurological diseases including cerebral venous thrombosis,10 subarachnoid hemorrhage,11 epileptic seizure,12 Parkinson’s disease,13 and ischemic stroke,14 etc. Iłżecki et al found that NSE could be a marker of reperfusion injury after carotid endarterectomy.15 Capoccia et al reported that perioperative microembolization may elevate NSE levels at 24 h after carotid artery stenting.16 What is more, one previous study showed that lower levels of NSE may be related to favorable outcome after treatment with intravenous rt-PA therapy.17 However, the association of NSE with patients undergoing MT has not been investigated before. Considering that MT has become an important reperfusion therapy nowadays, we undertook this study to explore the relationship between NSE and clinical outcomes including functional outcome and sICH in patients receiving MT therapy.
Patients and Methods
Study Population
We retrospectively collected the clinical and laboratory data of acute ischemic stroke patients between April 2015 and December 2020 in Nanjing First Hospital, Nanjing Medical University. The inclusion criteria were as follows: (1) over 18 years old; (2) time of symptom onset less than 6 h; (3) anterior circulation infarction treated with MT; (4) with serum NSE levels measured and 3-month follow-up. The exclusion criteria were as follows: (1) with intracranial hemorrhage or subarachnoid hemorrhage on initial CT; (2) with serious heart, liver or kidney dysfunction; (3) with craniocerebral tumor, pulmonary tumor or other diseases that may influence blood NSE levels.18 Due to the retrospective nature of this study, written informed consent to participate was waived by the ethics committee of Nanjing First Hospital, Nanjing Medical University. This work was conducted in conformity to the guidelines of the Declaration of Helsinki.
Baseline Characteristics Collection
Baseline characteristics of each participant were collected, including demographic characteristics, underlying diseases, smoking and drinking status, previous medication use (including antiplatelet and statin), stroke severity, blood pressure at admission, procedural-related characteristics, stroke subtype, and laboratory data. All the patients accepted equipment inspection as needed for assessing the stroke etiology. Stroke subtype was classified according to Trial of ORG 10172 in Acute Stroke Treatment (TOAST) criteria.19 Based on digital substraction angiography, successful recanalization was defined as a TICI score of 2b-3.20 Poor collateral status was defined as grade 0 to 1, and moderate to excellent collateral status was defined as grade 2 to 4 using the American Society of Interventional and Therapeutic Neuroradiology/Society of Interventional Radiology grading system.21 Stroke severity at admission was assessed using National Institutes of Health Stroke Scale (NIHSS) score. sICH was defined as any hemorrhagic transformation associated with total NIHSS score worsening ≥4 points or worsening ≥2 points in one NIHSS category or deterioration led to intubation, hemicraniectomy, external ventricular drain placement, or any other major interventions, which used the Heidelberg bleeding classification.5,22 The assessment of 3-month functional outcome after discharge were conducted through face-to-face or phone follow-up using the modified Rankin Scale (mRS) (ranging from 0 to 6). The favorable outcome was defined as mRS 0–223,24 and the poor outcome was defined as mRS 3–6.23,25 In our study, the primary clinical outcome was 3-month functional outcome, and the secondary clinical outcome was sICH after MT.
Measurement of Serum NSE
The peripheral blood was drawn within 24 h after receiving MT for laboratory examination. The measurement of serum NSE levels were conducted in a certificated laboratory of Nanjing First Hospital with a chemiluminescence detection assay by a Cobas 8000 automatic biochemical analyzer (Roche Diagnostics, Basel, Switzerland). The normal NSE value in healthy adults was <16.3 ng/mL.26,27
Statistical Analysis
Statistical analysis was conducted using the Statistical Package for the Social Sciences (SPSS) version 21.0 and GraphPad Prism version 9.0.0. Continuous variables were described as means ± standard deviations or medians (interquartile range), as appropriate, and categorical variables were described as frequencies (percentages). For continuous variables, comparisons in baseline characteristics between subgroups were performed with independent sample t-test, Mann–Whitney U-test or Kruskal–Wallis test, as appropriate. For binary and categorical variables, comparisons between subgroups were performed with χ2 test or Fisher’s exact test, as appropriate. The Spearman test was applied to examine the correlation between continuous variables or ranking variables. Receiver operating characteristic (ROC) curve was applied for calculating the area under the curve (AUC) to assess the discriminating value of NSE on clinical outcomes after MT and to determine the optimal cutoff point. The value above or below the optimal cutoff point was regarded as the high and normal NSE levels in stroke patients receiving MT, respectively. The sensitivity and specificity of NSE were calculated based on the definition of 3-month functional outcome and sICH. MedCalc software version 20.014 was used to obtain ROCs. Variables with statistical significance in univariate analysis were included in multivariable analysis to adjust for confounding factors. A two-tailed value of P < 0.05 was regarded as statistically significant.
Results
A total of 426 patients from April 2015 to December 2020 were finally enrolled in our study. Among these patients, 40 (9.4%) patients developed sICH. Favorable outcome and poor outcome were observed in 160 (37.6%) and 266 (62.4%) patients, respectively. Baseline characteristics of study patients according to 3-month outcome are shown in Table 1. Variables that significantly differed between the two groups are depicted as follows: age (P < 0.001), male (P = 0.005), smoking (P = 0.006), drinking alcohol (P = 0.009), initial NIHSS score (P < 0.001), time from onset to puncture (P < 0.001), number of device passes (P < 0.001), poor collateral status (P = 0.027), successful recanalization (P < 0.001), sICH (P < 0.001), stroke subtype (P = 0.003), FBG (P < 0.001) and NSE levels (P < 0.001). Baseline characteristics between sICH and non-sICH patients were also compared, shown in Supplementary Table 1.
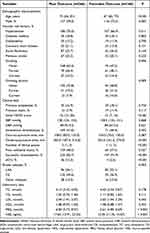 |
Table 1 Baseline Characteristics of Patients According to 3-Month Outcome |
Comparisons of NSE levels based on recanalization status, atrial fibrillation, sICH and intravenous thrombolysis are exhibited in Figure 1. Patients with sICH had significantly higher NSE levels than those without sICH (23.47 [17.70, 31.66] vs 15.16 [12.58, 19.55] ng/mL, P < 0.001). What is more, NSE levels were also higher in patients who had an atrial fibrillation than those without atrial fibrillation (18.46 [13.37, 23.82] vs 14.79 [12.57, 18.83] ng/mL, P < 0.001). Patients with successful recanalization had lower NSE levels than patients with unsuccessful recanalization, but the difference was not significant between the two subgroups (15.42 [12.56, 20.14] vs.17.42 [13.51, 21.18] ng/mL, P = 0.134). Also, there was no significant difference in NSE levels between patients with and without intravenous thrombolysis before MT (15.30 [12.67, 20.06] vs 15.68 [12.93, 20.50] ng/mL, P = 0.499). The Spearman test in Table 2 shows that serum NSE levels were significantly and positively correlated with stroke severity assessed by initial NIHSS score (R = 0.294), number of device passes during MT (R = 0.177), FBG (R = 0.215) and 3-month mRS score (R = 0.473) (all P < 0.001). Further analysis showed that patients with severe stroke had significantly higher NSE levels than mild or moderate stroke (P < 0.001). Also, patients with more than 3 passes during MT had significantly higher NSE levels compared with less than 3 passes (20.03 [13.29, 28.76] vs 15.15[12.59, 19.45] ng/mL, P < 0.001), shown in Supplementary Figures 1 and 2.
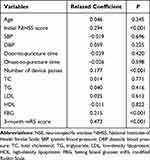 |
Table 2 Spearman Correlation of NSE with Baseline Characteristics and mRS Score |
The ROC curves showed the discriminative ability of NSE in 3-month poor outcome and sICH after MT (Figure 2). The optimal cutoff value for NSE as a predictor of 3-month poor outcome was determined to be 15.29 ng/mL, which yielded a sensitivity of 66.5% and a specificity of 72.5%, with the AUC at 0.724 (95% confidence interval [CI] 0.676–0.772). While the optimal cutoff value for NSE to discriminate sICH after MT was determined to be 23.12 ng/mL, which yielded a sensitivity of 55.0% and a specificity of 86.5%, with the AUC at 0.716 (95% CI 0.616–0.816).
 |
Figure 2 Receiver operating characteristic curves of serum NSE for predicting 3-month poor outcome (a) and discriminating sICH (b) after MT. Abbreviation: AUC, area under curve. |
Table 3 manifests the results of logistic regression analysis. In univariate logistic regression analysis, serum NSE levels were relevant to 3-month poor outcome and sICH. After adjusting for potential confounders, serum NSE as dichotomous variables according to the optimal cutoff value remained independently associated with 3-month poor outcome (odds ratio [OR] 5.049, 95% CI 2.933–8.689, P < 0.001) and sICH (OR 5.111, 95% CI 2.210–11.820, P < 0.001). Meanwhile, serum NSE as continuous variables was also independently associated with 3-month poor outcome (OR 1.151, 95% CI 1.087–1.219, P < 0.001), as well as sICH after undergoing MT (OR 1.036, 95% CI 1.008–1.065, P = 0.012).
 |
Table 3 Logistic Regression Analysis for NSE with 3-Month Poor Outcome and sICH |
Discussion
Although previous studies have examined the association of NSE with ischemic stroke,14,17,28 the clinical value of NSE in patients receiving MT remains unclear. In most studies, serum NSE was considered as a biomarker for severity and clinical outcome of ischemic stroke, while in stroke patients receiving reperfusion therapy, the clinical value of NSE could be complicated by more confounding factors. Gunawan et al found that NSE was significantly elevated after reperfusion in hypoxic ischemia rat model.29 The prospective study by Lu et al suggested a correlation between serum NSE > 13.90 ng/mL and 90-day outcome in patients treated with intravenous thrombolysis, but after adjustment for NIHSS scores and intracranial hemorrhage, the correlation was found not significant.17 As an observational study, our results showed that serum NSE levels were independently associated with 3-month outcome in patients undergoing MT. Serum NSE levels was significantly correlated with 3-month mRS scores. Serum NSE levels have a good predictive ability for poor outcome with a threshold of 15.29 ng/mL according to the ROC analysis. Patients with high NSE levels (>15.29 ng/mL) had about 4 times higher risk of poor functional outcome after 3 months. Interestingly, the threshold 15.29 ng/mL is very close to the upper limit of normal NSE value (16.3 ng/mL) in clinical practice. In this study, the incidence of sICH and poor outcome were in high agreement with the results of prior researches,6,30,31 such as DIRECT-MT trial, which was conducted by Chinese scholars focusing on MT therapy.
We found that serum NSE levels within 24 h were highly correlated with sICH after undergoing MT. Patients developing sICH possessed significantly higher serum NSE levels than non-sICH patients, since BBB disruption is the central factor of hemorrhagic transformation in ischemic stroke.32 Meanwhile, mechanical compression from hematoma and edema may decrease cerebral perfusion, and the infiltration of inflammatory and pro-apoptotic factors from blood could cause further brain damage.33 Moreover, hemorrhage transformation occurs more frequently in areas of grey matter, where NSE is highly localized.29,34 Kim et al continuously measured serum NSE in acute ischemic stroke patients, and the second elevation of NSE peak as well as lesion volume was found to be an independent predictor of hemorrhagic transformation, which was probably caused by the secondary brain injury.35 Our study indicated that serum NSE might also be a good predictor of hemorrhagic transformation after ischemic stroke patients receive MT therapy.
In line with prior research, there was a positive association between serum NSE levels and stroke severity on admission assessed by the NIHSS scores.28 Firstly, more severe stroke means more initial neuronal damage and disruption of the BBB. Secondly, stroke severity was considered to be an independent factor of hemorrhage transformation after thrombectomy.36 Therefore, postprocedural serum NSE levels were susceptible to the initial severity of stroke. In our study, patients with successful recanalization tended to have lower NSE levels. However, the difference was not significant, which may partly due to BBB disruption from reperfusion injury, and some of the patients with successful recanalization suffered early reocclusion within 24 h.37,38 According to Iłżecki et al, both cerebral hyper-perfusion and hypo-perfusion could be responsible for serum NSE increase.15 So the early serum NSE levels may not reflect the recanalization status well. Consistent with previous studies, patients with atrial fibrillation also had higher NSE levels.17,35 Besides the different severity of stroke, a clinical trial revealed that patients with atrial fibrillation were likely to undergo more retrieval attempts.39 Similarly, we found that serum NSE was positively associated with the number of thrombectomy device passes. Recent studies showed that over 3 retrieval attempts significantly increased the risk of sICH,40 and even in patients with recanalization achieved, more thrombectomy device passes reduced the rate of good outcome,41 which could be partly attributed to vascular endothelial injury, compromising BBB integrity.42 In addition, we found that serum NSE levels after receiving MT increased with fasting blood glucose, which agreed with the study by Pandey et al43 Our result supports the importance of early blood glucose management after endovascular treatment.
Of note, we did not find a positive correlation between NSE levels after MT and onset or door-to-puncture time. This might be explained by the inclusion criteria that time of symptom onset was less than 6 h. A systematic review by Anand et al demonstrated that the time frame of the earliest detectable increase in serum NSE ranged from 4 to 8 h after stroke onset.44 In our study, the median onset-to-puncture time was 241 min in all the patients included, so the impact on NSE levels after MT by onset or door-to-puncture time might be confounded because of procedure-related factors. In addition, we found no difference in NSE levels between patients with and without intravenous thrombolysis before receiving MT. One possible explanation is that although there could be a potential risk of hemorrhagic transformation, intravenous thrombolysis treatment may relieve nerve injury and improve prognosis, reducing serum NSE levels of ischemic stroke patients.4,45 Another interesting result was that the poor outcome group contained a larger number of female patients compared with the favorable outcome group, which agreed with the finding of a prospective cohort study by Madsen et al46 This is probably attributed to the older age and higher ratio of atrial fibrillation as well as cardioembolic stroke among the female patients.47,48 More detailed researches are warranted to ascertain the gender difference in endovascular treatment to help formulate the optimal management.
Furthermore, elevated NSE might not just be the result of neuronal injury but also involved in pathological process. It has been found that enolase raised BBB permeability by inducing apoptosis of brain microvascular endothelial cells.49 Inflammation may play an important role in neuronal injury, and previous study showed that serum NSE was remarkably increased in acute encephalitis syndrome.50 Matrix metalloproteinase-9 is a gelatinase linked to BBB extracellular matrix degradation released by inflammatory cells.51 Haque et al reported that inhibition of NSE activity decreased matrix metalloproteinase-9 expression and inflammatory reaction in spinal cord injury.52–54 By contrast, a recent study indicated that NSE may cooperate with α-enolase against neuronal injury in stroke models.55 Nevertheless, the pathophysiological role of NSE in ischemic stroke is still not clear.
Given the fact that NSE has been widely used as a well-known tumor marker, it is easy to be obtained in clinical practice.18,50 The connection between NSE after MT and clinical outcomes suggests that we should pay more attention to patients with high NSE levels in early stage, and good postprocedural care and treatment of complications such as hemorrhagic transformation should be stressed for these patients. In the present study, the NSE levels were not measured before MT, but brain damage had occurred when the patients arrived at the emergency room. Therefore, in the future study, we aim to focus on the clinical significance of serum NSE before MT and the mechanism how the reperfusion therapy affects serum NSE levels.
There are several restrictions to be considered in this study. First, this is a single-center study in China, and the number of participants in our study is relatively small, so our conclusions may be limited to be extended to other populations. Second, this is a retrospective and observational study, which may probably lead to selection bias. Some potential confounding factors may not be controlled. Third, it is revealed that NSE levels may change dynamically over time after brain injury,56 but in our study, the NSE levels were not measured continuously for each patient. Thus, the results in our study must be interpreted with caution.
Conclusions
To sum up, the present study suggested that a high serum NSE level within 24 h after receiving MT independently associates with the poor clinical outcomes in ischemic stroke patients. Serum NSE may be a good predictor of 3-month functional outcome and hemorrhagic transformation for patients treated with MT therapy. More severe stroke, atrial fibrillation, more retrieval attempts and high blood glucose could be related to early elevation of serum NSE in patients undergoing MT. Patients with high NSE levels after MT may need more attention from neurologists.
Abbreviations
NSE, neuron-specific enolase; MT, mechanical thrombectomy; NIHSS, National Institutes of Health Stroke Scale; SBP, systolic blood pressure; DBP, diastolic blood pressure; sICH, symptomatic intracranial hemorrhage; LAA, large-artery atherosclerosis; CE, cardioembolism; TC, total cholesterol; TG, triglyceride; LDL, low-density lipoprotein; HDL, high-density lipoprotein; FBG, fasting blood glucose; AUC, area under curve; BBB, blood–brain barrier; CI, confidence interval; CT, computed tomography; mRS, modified Rankin Scale; OR, odds ratio; ROC, receiver operating characteristic curve; TICI, thrombolysis in cerebral infarction.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics Statement
This work was conducted in conformity to the guidelines of the Declaration of Helsinki and the protocol was approved by the ethics committee of Nanjing First Hospital, Nanjing Medical University. Written informed consent to participate was waived due to the retrospective nature of the study, and all the procedures being performed were part of the routine care.
Acknowledgments
We are very grateful to all medical staff and patients for participating in and supporting this work.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the National Science and Technology Innovation 2030 – Major program of “Brain Science and Brain-Inspired Intelligence Research” (2021ZD0201807), the Natural Science Foundation of Jiangsu Province (BK20201117), Nanjing International Joint Research and Development Project (202201030).
Disclosure
The authors declare that they have no actual or potential conflict of interest for this work.
References
1. Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344–e418. doi:10.1161/STR.0000000000000211
2. Campbell BC, Mitchell PJ, Kleinig TJ, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372(11):1009–1018. doi:10.1056/NEJMoa1414792
3. Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372(11):1019–1030. doi:10.1056/NEJMoa1414905
4. Spronk E, Sykes G, Falcione S, et al. Hemorrhagic transformation in ischemic stroke and the role of inflammation. Front Neurol. 2021;12:661955. doi:10.3389/fneur.2021.661955
5. Zhang X, Yuan K, Wang H, et al. Nomogram to Predict mortality of endovascular thrombectomy for ischemic stroke despite successful recanalization. J Am Heart Assoc. 2020;9(3):e014899. doi:10.1161/JAHA.119.014899
6. Yang P, Zhang Y, Zhang L, et al. Endovascular thrombectomy with or without intravenous alteplase in acute stroke. N Engl J Med. 2020;382(21):1981–1993. doi:10.1056/NEJMoa2001123
7. Marangos PJ, Schmechel DE. Neuron specific enolase, a clinically useful marker for neurons and neuroendocrine cells. Annu Rev Neurosci. 1987;10:269–295. doi:10.1146/annurev.ne.10.030187.001413
8. Casmiro M, Maitan S, De Pasquale F, Cova V, Scarpa E, Vignatelli L. Cerebrospinal fluid and serum neuron-specific enolase concentrations in a normal population. Eur J Neurol. 2005;12(5):369–374. doi:10.1111/j.1468-1331.2004.01021.x
9. Selakovic V, Raicevic R, Radenovic L. The increase of neuron-specific enolase in cerebrospinal fluid and plasma as a marker of neuronal damage in patients with acute brain infarction. J Clin Neurosci. 2005;12(5):542–547. doi:10.1016/j.jocn.2004.07.019
10. Hu Y, Meng R, Zhang X, et al. Serum neuron specific enolase may be a marker to predict the severity and outcome of cerebral venous thrombosis. J Neurol. 2018;265(1):46–51. doi:10.1007/s00415-017-8659-9
11. Tawk RG, Grewal SS, Heckman MG, et al. The relationship between serum neuron-specific enolase levels and severity of bleeding and functional outcomes in patients with nontraumatic subarachnoid hemorrhage. Neurosurgery. 2016;78(4):487–491. doi:10.1227/NEU.0000000000001140
12. Shaik AJ, Reddy K, Mohammed N, Tandra SR, Rukmini Mridula K, Baba Kss S. Neuron specific enolase as a marker of seizure related neuronal injury. Neurochem Int. 2019;131:104509. doi:10.1016/j.neuint.2019.104509
13. Papuć E, Rejdak K. Increased cerebrospinal fluid S100B and NSE reflect neuronal and glial damage in Parkinson’s disease. Front Aging Neurosci. 2020;12:156. doi:10.3389/fnagi.2020.00156
14. Zaheer S, Beg M, Rizvi I, Islam N, Ullah E, Akhtar N. Correlation between serum neuron specific enolase and functional neurological outcome in patients of acute ischemic stroke. Ann Indian Acad Neurol. 2013;16(4):504–508. doi:10.4103/0972-2327.120442
15. Iłżecki M, Iłżecka J, Przywara S, et al. Serum neuron-specific enolase as a marker of brain ischemia-reperfusion injury in patients undergoing carotid endarterectomy. Acta Clin Croat. 2016;55(4):579–584. doi:10.20471/acc.2016.55.04.07
16. Capoccia L, Speziale F, Gazzetti M, et al. Comparative study on carotid revascularization (endarterectomy vs stenting) using markers of cellular brain injury, neuropsychometric tests, and diffusion-weighted magnetic resonance imaging. J Vasc Surg. 2010;51(3):584–591, 591.e581–583; discussion 592. doi:10.1016/j.jvs.2009.10.079
17. Lu K, Xu X, Cui S, Wang F, Zhang B, Zhao Y. Serum neuron specific enolase level as a predictor of prognosis in acute ischemic stroke patients after intravenous thrombolysis. J Neurol Sci. 2015;359(1–2):202–206. doi:10.1016/j.jns.2015.10.034
18. Xu CM, Luo YL, Li S, et al. Multifunctional neuron-specific enolase: its role in lung diseases. Biosci Rep. 2019;39(11). doi:10.1042/BSR20192732
19. Adams HP
20. Fernández MS, Murias QE, Vega VP, et al. Efficacy and safety of endovascular treatment in acute tandem carotid occlusions: analysis of a single-center cohort. Cerebrovasc Dis Extra. 2020;10(2):50–58. doi:10.1159/000507919
21. Zaidat OO, Yoo AJ, Khatri P, et al. Recommendations on angiographic revascularization grading standards for acute ischemic stroke: a consensus statement. Stroke. 2013;44(9):2650–2663. doi:10.1161/STROKEAHA.113.001972
22. von Kummer R, Broderick JP, Campbell BC, et al. The Heidelberg bleeding classification: classification of bleeding events after ischemic stroke and reperfusion therapy. Stroke. 2015;46(10):2981–2986. doi:10.1161/STROKEAHA.115.010049
23. Hill MD, Goyal M, Menon BK, et al. Efficacy and safety of nerinetide for the treatment of acute ischaemic stroke (ESCAPE-NA1): a multicentre, double-blind, randomised controlled trial. Lancet. 2020;395(10227):878–887. doi:10.1016/S0140-6736(20)30258-0
24. Tsivgoulis G, Katsanos AH, Malhotra K, et al. Thrombolysis for acute ischemic stroke in the unwitnessed or extended therapeutic time window. Neurology. 2020;94(12):e1241–e1248. doi:10.1212/WNL.0000000000008904
25. Karki P, Sharma GR, Joshi S, Paudel P, Shah DB. Retrospective study and outcome predictor after microsurgical resection of cerebral arteriovenous malformations in Nepal. Asian J Neurosurg. 2021;16(2):355–362. doi:10.4103/ajns.AJNS_509_20
26. Ding X, Hou Y, Ma X, Huipeng Z, Wang C, Wang Y. Adult adrenal ganglioneuroblastoma: a rare case report. Can Urol Assoc J. 2015;9(1–2):e75–77. doi:10.5489/cuaj.2410
27. Suh KJ, Keam B, Kim M, et al. Serum neuron-specific enolase levels predict the efficacy of first-line epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors in patients with non-small cell lung cancer Harboring EGFR mutations. Clin Lung Cancer. 2016;17(4):245–252.e241. doi:10.1016/j.cllc.2015.11.012
28. Singh HV, Pandey A, Shrivastava AK, Raizada A, Singh SK, Singh N. Prognostic value of neuron specific enolase and IL-10 in ischemic stroke and its correlation with degree of neurological deficit. Intl J Clin Chem. 2013;419:136–138. doi:10.1016/j.cca.2013.02.014
29. Indra Gunawan P, Noviandi R, Mariana Samosir S. Increased neuron specific enolase in a hypoxic ischemia rat model. Biomed Res Ther. 2022;9(6):5161–5165. doi:10.15419/bmrat.v9i7.752
30. Albers GW, Lansberg MG, Kemp S, et al. A multicenter randomized controlled trial of endovascular therapy following imaging evaluation for ischemic stroke (DEFUSE 3). Int J Stroke. 2017;12(8):896–905. doi:10.1177/1747493017701147
31. Mundiyanapurath S, Tillmann A, Möhlenbruch MA, Bendszus M, Ringleb PA. Endovascular stroke therapy may be safe in patients with elevated international normalized ratio. J Neurointerv Surg. 2017;9(12):1187–1190. doi:10.1136/neurintsurg-2016-012757
32. Arba F, Rinaldi C, Caimano D, Vit F, Busto G, Fainardi E. Blood-brain barrier disruption and hemorrhagic transformation in acute ischemic stroke: systematic review and meta-analysis. Front Neurol. 2020;11:594613. doi:10.3389/fneur.2020.594613
33. Wang X, Lo EH. Triggers and mediators of hemorrhagic transformation in cerebral ischemia. Mol Neurobiol. 2003;28(3):229–244. doi:10.1385/MN:28:3:229
34. Álvarez-sabín J, Maisterra O, Santamarina E, Kase CS. Factors influencing haemorrhagic transformation in ischaemic stroke. Lancet Neurol. 2013;12(7):689–705. doi:10.1016/S1474-4422(13)70055-3
35. Kim BJ, Kim YJ, Ahn SH, et al. The second elevation of neuron-specific enolase peak after ischemic stroke is associated with hemorrhagic transformation. J Stroke Cerebrovasc Dis. 2014;23(9):2437–2443. doi:10.1016/j.jstrokecerebrovasdis.2014.05.020
36. Tian B, Tian X, Shi Z, et al. Clinical and imaging indicators of hemorrhagic transformation in acute ischemic stroke after endovascular thrombectomy. Stroke. 2022;53(5):1674–1681. doi:10.1161/STROKEAHA.121.035425
37. Al-Mufti F, Amuluru K, Roth W, Nuoman R, El-Ghanem M, Meyers PM. Cerebral ischemic reperfusion injury following recanalization of large vessel occlusions. Neurosurgery. 2018;82(6):781–789. doi:10.1093/neuros/nyx341
38. Marto JP, Strambo D, Hajdu SD, et al. Twenty-four-hour reocclusion after successful mechanical thrombectomy: associated factors and long-term prognosis. Stroke. 2019;50(10):2960–2963. doi:10.1161/STROKEAHA.119.026228
39. Huang K, Zha M, Gao J, Du J, Liu R, Liu X. Increased intracranial hemorrhage of mechanical thrombectomy in acute ischemic stroke patients with atrial fibrillation. J Thromb Thrombolysis. 2021;51(2):536–544. doi:10.1007/s11239-020-02269-3
40. Maros ME, Brekenfeld C, Broocks G, et al. Number of retrieval attempts rather than procedure time is associated with risk of symptomatic intracranial hemorrhage. Stroke. 2021;52(5):1580–1588. doi:10.1161/STROKEAHA.120.031242
41. García-Tornel Á, Requena M, Rubiera M, et al. When to Stop. Stroke. 2019;50(7):1781–1788. doi:10.1161/STROKEAHA.119.025088
42. Abraham P, Scott Pannell J, Santiago-Dieppa DR, et al. Vessel wall signal enhancement on 3-T MRI in acute stroke patients after stent retriever thrombectomy. Neurosurg Focus. 2017;42(4):E20. doi:10.3171/2017.1.FOCUS16492
43. Pandey A, Saxena K, Verma M, Bharosay A. Correlative study between neuron-specific enolase and blood sugar level in ischemic stroke patients. J Neurosci Rural Pract. 2011;2(1):50–54. doi:10.4103/0976-3147.80099
44. Anand N, Stead LG. Neuron-specific enolase as a marker for acute ischemic stroke: a systematic review. Cerebrovasc Dis. 2005;20(4):213–219. doi:10.1159/000087701
45. Wang J, Fang X, Wang D, Xiao Y. Effect of intravenous thrombolysis with alteplase on clinical efficacy, inflammatory factors, and neurological function in patients with acute cerebral infarction. Braz J Med Biol Res. 2021;54(5):e10000. doi:10.1590/1414-431x202010000
46. Madsen TE, DeCroce-Movson E, Hemendinger M, et al. Sex differences in 90-day outcomes after mechanical thrombectomy for acute ischemic stroke. J Neurointerv Surg. 2019;11(3):221–225. doi:10.1136/neurintsurg-2018-014050
47. Tan BYQ, Siow I, Lee KS, et al. Effect of sex on outcomes of mechanical thrombectomy in basilar artery occlusion: a multicentre cohort study. Cerebrovasc Dis. 2022;51(5):639–646. doi:10.1159/000524048
48. Casetta I, Fainardi E, Pracucci G, et al. Sex differences in outcome after thrombectomy for acute ischemic stroke. A propensity score-matched study. Eur Stroke J. 2022;7(2):151–157. doi:10.1177/23969873221091648
49. Liu H, Lei S, Jia L, et al. Streptococcus suis serotype 2 enolase interaction with host brain microvascular endothelial cells and RPSA-induced apoptosis lead to loss of BBB integrity. Vet Res. 2021;52(1):30. doi:10.1186/s13567-020-00887-6
50. Pratamastuti D, Indra Gunawan P, Saharso D. Serum neuron specific enolase is increased in pediatric acute encephalitis syndrome. Korean J Pediatr. 2017;60(9):302–306. doi:10.3345/kjp.2017.60.9.302
51. Nikolakopoulou AM, Wang Y, Ma Q, et al. Endothelial LRP1 protects against neurodegeneration by blocking cyclophilin A. J Exp Med. 2021;218:4. doi:10.1084/jem.20202207
52. Haque A, Capone M, Matzelle D, Cox A, Banik NL. Targeting enolase in reducing secondary damage in acute spinal cord injury in rats. Neurochem Res. 2017;42(10):2777–2787. doi:10.1007/s11064-017-2291-z
53. Haque A, Polcyn R, Matzelle D, Banik NL. New insights into the role of neuron-specific enolase in neuro-inflammation, neurodegeneration, and neuroprotection. Brain Sci. 2018;8(2):33. doi:10.3390/brainsci8020033
54. Polcyn R, Capone M, Matzelle D, et al. Enolase inhibition alters metabolic hormones and inflammatory factors to promote neuroprotection in spinal cord injury. Neurochem Int. 2020;139:104788. doi:10.1016/j.neuint.2020.104788
55. Jiang W, Stingelin L, Zhang P, et al. Enolase2 and enolase1 cooperate against neuronal injury in stroke model. Neurosci Lett. 2021;747:135662. doi:10.1016/j.neulet.2021.135662
56. Rafecas A, Bañeras J, Sans-Roselló J, et al. Change in neuron specific enolase levels in out-of-hospital cardiopulmonary arrest survivors as a simple and useful tool to predict neurological prognosis. Rev Esp Cardiol. 2020;73(3):232–240. doi:10.1016/j.recesp.2019.01.014
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

