Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 17
The Regulation Role of the Gut-Islets Axis in Diabetes
Authors Yang S , Cao J, Sun C, Yuan L
Received 14 December 2023
Accepted for publication 3 March 2024
Published 22 March 2024 Volume 2024:17 Pages 1415—1423
DOI https://doi.org/10.2147/DMSO.S455026
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Konstantinos Tziomalos
Songtao Yang,1 Jie Cao,1 Chuan Sun,2 Li Yuan1
1Department of Endocrinology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, 430022, People’s Republic of China; 2Department of Emergency Medical, Wuhan ASIA GENERAL Hospital, Wuhan, 430000, People’s Republic of China
Correspondence: Li Yuan, Email [email protected]
Abstract: The gut-islets axis is an important endocrine signaling axis that regulates the function of islets by modulating the gut micro-environment and its endocrine metabolism. The discovery of intestinal hormones, such as GLP-1 and GIP, has established a preliminary link between the gut and the islet, paving the way for the development of GLP-1 receptor agonists based on the regulation theory of the gut-islets axis for diabetes treatment. This discovery has created a new paradigm for diabetes management and rapidly made the regulation theory of the gut-islets axis a focal point of research attention. Recent years, with in-depth study on gut microbiota and the discovery of intestinal-derived extracellular vesicles, the concept of gut endocrine and the regulation theory of the gut-islets axis have been further expanded and updated, offering tremendous research opportunities. The gut-islets axis refers to the complex interplay between the gut and the islet, which plays a crucial role in regulating glucose homeostasis and maintaining metabolic health. The axis involves various components, including gut microbiota, intestinal hormones, amino acids and ACE2, which contribute to the communication and coordination between the gut and the islet.
Keywords: islets β cell, diabetes, GLP-1, gut microbiota, ACE2
Introduction
As one of the largest organs in the human body, the intestine has always attracted significant attention, and in recent years, more and more research has focused on the gut microbiota. Apart from its digestive function, the diversity of gut microbiota and gut endocrine function provided new directions for the pathophysiological research of various diseases, such as diabetes. The pancreas, adjacent to the intestine, not only has anatomical connections with the gut but also possesses exocrine functions for the secretion of pancreatic juice. Additionally, its endocrine function is equally crucial. Regardless of type 1 or type 2 diabetes(T2DM), the deterioration of islets β-cell function is an important factor in the progression of diabetes that cannot be ignored. Therefore, the preservation of pancreatic β-cell function has always been a key topic in diabetes prevention and treatment. Research has found that endocrine cells in the digestive system have a unique role in regulating islets function. Hormones secreted by intestinal L cells, such as GLP-1, and by K cells, such as GIP, can exert incretin effects on the islets.1 Their presence can enhance glucose-stimulated insulin secretion in islets β-cells. Moreover, studies have shown that the islets and intestinal endocrine cells may share common transcriptional signals and regulatory programs, implying their homogeneity and providing a basis for the reprogramming of gastrointestinal organs into insulin-secreting cells.2
As a key component of the digestive tract, the intestine exhibits complex structural and physiological characteristics that are critical for efficient nutrient absorption and maintenance of overall intestinal homeostasis. The intestine consists of two main parts: the small intestine and the large intestine. The small intestine, which includes the duodenum, jejunum, and ileum, has a highly specialized structure characterized by finger-like projections called villi, which are covered with microvilli collectively known as the brush border. This refined surface area expansion mechanism helps enhance nutrient absorption. In addition, the barrier structure of the intestine can also prevent the intrusion of harmful substances.3 The large intestine, which consists of the colon and rectum, serves primarily to absorb water and electrolytes from undigested residues. In addition, the large intestine is home to a diverse and beneficial intestinal microbiota that aids in the fermentation process and the synthesis of certain vitamins. The symbiotic relationship between the host and intestinal microbes is critical for immune regulation, metabolism, and overall intestinal health.4 In addition to its exocrine function, research on the endocrine function of the gastrointestinal tract has been gradually elucidated in recent years. For example, K cells in the small intestine secrete glucagon-dependent insulin secretagogue (GIP) and glucagon-like peptide 1 (GLP-1), both of which have regulatory effects on pancreatic islets.5 L cells in the colon can secrete GLP-1, which also affects pancreatic islet function. These findings further expand our understanding of the functional complexity of the gastrointestinal tract and provide new insights into the regulatory relationship between the gut and pancreatic islets. Overall, the delicate structure and physiology of the intestine are carefully tuned to optimize nutrient absorption, regulate digestion, maintain water and electrolyte balance, and support symbiotic interactions between the host and intestinal microbes, thus ensuring the integrity of the gastrointestinal system. and functionality. The integrity of the structure and function of the gastrointestinal system is also the basis for maintaining the body’s metabolic balance.
Pancreatic islets occupy about 1% of the pancreas, and although the proportion is small, they account for 15–20% of the organ’s blood flow.6 Pancreatic islets are composed of different types of cells, and endocrine cells include alpha cells, beta cells, delta cells, gamma cells, and epsilon cells, each with unique functions. The main function of pancreatic islets is to regulate blood sugar levels and maintain metabolic balance in the body. Beta cells are the most important cell type in the pancreatic islets, responsible for the synthesis and secretion of insulin, a hormone critical for glucose metabolism and absorption. In contrast, alpha cells produce glucagon, which acts in opposition to insulin and regulates glucose release from the liver.7 Delta cells secrete somatostatin, which inhibits the release of insulin and glucagon.8 Non-endocrine cells may also play an important role in islet homeostasis, such as endothelial cells that maintain the islet capillary network, as well as neural and immune cells that may help coordinate insulin secretion and responses to other stimuli.6 These non-endocrine cells may ensure precise regulation of blood glucose levels and maintain overall metabolic balance by transporting nutrients and soluble factors or providing signals that affect the health and function of islet cells.9 The importance of pancreatic islets lies in their critical regulatory role in maintaining normal blood glucose levels and energy metabolism in the body. Although the precise regulation of islet function is still not fully understood, the introduction of the concept of the gut-islet axis undoubtedly provides new insights into the regulation of islet function.
In recent years, with the emergence of drugs like GLP-1 receptor agonists, the connection between the intestine and the islets has once again been closely linked. We refer to the important endocrine signaling axis that involves the reciprocal regulation of gut microbiota, gut endocrine metabolism, and islets function as the gut-islets axis. This review introduces several aspects of the interaction between the intestine and the islets, including the effects of intestinal endocrine hormones on the islets, the effects of gut microbiota on the islets, and the effects of intestinal amino acids on the islets. This provides new insights for exploring strategies to protect islets β-cell function and offers theoretical value and research prospects in exploring new pathways and intervention targets for gut-islets regulation.
The Intestine and the Islets
The Intestine
The intestine, as a critical component of the gastrointestinal tract, exhibits a complex structure and physiology essential for efficient nutrient absorption and maintaining overall gut homeostasis. It consists of two major divisions: the small intestine and the large intestine. The small intestine, comprising the duodenum, jejunum, and ileum, exhibits a highly specialized architecture characterized by finger-like projections called villi, lined with microvilli, collectively known as the brush border. This elaborate surface area amplification allows for enhanced nutrient absorption. The barrier structure of the intestine also prevents the invasion of harmful substances.
The large intestine, comprising the colon and rectum, primarily serves as a site for water and electrolyte absorption from undigested residues. Additionally, the large intestine harbors a diverse population of beneficial gut microbiota, contributing to fermentation processes and the synthesis of certain vitamins. This symbiotic relationship between the host and gut microbiota is vital for immune regulation, metabolism, and overall intestinal health.
In addition to its exocrine function, the endocrine role of the gastrointestinal tract has been increasingly elucidated in recent years. For instance, K cells in the small intestine secrete GIP and GLP1, which act on the pancreatic islets. L cells located in the colon can secrete GLP1 that also acts on the pancreatic islets. These research findings have further expanded our understanding of the functional complexity of the gastrointestinal tract and provided new insights into the regulatory relationship between the intestine and the pancreatic islets.
Overall, the intricate structure and physiology of the intestine are finely tuned to optimize nutrient absorption, regulate digestion, maintain water and electrolyte balance, and support the mutualistic interaction between the host and gut microbiota, thereby ensuring the integrity and functionality of the gastrointestinal system. The integrity of the structure and function of the gastrointestinal system is also fundamental to maintaining metabolic homeostasis in the body.
The Islets
Islets, constituting approximately 1% of the pancreas, play a crucial role despite their small proportion, requiring 15–20% of the organ’s blood flow.10 Islets are composed of different cell types, with the endocrine cells including α cells, β cells, δ cells, γ cells, and ε cells, each having distinct functions. The main function of islets is to regulate blood glucose levels and maintain metabolic balance within the body. β cells, the most important cell type in the islets, are responsible for synthesizing and secreting insulin, a hormone essential for glucose metabolism and absorption. In contrast, α cells produce glucagon, which opposes the actions of insulin and regulates the release of glucose from the liver. δ cells secrete somatostatin, which inhibits the release of both insulin and glucagon.6 Non-endocrine cells may also play a significant role in islet homeostasis, such as the endothelial cells that maintain the islet capillary network, as well as neuronal and immune cells that potentially contribute to coordinating insulin secretion and other stimulus responses. These non-endocrine cells likely regulate islet homeostasis by transporting nutrients and soluble factors or providing signals that influence the health and function of islet cells, ensuring precise regulation of blood glucose levels and maintaining overall metabolic balance.9,11 The importance of islets lies in their critical regulatory role in maintaining normal blood glucose levels and energy metabolism within the body, although the precise regulation of islet function remains incompletely understood. The introduction of the concept of the gut-islets axis undoubtedly provides new insights into the regulation of islets function.
Intestinal Endocrine Hormones
GLP-1 and Islets β Cells
GLP-1 is secreted from intestinal endocrine L cells. The most significant effect of GLP-1 on islets β cells is to enhance glucose-dependent stimulation of insulin secretion. This effect is primarily mediated by the activation of PKA and Epac after GLP-1R activation on islets β cells.12 Furthermore, GLP-1 can regulate the proliferation, apoptosis, and differentiation of β cells, thereby improving their function. Even under conditions of hyperglycemia, hyperlipidemia, inflammatory cytokines and oxidative stress, GLP-1 can promote the survival of β cells.13 Emerging evidence from multiple research groups utilizing diverse rodent and cellular models indicates that GLP-1R agonists have the potential to modulate β cell mass by attenuating β cell apoptosis. Similarly, the GLP-1R agonist liraglutide has demonstrated the ability to decrease apoptosis rate and enhance the viability of transplanted islets.14 Additionally, both GLP-1 and GIP, individually and in combination, have been found to increase the expression of insulin, Bcl-2, and Pdx-1 genes in human islets.15,16 Moreover, activation of the GLP-1R exerts anti-inflammatory effects in human islets., as evidenced by the suppression of chemokine and cytokine expression, possibly through the downregulation of STAT-1 expression in response to the GLP-1R agonist Exendin-4.17,18
GLP-1 regulates β-cell mass not only by inhibiting apoptosis but also by controlling β-cell proliferation. Research investigations have identified the indispensable role of Skp2 (S phase kinase-associated protein 2) as a crucial downstream mediator required for the proliferative actions of GLP-1 in β-cells. Treatment with exendin-4 for 7 days in 8-week-old Skp2-/- mice resulted in a 2.5-fold increase in β-cell mass and a 7.2-fold increase in Ki67+ β-cells in control ± mice, whereas no such effects were observed in Skp2-/- mice. Histochemical analysis revealed reduced β-cell p27 expression. Furthermore, administration of exendin-4 to 4-week-old db/db mice for a duration of 7 days resulted in heightened β-cell proliferation. This effect was accompanied by a reduction in the expression of the cell cycle inhibitor p27 and an elevation in Skp2 expression within the β-cells.19,20 Similarly, exendin-4 administering treatment for two weeks to 6-week-old prediabetic mice prevented the progression to severe diabetes. As a consequence of this treatment, there was an enlargement in the mass of the islets, a promotion of β-cell proliferation, and enhancements in glucose tolerance and insulin secretion.21
GLP-1 and Islets α Cells
The most well-known effect of GLP-1 on α cells is the inhibition of glucagon secretion. Nevertheless, two questions remain regarding GLP-1 and islet α cells: a) how GLP-1 suppresses glucagon secretion from α cells, and b) how and under what circumstances do islet α cells begin to produce genuine bioactive GLP-1? According to classical concepts, uninjured α cells within a healthy pancreas do not typically produce GLP-1. However, multiple reports suggest that islet injury, metabolic stress, exposure to cytokines, or pancreatic and/or islet injury or inflammation can induce the expression of the PC1/3 gene and the production of GLP-1 in α cells.22–24
How does GLP-1 inhibit glucagon secretion? Although direct inhibition of GLP-1Rs expressed and functional in individual α cells might seem like an obvious mechanism, studies have shown low expression of the GLP-1r on α cells.25 Therefore, the available data suggests that the effects of GLP-1 in inhibiting glucagon secretion are likely indirect. These indirect mechanisms may involve the central nervous system (CNS), the β cell, the δ cell, or other additional pathways. Furthermore, GLP-1 has the potential to increase β cell regeneration by promoting α-to-β cell transdifferentiation, although this effect is likely observed only in cases of extreme β cell exhaustion.26,27
Gip
GIP is released from K cells in the intestinal epithelium and exhibits robust incretin activity in both rodents and human subjects. Unlike GLP-1, which exhibits various non-incretin effects in the regulation of blood glucose, GIP primarily stimulates insulin secretion in a glucose-dependent manner. Additionally, GIP acts on β cells to stimulate β-cell proliferation and exert an anti-apoptotic effect to preserve β-cell mass.28 Another important difference between GIP and GLP-1 is that GIP stimulates glucagon secretion from α cells.29 The number of GIP receptors (GIPR) on α and β cells is similar, and studies have shown that glucagon secreted by GIP-stimulated α cells can enhance insulin secretion by β cells beyond direct GIPR activation in β cells alone.30
GLP-1 and GIP are well-studied incretins with known insulin-stimulating properties. While these properties have been identified many years ago, new functions of incretins continue to be discovered. Simultaneously, the development and application of drugs such as GLP-1R agonists and GLP-1/GIP dual receptor agonists will provide fresh insights into the theory of the gut-islets axis.
Gut Metabolites and Microbiota
Gut Metabolites
Following a nutrient-rich meal, islets β cells intricately integrate a multitude of external signals to meticulously modulate the release of insulin, thus precisely orchestrating the regulation of blood glucose levels in accordance with the metabolic requirements. Among these signaling molecules, amino acids assume paramount significance as pivotal stimulatory agents for insulin secretion. Notably, amino acids exert their profound influence by effectively stimulating islets β cells to promptly secrete insulin in response to fluctuations in blood glucose concentrations. In addition, some amino acids, especially branched-chain amino acids (BCAAs), have been shown to contribute to the pathogenesis of insulin resistance and T2DM by inhibiting insulin signaling and promoting inflammation. BCAAs are essential amino acids, including leucine, isoleucine, and valine, that possess the ability to directly stimulate insulin secretion in pancreatic beta cells. Currently, the exclusive source of BCAAs is through dietary intake, followed by subsequent absorption in the intestines. It’s reported that circulating BCAAs levels rise about 2–3 fold after a meal.31 Low-dose BCAAs therapy has been shown to significantly improve the expression of important transcription factors such as pdx1, a marker for islets β cells, in diabetic rats. This therapy also inhibits the elevation of plasma glucose levels in diabetic rats by increasing circulating insulin levels.32 However, sustained elevation of circulating BCAAs levels is associated with insulin resistance. Studies have seen plasma BCAAs elevations as the strongest predictor for developing diabetes in the next ten years or more.33,34 And when infused BCAAs into the circulation, healthy volunteers are found impairment in glucose disposal.35 Similarly, adding BCAAs to a high-fat diet worsens glucose tolerance in rodent models,31 while BCAAs-deficient diets can intervene in the progression of type 2 diabetes in db/db mice.36 Overall, these data suggest a close association between BCAAs and the development of insulin resistance. Insulin resistance itself may lead to elevated levels of BCAAs. Although the mechanisms behind this relationship are not yet clear, it can be anticipated that an excessive amount of BCAAs and insulin resistance may form a vicious cycle, metabolic disorders can act as both driving factors impairing functionality and as outcomes of impaired functionality.
Mice fed phenylalanine-rich chow or phenylalanine-producing aspartame or overexpressing human phenylalanyl-tRNA synthetase develop insulin resistance and T2DM symptoms. In addition, providing mice with a phenylalanine-rich diet or administering aspartame, which generates phenylalanine, leads to the inactivation of insulin receptor β and impairs insulin-stimulated glucose uptake, resulting in insulin resistance and symptoms of T2DM in mice;37 Tyrosine can cause abnormal glucose tolerance by altering β cell insulin secretion;38 Following weight-loss surgery, levels of intestinal glycine, histidine, glutamate, and glutamine are significantly increased and associated with improved β cell function and metabolism;39 Glutamate improves glucose metabolism by enhancing incretin-induced insulin secretion.40,41 In summary, amino acids play a crucial role in the gut-islets axis by directly and indirectly influencing the secretion and action of intestinal endocrine hormones and insulin, thereby regulating blood glucose levels and energy metabolism.
Gut Microbiota
The microorganisms residing in the human gut play a crucial role in host metabolism. The gut microbiota comprises not only bacteria but also viruses, bacteriophages, yeast, and fungi, among others, forming a symbiotic relationship with the host.42 Imbalance in the gut microbiota can lead to various metabolic disorders such as metabolic syndrome, type 2 diabetes mellitus (T2DM), and gestational diabetes mellitus (GDM).43,44 Mechanistically, the interaction between the gut microbiota and islets function has been demonstrated to be mediated by multiple factors, including the regulation of insulin secretion and insulin sensitivity, the modulation of intestinal endocrine hormones, and the influence of microbial-derived components. In a cross-over, randomized double-blind placebo-controlled trial, supplementing with Bifidobacterium can simultaneously improve both fasting and postprandial insulin sensitivity of the volunteers.45 Butyrate-producing bacteria, such as F. prausnitzii, have appeared to alleviate insulin resistance by inducing GLP-1 secretion from the enteroendocrine L cells.46 The bile salt hydrolases produced by Bifidobacterium and Lactobacillus is able to convert primary conjugated bile salts into deconjugated bile acids which subsequently induce the production of GLP-1.47 Moreover, metabolites produced by microbial fermentation of fibers induced the production of the endogenous peptides GLP-1 and GLP-2.44,48 In addition, gut microbiota can affect intestinal amino acid absorption and metabolism through various pathways, thereby influencing islets function. Prevotella copri and Bacteroides vulgatus have been identified as the key species responsible for the correlation between the biosynthesis of branched-chain amino acids, insulin resistance, and glucose intolerance.49 However, the effects of gut microbiota on islets function are mostly indirect, mediated through gut hormones and metabolites. The improvement of insulin resistance in target organs, such as the liver, skeletal muscle, and adipose tissue, is mainly observed. Further research is needed to understand the direct regulation of islets cells by gut microbiota. Recent studies have opened up new perspectives on the gut-islets axis. It has been discovered that extracellular vesicles derived from gut microbiota can transfer microbial DNA to islets β cell, leading to islets inflammation and β cell damage.50 Under normal conditions, islets Vsig4+ macrophages can prevent the infiltration of gut microbiota and its derivatives into the host circulation and distant organs through a mechanism mediated by complement protein C3. However, in obese individuals with chronic low-grade inflammation, there is a significant reduction in the number of Vsig4+ macrophages, leading to intestinal barrier impairment. As a result, gut microbiota-derived extracellular vesicles spread and microbial DNA accumulates in β cells of obese mice, exacerbating islet inflammation and triggering more severe metabolic syndrome. This suggests that in addition to regulating gut-derived incretins like GIP and GLP-1 on the islets, gut microbiota can also secrete extracellular vesicles for long-distance transmission to the islets. These vesicles contain bioactive substances that regulate the function of islets α and β cells.
Ace2
The classical understanding of ACE2 primarily revolves around its role as a crucial component of the ACE2/Ang1-7/Mas axis, which antagonizes the ACE signaling pathway in the renin-angiotensin system(RAS). It has garnered significant attention due to its dual action of reducing the levels of angiotensin II (Ang II) and counteracting the effects of Ang II, resembling the inhibitory effects of RAS inhibitors such as ACE inhibitors (ACEI) and ARB.51 Recent studies have revealed the presence of a complete set of ACE2/Ang1-7/Mas axis components within islets, indicating its role in local regulation. ACE2 exerts beneficial effects in islets by improving islet microcirculation and preserving endothelial cell function through its interaction with the Mas receptor. Additionally, it inhibits oxidative stress and inflammatory responses, reduces the dedifferentiation of islets β cells under metabolic stress, positively regulates the function and differentiation state of β cells, and promotes β cell proliferation.52–54 Moreover, ACE2 is widely distributed in the intestine, with the highest expression observed in the small intestine. It plays beneficial roles such as anti-inflammatory effects, immune regulation, and maintenance of intestinal barrier function, contributing to the homeostasis of the intestinal environment. Viral infections and inflammatory conditions significantly decrease the expression of ACE2 in the intestines, inhibiting its protective function, compromising intestinal barrier function, and increasing susceptibility to inflammation.55,56 Recent research from our research group has confirmed that ACE2 serves as a membrane structural ligand for amino acid transport.57 It is involved in the regulation of islets function by modulating the transport and absorption of amino acids in the upstream of the intestine. This suggests that ACE2 may regulate islets cell function through both local islets regulation and the gut-islets axis. This also suggests that there may be new regulatory pathways and mechanisms in the gut-islets axis, specifically mediated by gut-derived extracellular vesicles, to modulate the function of distant islets. This will provide a new bridge for communication between the gut and the islets, serving as a novel pathway for regulating the gut-islets axis and injecting new meaning into the theory of gut-islets axis regulation.
Conclusion
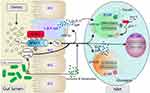
Recent years, an increasing number of studies have discovered the significant role of the gut-islets axis in the pathogenesis of metabolic and digestive system disorders such as diabetes, obesity, and inflammatory bowel disease. Therefore, investigating the mechanisms and regulatory pathways of the gut-islets axis is of great significance for the prevention and treatment of these diseases. The proposal of the gut-islets axis undoubtedly enhances people’s understanding of the close connection and interaction between the intestine and islets, as well as the role of gut microbiota in the regulation of islets function(Figure 1).
Research on the gut-islets axis has delved into the micro level, revealing the correlation between factors such as gut microbiota, enteroendocrine hormones, and amino acids with islets function. These intestinal-derived molecules affect the proliferation and apoptosis of pancreatic tissue cells through direct or indirect pathways and produce a moderately ameliorating effect on the differentiation and proliferative survival of pancreatic islet β-cells. In the same time, there exists a partial synergy between these molecules, which in turn comprehensively and accurately regulates the proliferation and endocrine functionality of pancreatic islet tissues. These research findings are of great significance for addressing intestinal and metabolic diseases, especially in the treatment and prevention of conditions like diabetes. Future research needs to deeply analyze the molecular mechanisms of the “gut-islet axis”, including hormone signaling, microbial-mediated effects, immune regulation, etc. This will help to more comprehensively understand and improve the regulatory network of the “gut-islet axis”. Secondly, treatment methods based on the “gut-islet axis” theory also need to focus on. An in-depth understanding of the role of the “gut-islet axis” can provide new directions for nutritional intervention. By adjusting dietary structure and nutrient intake, it may help maintain the balance of the “gut-islet axis” and prevent the occurrence of metabolic diseases. At the same time, an in-depth understanding of the “gut-islet axis” may provide new targets for the treatment of metabolic diseases such as diabetes and obesity. The development of drugs that can regulate the “gut-islet axis” may become part of future treatment strategies, combined with continuous Updated gene regulation technology and nanobiological means, various treatment methods and intervention methods derived from the “gut-islet axis” will also provide effective guarantees for the prevention, diagnosis and treatment of various related diseases. Research on the “gut-islet axis” should not ignore the existence of individual differences. Future research should pay more attention to individualized treatment to develop more precise treatment plans based on individual microbial composition, genetic background and other factors. Overall, the study of the “gut-islet axis” provides us with a more comprehensive and complex perspective on the regulation of islet function. Future research will continue to delve deeper into this field to provide new ideas and strategies into the treatment and prevention of metabolic diseases.
Acknowledgments
Dr Songtao Yang, Cao Jie and Sun Chuan contributed equally as co-first authors.
Funding
This research was funded by National Natural Science Foundation of China, grant number 82170812 and 81974104.
Disclosure
The authors declare that there are no conflicts of interest.
References
1. Holst JJ. The incretin system in healthy humans: the role of GIP and GLP-1. Metabolism. 2019;96:46–55. doi:10.1016/j.metabol.2019.04.014
2. Lavergne A, Tarifeno-Saldivia E, Pirson J, et al. Pancreatic and intestinal endocrine cells in zebrafish share common transcriptomic signatures and regulatory programmes. BMC Biol. 2020;18(1):109. doi:10.1186/s12915-020-00840-1
3. Camilleri M. Leaky gut: mechanisms, measurement and clinical implications in humans. Gut. 2019;68(8):1516–1526. doi:10.1136/gutjnl-2019-318427
4. Schoeler M, Caesar R. Dietary lipids, gut microbiota and lipid metabolism. Reviews Endocrin Metabolic Disord. 2019;20(4):461–472. doi:10.1007/s11154-019-09512-0
5. Gribble FM, Reimann F. Enteroendocrine Cells: chemosensors in the intestinal epithelium, annual review of physiology 78; 2016;277–299.
6. Walker J, Saunders D, Brissova M, Powers A. The human islet: mini-organ with mega-impact. Endocrine Reviews. 2021;42(5):605–657. doi:10.1210/endrev/bnab010
7. Wendt A, Eliasson L. Pancreatic α-cells - The unsung heroes in islet function. Seminars Cell Develop Biology. 2020;103:41–50. doi:10.1016/j.semcdb.2020.01.006
8. Barbieux C, Parnaud G, Lavallard V, et al. Asymmetrical distribution of δ and PP cells in human pancreatic islets. J Endocrinol. 2016;229(2):123–132. doi:10.1530/JOE-15-0542
9. Li W, Yu G, Liu Y, Sha L. Intrapancreatic ganglia and neural regulation of pancreatic endocrine secretion. Front Neurosci. 2019;13:21. doi:10.3389/fnins.2019.00021
10. Vlahos AE, Cober N, Sefton MV. Modular tissue engineering for the vascularization of subcutaneously transplanted pancreatic islets. Proc Natl Acad Sci. 2017;114(35):9337–9342. doi:10.1073/pnas.1619216114
11. Faber CL, Deem JD, Campos CA, G.j. T, Morton GJ. CNS control of the endocrine pancreas. Diabetologia. 2020;63(10):2086–2094. doi:10.1007/s00125-020-05204-6
12. Rowlands J, Heng J, Newsholme P, Carlessi R. Pleiotropic effects of glp-1 and analogs on cell signaling, metabolism, and function. Front Endocrinol. 2018;9:672. doi:10.3389/fendo.2018.00672
13. Dj D. Incretin action in the pancreas Potential promise, possible perils, and pathological. Diabetes. 2013;62(10):3316–3323. doi:10.2337/db13-0822
14. Toso C, McCall M, Emamaullee J, et al. Liraglutide, a long-acting human glucagon-like peptide 1 analogue, improves human islet survival in culture. Transpl Int. 2010;23(3):259–265. doi:10.1111/j.1432-2277.2009.00984.x
15. Suleiman M, Marselli L, Cnop M, et al. The role of beta cell recovery in type 2 diabetes remission. Int J Mol Sci. 2022;23(13):7435. doi:10.3390/ijms23137435
16. Lupi R, Del Guerra S, D’Aleo V, Boggi U, Filipponi F, Marchetti P. The direct effects of GLP-1 and GIP, alone or in combination, on human pancreatic islets. Regul Pept. 2010;165(2–3):129–132. doi:10.1016/j.regpep.2010.04.009
17. Luo Y, Yang P, Li Z, et al. Liraglutide improves non-alcoholic fatty liver disease in diabetic mice by modulating inflammatory signaling pathways. Drug Des Devel Ther. 2019;13:4065–4074. doi:10.2147/DDDT.S224688
18. Pugazhenthi U, Velmurugan K, Tran A, Mahaffey G, Pugazhenthi S. Anti-inflammatory action of exendin-4 in human islets is enhanced by phosphodiesterase inhibitors: potential therapeutic benefits in diabetic patients. Diabetologia. 2010;53(11):2357–2368. doi:10.1007/s00125-010-1849-y
19. Tschen SI, Georgia S, Dhawan S, Bhushan A. Skp2 is required for incretin hormone-mediated beta-cell proliferation. Mol Endocrinol. 2011;25(12):2134–2143. doi:10.1210/me.2011-1119
20. Song WJ, Schreiber WE, Zhong E, et al. Exendin-4 stimulation of cyclin A2 in beta-cell proliferation. Diabetes. 2008;57(9):2371–2381. doi:10.2337/db07-1541
21. Ren L, Cui Q, Liu W, et al. Novel GLP-1 analog supaglutide stimulates insulin secretion in mouse and human islet beta-cells and improves glucose homeostasis in diabetic mice. Front Physiol. 2019;10:930. doi:10.3389/fphys.2019.00930
22. Saikia M, Holter MM, Donahue LR, et al. GLP-1 receptor signaling increases PCSK1 and beta cell features in human alpha cells. JCI Insight. 2021;6(3). doi:10.1172/jci.insight.141851
23. Zhu L, Dattaroy D, Pham J, et al. Intra-islet glucagon signaling is critical for maintaining glucose homeostasis. JCI Insight. 2019;5(10):1.
24. Fernandez-Millan E, de Toro-Martin J, Lizarraga-Mollinedo E, Escriva F, Alvarez C. Role of endogenous IL-6 in the neonatal expansion and functionality of Wistar rat pancreatic alpha cells. Diabetologia. 2013;56(5):1098–1107. doi:10.1007/s00125-013-2862-8
25. Davis EM, Sandoval DA. Glucagon-like peptide-1: actions and influence on pancreatic hormone function. Compr Physiol. 2020;10(2):577–595.
26. Lee YS, Lee C, Choung JS, Jung HS, Jun HS. Glucagon-like peptide 1 increases beta-cell regeneration by promoting alpha- to beta-cell transdifferentiation. Diabetes. 2018;67(12):2601–2614. doi:10.2337/db18-0155
27. Thorel F, Nepote V, Avril I, et al. Conversion of adult pancreatic alpha-cells to beta-cells after extreme beta-cell loss. Nature. 2010;464(7292):1149–1154. doi:10.1038/nature08894
28. Campbell JE, Drucker DJ. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab. 2013;17(6):819–837. doi:10.1016/j.cmet.2013.04.008
29. El K, Campbell JE. The role of GIP in alpha-cells and glucagon secretion. Peptides. 2020;125:170213. doi:10.1016/j.peptides.2019.170213
30. El K, Gray SM, Capozzi ME, et al. GIP mediates the incretin effect and glucose tolerance by dual actions on α cells and β cells. Sci Adv. 2021;12(7):11.
31. Neinast M, Murashige D, Arany Z. Branched chain amino acids. Annu Rev Physiol. 2019;81(1):139–164. doi:10.1146/annurev-physiol-020518-114455
32. Lu M, Zhang X, Zheng D, Jiang X, Chen Q. Branched-chain amino acids supplementation protects streptozotocin-induced insulin secretion and the correlated mechanism. Biofactors. 2015;41(2):127–133. doi:10.1002/biof.1188
33. Liu J, Semiz S, van der Lee SJ, et al. Metabolomics based markers predict type 2 diabetes in a 14-year follow-up study. Metabolomics. 2017;13(9):104. doi:10.1007/s11306-017-1239-2
34. Guasch-Ferré M, Hruby A, Toledo E, et al. Metabolomics in prediabetes and diabetes a systematic review and meta-analysis. Diabetes Care. 2016;39(5):833–846. doi:10.2337/dc15-2251
35. Harris LLS, Smith GI, Patterson BW, et al. Alterations in 3-hydroxyisobutyrate and FGF21 metabolism are associated with protein ingestion-induced insulin resistance. Diabetes. 2017;66(7):1871–1878. doi:10.2337/db16-1475
36. Wei S, Zhao J, Wang S, Huang M, Wang Y, Chen Y. Intermittent administration of a leucine-deprived diet is able to intervene in type 2 diabetes in db/db mice. Heliyon. 2018;4(9):e00830. doi:10.1016/j.heliyon.2018.e00830
37. Zhou WW, Sun JC, Chen HL. Phenylalanine impairs insulin signaling and inhibits glucose uptake through modification of IRbeta. Nat Commun. 2022;13(1):4291. doi:10.1038/s41467-022-32000-0
38. Korner J, Cline GW, Slifstein M, et al. A role for foregut tyrosine metabolism in glucose tolerance. Mol Metab. 2019;23:37–50. doi:10.1016/j.molmet.2019.02.008
39. Tan C, Zheng Z, Wan X, Cao J, Wei R, Duan J. The role of gut microbiota and amino metabolism in the effects of improvement of islet beta-cell function after modified jejunoileal bypass. Sci Rep. 2021;11(1):4809. doi:10.1038/s41598-021-84355-x
40. Gheni G, Ogura M, Iwasaki M, et al. Glutamate acts as a key signal linking glucose metabolism to incretin/cAMP action to amplify insulin secretion. Cell Rep. 2014;9(2):661–673. doi:10.1016/j.celrep.2014.09.030
41. Yokoi N, Gheni G, Takahashi H, Seino S. beta-Cell glutamate signaling: its role in incretin-induced insulin secretion. J Diabetes Investig. 2016;1(Suppl 1):38–43. doi:10.1111/jdi.12468
42. Gomes AC, Hoffmann C, Mota JF. The human gut microbiota: metabolism and perspective in obesity. Gut Microbes. 2018;9(4):308–325. doi:10.1080/19490976.2018.1465157
43. Kuang YS, Lu JH, Li SH, et al. Connections between the human gut microbiome and gestational diabetes mellitus. Gigascience. 2017;6(8):1–12. doi:10.1093/gigascience/gix058
44. Dabke K, Hendrick G, Devkota S. The gut microbiome and metabolic syndrome. J Clin Invest. 2019;129(10):4050–4057. doi:10.1172/JCI129194
45. Solito A, Cionci NB, Calgaro M, et al. Supplementation with bifidobacterium breve BR03 and B632 strains improved insulin sensitivity in children and adolescents with obesity in a cross-over, randomized double-blind placebo-controlled trial. Clin Nutr. 2021;40(7):4585–4594. doi:10.1016/j.clnu.2021.06.002
46. Lee CJ, Sears CL, Maruthur N. Gut microbiome and its role in obesity and insulin resistance. Ann N Y Acad Sci. 2020;1461(1):37–52. doi:10.1111/nyas.14107
47. Gurung M, Li Z, You H, et al. Role of gut microbiota in type 2 diabetes pathophysiology. EBio Med. 2020;51:102590. doi:10.1016/j.ebiom.2019.11.051
48. Thaiss CA, Levy M, Grosheva I. Hyperglycemia drives intestinal barrier dysfunction and risk for enteric infection. Science. 2018;359(6382):1376–1383. doi:10.1126/science.aar3318
49. Pedersen HK, Gudmundsdottir V, Nielsen HB, et al. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature. 2016;535(7612):376–381. doi:10.1038/nature18646
50. Gao H, Luo Z, Ji Y, et al. Accumulation of microbial DNAs promotes to islet inflammation and beta cell abnormalities in obesity in mice. Nat Commun. 2022;13(1):565. doi:10.1038/s41467-022-28239-2
51. Verano-Braga T, Martins ALV, Motta-Santos D, Campagnole-Santos M, Santos RS; Campagnole-Santos, R.A.S. Santos, ACE2 in the renin-angiotensin system. Clin Sci (Lond). 2020;134(23):3063–3078. doi:10.1042/CS20200478
52. Xuan X, Gao F, Ma X, et al. Activation of ACE2/angiotensin (1-7) attenuates pancreatic beta cell dedifferentiation in a high-fat-diet mouse model. Metabolism. 2018;81:83–96. doi:10.1016/j.metabol.2017.12.003
53. Yuan L, Wang Y, Lu C, Li X. Angiotensin-converting enzyme 2 deficiency aggravates glucose intolerance via impairment of islet microvascular density in mice with high-fat diet. J Diabetes Res. 2013;2013:405284. doi:10.1155/2013/405284
54. Ma X, Gao F, Chen Q, et al. ACE2 modulates glucose homeostasis through GABA signaling during metabolic stress. J Endocrinol. 2020;246(3):223–236. doi:10.1530/JOE-19-0471
55. Garg M, Royce SG, Tikellis C, et al. Imbalance of the renin-angiotensin system may contribute to inflammation and fibrosis in IBD: a novel therapeutic target? Gut. 2020;69(5):841–851. doi:10.1136/gutjnl-2019-318512
56. Jaworska K, Koper M, Ufnal M. Gut microbiota and renin-angiotensin system: a complex interplay at local and systemic levels. Am J Physiol Gastrointest Liver Physiol. 2021;321(4):G355–G366. doi:10.1152/ajpgi.00099.2021
57. Chen Q, Gao F, Gao Y, et al. Intestinal ACE2 regulates glucose metabolism in diet-induced obese mice through a novel gut-islet axis mediated by tryptophan. Obesity. 2023;31(5):1311–1325. doi:10.1002/oby.23719
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.