Back to Journals » Journal of Inflammation Research » Volume 16
The Potential of Natural Compounds Regulating Autophagy in the Treatment of Osteoporosis
Authors Zhao Y, Qu Z, Zhao S, Zhang Y, Gong Y, Zhang B, Gao X, Wang D, Yan L
Received 25 August 2023
Accepted for publication 28 November 2023
Published 8 December 2023 Volume 2023:16 Pages 6003—6021
DOI https://doi.org/10.2147/JIR.S437067
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Yiwei Zhao,* Zechao Qu,* Songchuan Zhao, Yong Zhang, Yining Gong, Bo Zhang, Xiangcheng Gao, Dong Wang, Liang Yan
Department of Spine Surgery, Honghui Hospital, Xi’an Jiao Tong University, Xi’an, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Liang Yan, Department of Spine Surgery, Honghui Hospital, Xi’an Jiao Tong University, Xi’an, People’s Republic of China, Email [email protected]
Abstract: The maintenance of bone homeostasis is dynamically regulated by osteoblast-mediated bone formation and osteoclast-mediated bone resorption. Abnormal differentiation of osteoclast and insufficient osteoblast production can cause bone diseases such as osteoporosis. As one of the highly conserved catabolic pathways in eukaryotic cells, autophagy plays an important role in maintaining cell homeostasis, stress injury repair, proliferation and differentiation. Numerous studies have found that autophagy activity is essential for the survival, differentiation and function of bone cells, and that regulation of autophagy can affect the metabolism of osteoblasts and osteoclasts, thus affecting bone homeostasis. Therefore, using autophagy as a theme, this review outlines the basic process of autophagy, the relationship between autophagy and osteoblasts and osteoclasts, and summarizes the latest research progress of common autophagic signaling pathways in osteoblasts and osteoclasts. The regulatory effects of protein molecules and natural compounds on the autophagy pathway of osteoblasts and osteoclasts discovered in current research are summarized and discussed. This will help to further clarify the mechanism of osteoporosis, understand the relationship between autophagy and osteoporosis, and propose new therapeutic strategies and new ideas for anti-osteoporosis.
Keywords: osteoporosis, osteoblast, osteoclast, autophagy signaling pathway, natural compounds
Introduction
Osteoporosis (OP) is a multifactorial systemic bone disease that is characterized by decreased bone mass, destruction of bone microarchitecture, and increased bone fragility.1 According to a myriad of scientific investigations, it has been established that OP can be attributed to a wide array of factors. At the cellular level, two principal categories of cells have been identified, as elucidated by numerous scholarly studies: Osteoblasts (OBs), originating from bone marrow mesenchymal stem cells, and osteoclasts (OCs), formed through the fusion of mononuclear macrophages, constitute two distinct cell types that hold significant relevance in the context of osteoporosis. Notably, researchers have shown a keen interest in understanding the mechanisms behind OP resulting from inadequate bone formation, orchestrated by OBs, as well as excessive bone resorption, orchestrated by OCs.2 At the pathogenic level, a multitude of factors, including inflammation, endocrine dysfunction, aging, oxidative stress, among others, have been identified as disruptors of the delicate equilibrium between OBs and OCs in bone metabolism.3,4 Hence, the intricate interplay and harmonious equilibrium between OBs and OCs assume paramount importance and must not be disregarded.
Autophagy, a highly conserved self-degradative process in cells, plays a vital role in preserving cellular homeostasis during nutrient scarcity or stressful conditions. It encompasses a distinctive series of processes, including the orchestrated recruitment of autophagy-related proteins, the initiation of autophagic flux, the formation of autophagosomes, and the fusion of autophagosomes with lysosomes.5 Therefore, any intervention that promotes or disrupts any aspect of this intricate process can modulate the level of autophagy and impact cellular homeostasis. For instance, the autophagy inhibitor bafilomycin A1 has demonstrated the ability to effectively impede the fusion of autophagosomes with lysosomes, thereby attenuating autophagy.6,7 Recent accumulating evidence substantiates that the modulation of autophagy can mitigate the detrimental effects triggered by hormonal fluctuations, nutrient insufficiency, chronic inflammation, aging, oxidative stress, and effectively safeguard cells against apoptosis.8 Moreover, dysregulated autophagic activity exerts a profound influence on the differentiation and functionality of OBs and OCs, rendering it inseparable from OP pathogenesis instigated by a myriad of factors, including aging, estrogen deficiency, glucocorticoid exposure, diabetes, and other pertinent etiological elements.9,10 Thus, modulating autophagy presents a promising therapeutic strategy for the management of bone-related disorders.
Natural compounds are the result of nature’s screening and evolution over a long period of time, and their rich stereostructures and functional groups are of great significance to the discovery of new drugs and the exploration of drug mechanisms, which have always been an important source of new drug discovery. Many clinically applied drugs are directly or indirectly derived from natural compounds, such as, penicillin, rapamycin, etc. The discovery of these natural compound drugs has made an indelible contribution to human health.11,12 In recent years, many natural compounds have been found to have great potential in regulating autophagy and treating osteoporosis.
Given the aforementioned discoveries, this article reviews the autophagy signaling pathways of OBs and OCs, furthermore, it explores the impact of protein molecules and natural compounds that regulate autophagy signaling pathways on the functionality of OBs and OCs, and this article offers a significant reference point for comprehending the pathogenesis of OP. Further provides novel insights for the treatment of OP.
Overview of Autophagy
Autophagy can be defined as the process by which cytoplasmic components are delivered to lysosomes for degradation and recycling. Currently, it is well-established that autophagy participates in various cellular metabolism, these include the clearance of misfolded proteins, removal of damaged organelles, elimination of intracellular pathogens, as well as the regulation of cell differentiation and specific functions.13 The essence lies in conferring survival advantages to cells with active autophagy compared to autophagy-deficient cells when confronted with environmental changes, nutrient scarcity, or energy deficiencies.14,15
Classification of Autophagy
In mammalian cells, three types of autophagy have been identified: macroautophagy, microautophagy and chaperone-mediated autophagy.16 In macroautophagy, the autophagosome, characterized by its double-membrane structure, engulfs damaged organelles, proteins, and invading pathogens, facilitating the degradation of these components and providing recycled materials for the cell’s continued utilization.8,17 Macroautophagy is widely recognized as the primary form of autophagy and is often closely associated with various diseases. Therefore, for the purpose of this paper, we will use the term “autophagy” to specifically refer to macroautophagy. Autophagy can be subdivided into two distinct forms: selective autophagy and non-selective autophagy. Extensive research has demonstrated that non-selective degradation of cellular cargo in response to autophagy signals, such as starvation, is crucial for maintaining cellular stability. But now there is also much evidence that highly specific and selective degradation of cargo (such as mitochondria, endoplasmic reticulum, ribosomes, etc.) is also active under nutrient-rich conditions. It can specifically remove dysfunctional organelles, proteins or intracellular pathogens when activated. The former is called non-selective autophagy and the latter is selective autophagy. However, when the damaged mitochondria are specifically cleared by autophagosomes, it is called mitophagy.18,19
The Process of Autophagy
The autophagosome, a closed lipid bilayer membrane structure, originates from endoplasmic reticulum and Golgi apparatus.8 However, the membrane formation entails a multi-stage process, characterized by the sequential involvement of various autophagy-related gene proteins (ATG) and their selective recruitment at distinct time points. Moreover, nearly all ATG, with rare exceptions, play a role in the formation of fully formed autophagosomes.17 This process encompasses several key steps, including initiation, nucleation, extension, closure, and fusion (Figure 1).
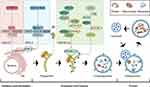 |
Figure 1 The main stage of autophagy process. The schematic diagram of the main stages of autophagy: initiation and nucleation, extension, closure and maturation, fusion. |
The ULK1 kinase complex (ULK1-C) consists of ULK1, RB1-inducible coiled-coil protein 1, ATG13, and ATG101.20 When the activation of mTORC1 is restricted, ULK1-C activation initiates autophagy.20–22 Type III phosphatidylinositol 3-kinase complexes I (PI3K-C1) is composed of beclin-1, The catalytic subunit vacuolar protein sorting 34 (VPS34), vacuolar protein sorting 15 (VPS15), ATG14, Autophagy and Beclin 1 regulator 1 (AMBRA1), and plays a central role in the autophagy nucleation stage.23 During phagophore elongation, PI3P effector protein WD repeat domain phosphoinositide interacting protein 2 (WIPI2) binds to the membrane,24 and WIPI2 binds directly to the ATG16L1 complex (ATG16L1-C: ATG12-ATG5-ATG16L).25 ATG16L1-C promotes the formation of LC3-II. LC3-II then binds to the phagosomal membrane, promotes the elongation of the autophagosome membrane, and helps autophagosome membrane closure and fusion with lysosomes.26 Finally, the small GTPase Rab7 assists the fusion of the autophagosome and the lysosome.27,28
The Involvement of Autophagy in OBs and OCs
The Involvement of Autophagy in OBs
OBs originate from mesenchymal stem cells in the bone marrow, under certain conditions, preosteoblasts differentiate into mature OBs. Mature OBs exhibit the expression of essential genes, including alkaline phosphatase and osteocalcin. Additionally, they actively secrete crucial components such as osteocalcin and osteonectin. These secreted molecules interact with inorganic salt components, facilitating mineralization and contributing to the process of osteogenesis. Autophagy contributes to the osteogenic differentiation of mesenchymal stem cells and facilitates the secretion of crucial components of OBs. In vitro studies have demonstrated that canonical Wnt signaling inhibits the differentiation of mesenchymal stem cells into adipocytes and promotes the differentiation of MSCs into OBs.29,30 Irisin, identified as an anti-inflammatory cytokine, has been shown to play a crucial role in promoting the differentiation of mesenchymal stem cells into OBs. This effect is achieved by activating autophagy through the Wnt/β-catenin signaling pathway.31 According to the literature, Runx2 is an OBs-specific transcription factor that plays a crucial role in regulating both the differentiation and function of OBs. During the process of OBs differentiation, Runx2 exhibits high expression levels in preosteoblasts.32,33 Elevating the expression of Runx2 has been shown to enhance OBs autophagy and increase the levels of p-p38MAPK. This upregulation influences the expression of osteogenesis-related genes such as alkaline phosphatase and osteocalcin.34 Runx2 possesses the capacity to enhance autophagy in OBs, a process that also can be achieved by regulating the expression of lysosomal-associated transmembrane protein 5,35 this regulatory mechanism influences OBs mineralization and its subsequent influences the overall bone formation process. In addition, the targeted knockout of ATG7, a specific autophagy-related gene, results in autophagy impairments, which in turn causes endoplasmic reticulum stress in OBs and subsequently hinders osteoblast mineralization in a DNA damage-induced transcript 3-mitogen-activated protein kinase 8-SMAD1/5/8-dependent manner, demonstrating the positive role of autophagy in maintaining OBs stability and mineralization.36
However, the energy generator of mitochondria plays an important role in osteoblast differentiation. In osteoblast cell line MC3T3-E1 and primary calvarial osteoblasts, the formation of mitochondria and supercomplex occurs continuously during osteoblast differentiation, and SIRT3 enhances SOD2 activity and regulates mitochondrial stress.37 PTEN-induced kinase 1 (PINK1) can trigger mitophagy and regulate the quality of mitochondria. The differentiation of osteoblasts is inhibited by the down-regulation of Pink1, accompanied by the damage of mitochondrial homeostasis and the increase of mitochondrial reactive oxygen species. The activation of PINK1 is a necessary condition for osteoblast differentiation.38 Due to the biological activity of exosomes, exosomes from bone marrow mesenchymal stem cells (BMSC-Exos) circular RNA (circRNA) promote the differentiation of MC3T3-E1 into osteoblasts, among them, circHIPK3 was verified to be the key to promoting osteoblast differentiation in BMSC-Exos, which plays a role in mitophagy by targeting miR-29a-5p and PINK1.39 In addition, the expression of Kruppel-like factor 2 (KLF2) and autophagy-related genes are significantly increased during osteoblast differentiation. When mitophagy is promoted, the levels of KLF2 and osteogenic differentiation-related molecules are increased. Chromatin immunoprecipitation analysis confirmed that KLF2 and active epigenetic markers (H3K27Ac and H3K4me3) are up-regulated in the promoter region of ATG7 and play a crucial role in regulating mitophagy and osteoblast differentiation.40
Furthermore, a plethora of studies have demonstrated that autophagy plays a huge role in the anti-aging and anti-apoptosis of OBs.10 A recent study has identified discoidin domain receptor 1 (DDR1) as a collagen receptor that actively contributes to bone development. Intriguingly, the knockout of the DDR1 gene has been shown to markedly diminish autophagy levels while concurrently triggering OBs apoptosis.41 Heat shock protein 60 (HSP60) has been recognized as a key regulator in preserving protein functionality within the cellular microenvironment. Notably, in OBs subjected to glucocorticoid treatment, HSP60 maintains the expression of specific autophagy-related genes, such as Atg4 and Atg12, and promotes autophagic flux. This process ultimately mitigates apoptosis in OBs, highlighting the cytoprotective effects of HSP60 in this cellular context.42 Similarly, in the galactose-induced OBs senescence model, glucosamine can improve the apoptosis of osteoblasts by promoting autophagy.43 However, in the context of hypoxia, OBs exhibit an elevation in the expression of cyclin-dependent kinase 8 (CDK8), and the overexpression of CDK8 has been found to induce both autophagy and apoptosis in this cellular setting.44
The Involvement of Autophagy in OCs
OCs, derived primarily from hematopoietic progenitor cells of the monocyte-macrophage lineage, serve as the exclusive cells responsible for bone resorption within the human body.45 Mature OCs secrete acidic substances and proteases, which facilitate the dissolution of minerals and the digestion of collagen fibers within the bone matrix, thereby promoting the process of bone resorption.46 During this intricate process, a large actin ring sealing region is formed in the cytoplasm of mature multinucleated OCs to degrade the bone matrix, and the actin ring surrounds a folded boundary.47 The edge of the fold undergoes fusion between lysosomes and the plasma membrane, resulting in the formation of an acidic microenvironment. Within this microenvironment, OCs secrete various proteolytic enzymes, including cathepsin K (CTSK), to efficiently degrade the bone matrix.48 Previous studies have reported that autophagy-related proteins, including Atg5, Atg7, Atg4B, and LC3, play a crucial role in governing the formation of fold boundaries in OCs. These proteins are instrumental in guiding the fusion process between lysosomes and the plasma membrane, which is essential for facilitating OCs secretion and enabling efficient bone resorption. Specifically, Atg5 has been identified as a key regulator in facilitating the localization of LC3-mediated lysosomes at the edges of OCs folds, thereby exerting a significant influence on the bone resorption function of OCs.49 In addition, the migration of OCs on the bone surface is crucial to the function of bone resorption, and the migration of OCs requires the continuous and rapid assembly and disassembly of podosomes.50 Nevertheless, when LC3-mediated autophagy is inhibited, there is an accumulation of kindlin3, a critical adapter protein. This accumulation amplifies the interaction between kindlin3 and integrins, leading to the hyperactivation of integrins. This, in turn, impedes the degradation of podosomes and disrupts OCs migration.51
During OCs differentiation, monocyte-macrophage colony stimulating factor (M-CSF) and receptor activator for nuclear factor-κB ligand (RANKL) are the key stimulating molecules to induce mature osteoclasts.52 RANK on the surface of OCs binds to RANKL released by OBs and promotes TNF receptor associated factor (TRAF)-mediated downstream signaling.53 As reported in the literature, silencing of the autophagy-related gene Beclin-1 has been shown to impede the degradation of TRAF3, thus affecting the regulation of osteoclastogenesis through the RANKL/Beclin-1/Autophagy/TRAF3 axis.54 In addition, in a study conducted by Ke et al it was discovered that RANKL stimulation leads to the phosphorylation of BCL2 at the Ser70 (S70) site. This phosphorylation event triggers autophagy in OCs precursors (OCPs), thereby promoting OCs differentiation. Interestingly, when the S70 site of BCL2 was mutated via site-directed mutagenesis, it led to the inhibition of autophagy activity in OCPs and instead promoted apoptosis in these precursor cells.55
Additionally, mitochondria are highly active during OCs formation, and mitochondrial dynamics (fusion, fission, and mitophagy) are at a high level. Mitofusin2 (MFN2) is a protein that can regulate adjacent mitochondrial fusion, promote mitochondrial calcium absorption, and regulate mitophagy. OCs differentiation is regulated by MFN2 through Ca2+-NFATc1 axis.56 Mitochondrial deacetylase sirtuin-3 (Sirt3) is associated with aging-induced bone loss. Although osteoclast progenitor cells in Sirt3-deficient senescent mice can differentiate into OCs, differentiated cells show decreased levels of mitophagy and impaired bone resorption.57 In vitro, PTEN-induced kinase 1 (PINK1) -dependent mitophagy is enhanced by inhibiting signal transducer and activator of transcription 3 (STAT3) and PTEN-induced kinase 1 (PINK1) -dependent mitophagy, which affects nlrp3 activation and regulates osteoclastogenesis.58 In addition, the fate of mature OCs is related to mitophagy. By inhibiting mitophagy, osteoclast division can be inhibited and osteoclast apoptosis can be induced. By enhancing mitophagy, osteoclast-osteomorph recycle can be promoted, and the bone resorption ability of fused OCs is proved.59
Autophagy of Osteoblasts and Osteoclasts in Osteoporosis
Bone is a living, dynamic organ that is constantly remodeling, and in this dynamic process, it is regulated by two important cellular activities: OBs and OCs.60 During the intricate process of bone remodeling, OCs activation initiates bone resorption, while OBs activation promotes mineralization. These two processes are intricately interconnected, constantly influencing each other’s activity. Furthermore, a series of cellular molecules come into play, orchestrating the delicate balance between bone catabolism and anabolism.61 However, the essence of autophagy involved in the pathogenesis of osteoporosis is to affect the differentiation and osteogenesis of OBs and the differentiation and bone resorption function of OCs. Baseline levels of autophagy exist in all cells, maintaining homeostasis and promoting cell survival. Abnormal autophagy of OBs and OCs caused by pathological environment may be an important factor in breaking the delicate balance of bone remodeling and leading to bone loosening (Figure 2).
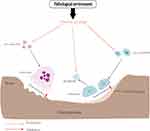 |
Figure 2 In the pathological environment, abnormal autophagy of OCs and OBs, increased bone resorption and insufficient bone formation lead to osteoporosis. |
Primary osteoporosis involves a variety of molecular mechanisms, but oxidative stress and estrogen reduction seem to be the two most critical factors. Two types of primary osteoporosis: postmenopausal OP and age-related OP are associated with advanced oxidation protein product (AOPP: an oxidative stress product) levels.62,63 In vitro, AOPP interacts with RANK and RAGE to activate nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, induce ROS production and induce osteoclast differentiation and maturation,64 and ROS induced mitochondrial dysfunction, endoplasmic reticulum stress and Ca2 + overload in osteoblasts by inducing phosphorylation of mitogen-activated protein kinases (MAPKs), leading to bone loss.65 More importantly, ROS is involved in the regulation of autophagy of osteoblasts and osteoclasts by activating downstream molecules FOXO3, AMPK, Akt, etc., as a therapeutic target for osteoporosis.10 In postmenopausal OP, the decline in estrogen levels results in reduced cellular autophagy and increased susceptibility of OBs to apoptosis, estrogen receptor antagonist (ICI18270) combined with estrogen receptor (ER) to reduce autophagy leads to osteoblast death, and estradiol activation of ER-ERK-mTOR pathway may be a pathway to save osteoblast apoptosis.66 17β-Estradiol can activate the downstream ERK signal to regulate osteoblast mitophagy through the G protein-coupled receptor 30 (GPR30) as one of the estrogen receptors.67 Estrogen regulatory protein RAB3GAP1 is a catalytic component of the RAB3GAP complex and plays a role in autophagosome biosynthesis. Estrogen deficiency and reduced interaction with RAB3GAP1 lead to reduced autophagy in human osteoblasts, with corresponding reductions in both osteoblast lifespan and osteoblast mineralization.68 Moreover, the hormone estrogen (specifically 17β-estradiol) directly amplifies pro-osteoclast autophagy while concurrently impeding OCs differentiation.69
Glucocorticoids, as the main force of anti-inflammatory, have been widely used for a long time. In secondary osteoporosis, the disadvantages of glucocorticoid-induced bone loss are gradually exposed.70 It has been reported that the expression of autophagy-related genes, such as ATG7 and Beclin 1, is reduced by 1–5 times in dexamethasone-induced OP in vivo and in vitro.62 The level and activity of autophagy in osteoblasts treated with different concentrations of dexamethasone are also different. Low level of autophagy induced by ground rice contributes to the survival of osteoblasts, while high level of dexamethasone can lead to autophagy inhibition and OBs function inhibition and apoptosis.63 In addition, Rankl-induced OCs formation was significantly higher in the glucocorticoid (1μM) treatment group than in the hormone-free treatment group, and dexamethasone-induced ROS played a key role in regulating autophagy. Interestingly, inhibition of autophagy by 3-MA did not cause ROS accumulation.64
Diabetes is also an important cause of secondary osteoporosis. It is known that hyperglycemia and insulin resistance may induce osteoblast death,71 and hyperglycemia and glucose fluctuation are two common states in diabetic patients, both of which inhibit osteoblast proliferation and activate autophagy, generating ROS leading to apoptosis induced by a decrease in the mitochondrial membrane potential of OBs.72 Non-imprinted in Prader-Willi/Angelman syndrome region protein 2 (NIPA2), a highly selective magnesium transporter, has been reported to be associated with type 2 diabetic osteoporosis, Among them, high glucose (35 mM glucose) decreased the expression of NIPA2 osteoblasts and induced mitophagy, while NIPA2 can regulate mitophagy and mitophagy is negatively correlated with osteogenic ability in high glucose environment.73
Signaling Pathways Involved in Autophagy in OBs and OCs
Autophagy in OBs and OCs: AMPK Signaling Pathway
AMPK is an AMP-dependent protein kinase, controlled by the AMP: ATP ratio and its upstream signal, and is a sensor of energy metabolism.74 The downstream of AMPK molecules, there are mainly two molecules, mTOR and ULK1, that regulate autophagy and participate in the metabolism of bone homeostasis.75 In the absence of energy, phosphorylated AMPK causes tuberous sclerosis complex 2 (TSC2) phosphorylation, resulting in the formation of TSC1/TSC2 complex, resulting in the inactivation of Rheb and the loss of the ability to activate mTOR.76 In addition, AMPK directly phosphorylates Raptor, preventing Raptor from binding to mTOR, which in turn leads to mTOR inactivation. The inactivation of mTOR eliminates its inhibition of ULK1 phosphorylation and induces the binding of ULK1 to AMPK, one of the main mechanisms by which AMPK activates autophagy.77 In OBs, AMPK activation induces autophagy, as evidenced by upregulation of LC3-II, downregulation of p62 and increased autophagosome density, while enhancing OBs differentiation and mineralization.78 Various factors can affect the AMPK signaling pathway in OBs. Under simulated negative pressure treatment conditions, activation of AMPK-ULK1 axis promotes MSCs differentiation into OBs.75 Metformin can target AMPK signaling in glucocorticoid-induced OBs apoptosis. As a small ribosomal 40s subunit S6 protein kinase, P70S6K enables the small ribosomal 40s subunit to easily bind to translation complexes and promote autophagy-related protein synthesis, metformin regulates AMPK signaling and affects downstream mTOR and P70S6K molecules to alter autophagy levels and reverse OBs apoptosis.79
AMPK signaling exhibits reliable regulation of OCs autophagy levels in a high level glucose environment, inhibition of AMPK/mTOR/ULK1 can reduce the high autophagy level of OCs mediated by high concentration of glucose, and inhibit the formation of OCs, elucidating the mechanism of glucose-mediated OCs formation.80 In addition, osteoprotegerin (OPG), a paracrine inhibitor derived from OBs, has been shown to be an important molecule involved in osteoclastogenesis. It can bind to RANK to block the binding of RANK to RANKL and inhibit OCs formation.48,81 According to reports in the literature, OPG inhibits OCs differentiation and bone resorption by enhancing autophagy in vitro, and the mechanism is that OPG activates AMPK protein, which regulates OCs autophagy through the AMPK/mTOR/p70S6K pathway. In the field of autophagy, the effect of OPG on OCs has been elucidated, and AMPK signaling regulates autophagy in OCs.82,83
Autophagy in OBs and OCs: PI3K/AKT/mTOR Signaling Pathway
Phosphatidylinositol 3-kinase (PI3K) is a lipid kinase with three types of I, II and III in mammals. It is mainly produced on the lipid membrane and recruits downstream lipid signals such as serine / threonine kinase (AKT) to participate in cell metabolism.84,85 Light has many biological effects, including promoting tissue repair, improving neuroinflammation, anti-apoptosis, etc.86 Photobiomodulation (PMB) treatment can reduce the phosphorylation levels of PI3K and AKT, enhance OBs autophagy by inhibiting PI3K/AKT/mTOR signaling pathway, alleviate OBs apoptosis and enhance OBs differentiation.87 The Jintiange (JTG) protein in the artificial tiger bone JTG can regulate the expression of key proteins in the PI3K/AKT and endoplasmic reticulum stress (ER) pathways in osteogenesis, and enhance the formation of autophagosomes, through PI3K/AKT/mTOR and ER stress signaling pathways enhance autophagy to promote OBs formation and inhibit OBs apoptosis.88
High levels of cholesterol can affect the expression of PI3K signaling and autophagy proteins in OCs, and inhibit autophagy and OCs differentiation through PI3K/AKT/mTOR.89 The expression of PI3K, p-Akt and p-mTOR was inhibited when excessive glucocorticoid induced OCs autophagy. The PI3K/Akt/mTOR signaling pathway can increase OCs autophagy and promote OCs formation.90 In addition, OPG regulates OCs differentiation by altering OCs autophagy not only through AMPK signaling,83 but also by regulating the AKT signaling pathway. According to Zhao et al reported that phosphorylated AKT and mTOR levels were decreased in OPG-treated OCs, and OPG regulates autophagy by activating PI3K/AKT/mTOR signaling pathway leading to OCs viability, differentiation ability and bone resorption both activities are weakened.91
Autophagy in OBs: FOXO1/3 Signaling Pathway
FOXO is a transcription factor that responds to various external stimuli, including oxidative stress, fluctuations in energy levels, and other factors.92 In mouse OBs, the deletion of FOXO1, FOXO3, and FOXO4 has been found to induce oxidative stress and elevate OBs apoptosis. Conversely, upregulation of FOXO3 expression in mature OBs has shown to confer resistance against oxidative stress and reduce cell death. Furthermore, FOXO3 can mitigate OBs senescence by regulating the methylation of FOXO3. These findings highlight the crucial role of FOXO-dependent oxidative defense in counteracting the detrimental effects of free radicals generated during the aerobic metabolism of OBs.93,94 According to literature reports, inhibiting the FOXO1 signaling pathway has been shown to decrease the autophagy level in OBs exposed to a high glucose environment, consequently impacting the overall function of OBs.95 On the other hand, it has been reported that FOXO3 induces autophagy in order to mitigate the generation of ROS during mitochondrial metabolism in OBs. This protective mechanism helps safeguard OBs from potential damage.96 In addition, Furthermore, it has been observed that treatment of MC3T3-E1 cells with low-dose dexamethasone leads to the upregulation of glucocorticoid-induced kinase-1 (SGK1), which subsequently facilitates the dephosphorylation of FOXO3. This dephosphorylation event triggers the activation of autophagy, thereby enhancing cell viability and improving the overall function of OBs.97
Autophagy in OBs: SIRT1/3 Signaling Pathway
Silent Information Regulator (SIRT) proteins belong to the NAD+-dependent deacetylase family. The subtypes SIRT1-7 have been implicated in human aging-related diseases and are known to regulate various biological responses in cells, including immune responses and autophagy.98,99 SIRT1 and SIRT3 have been found to have a close association with autophagy in osteoblasts. Notably, SIRT1 functions as an NAD+-dependent histone that offers cellular protection through the process of deacetylating target proteins.100 In the hFOB1.19 cell model, 17β-estradiol has been shown to induce an upregulation of SIRT1 gene expression. This increase in SIRT1 expression subsequently promotes OBs autophagy while inhibiting apoptosis.101 SIRT3, a prominent mitochondrial deacetylase, plays a significant role in oxidative metabolism and the response to oxidative stress. Reducing the signal of SIRT3 leads to weakening the differentiation of adipocytes into osteoblasts.102 According to the literature, increasing the expression of SIRT3 can reduce superoxide dismutase 2 (SOD2) acetylation and promote SOD2 activity, maintain mitochondrial stability, and regulate autophagy through SIRT3/SOD2 signaling pathway to resist titanium ion-induced osteoblast damage.103
Autophagy in OBs: PINK1/Parkin Signaling Pathway
PTEN-induced kinase 1 (PINK1), a serine/threonine kinase with a mitochondrial targeting sequence, plays a critical role in regulating mitochondrial quality control. During OBs differentiation, the knockout of the PINK1 gene disrupts mitochondrial homeostasis and leads to elevated production of ROS, thereby inhibiting OBs differentiation.38 In age-related OP, a large number of advanced oxidation protein products (AOPPs) promote the production of mitochondrial ROS (mROS), which aggravates oxidative stress and promotes OBs apoptosis, while PINK1/Parkin-mediated mitophagy can eliminate excessive mROS and damaged mitochondria to reverse AOPP-induced OBs apoptosis.104 Excess ROS can activate mitophagy, which is an important mechanism for controlling mitochondrial quality and cell homeostasis. Interestingly, when dibutyl phthalate (DBP) treated OBs, a large amount of ROS was generated and accompanied by the activation of mitophagy. However, silencing Parkin expression inhibited mitophagy and alleviated DBP-induced MC3T3 -E1 dysfunction and apoptosis.105 In addition, it has been reported that dexamethasone attenuates OBs mitophagy by downregulating the expression of PINK1, Parkin genes, resulting in the inhibition of OBs differentiation and mineralization in vitro.106
Autophagy in OCs: Beclin-1/Bcl-2 Signaling Pathway
Beclin-1 is an important autophagy-initiating protein with multiple roles in OCs differentiation. Interleukin-17A (IL-17A) can promote the differentiation of OCs precursors into OCs, inhibit the phosphorylation of extracellular regulated kinase (ERK) to increase the expression of Beclin-1, enhance the autophagy activity of OCs precursors through the ERK/mTOR/Beclin1 pathway, and promote OCs differentiation.107 Chung et al found that knockout of Beclin-1 inhibited RANKL-mediated activation of JNK and p38, thereby inhibiting the expression of OCs-specific gene NFATc1.108 In vitro, autophagy is activated during RANKL-induced OCs differentiation and requires TRAF6-mediated Beclin-1 ubiquitination, and Beclin-1 knockout mice exhibit impaired OCs bone resorption and thickened bone cortex.109 Beclin-1 inhibits OPG-induced osteoclastogenesis by increasing autophagy. In the process of OPG inhibiting OCs differentiation stimulated by RANKL and M-CSF, the expression of matrix metalloproteinase 9 (MMP-9) decreased and the expression of Beclin-1 increased. Beclin-1 knockdown can reduce the level of autophagy and resist the number of OCs inhibited by OPG.110 In addition, it has been reported that metformin can be used as an autophagy regulator and is widely involved in the protection of various tissues. Metformin-mediated down-regulation of E2F1 in ovariectomized mice reduces the expression levels of Beclin-1 and BNIP3, which subsequently interferes with the binding of Beclin-1 to Bcl-2, inhibits the formation of autophagosomes, and leads to the inhibition of osteoclast formation, thereby reducing OVX-induced bone loss.111
Autophagy in OCs: ROS Signaling Pathway
ROS are critical to cellular signaling and physiological functions. However, oxidative stress leading to excess ROS also induces autophagy and affects the metabolic level of osteoclasts.10,112 Cholesterol in human body is positively correlated with reactive oxygen species. Cholesterol metabolite 7-ketocholesterol (7-KC) enhances autophagy and increases the number and activity of osteoclast through ROS-TFEB signaling pathway.113 Doxorubicin (DOX) is a widely used chemotherapeutic drug that can induce excessive autophagy of osteoclasts, leading to bone loss and increasing the risk of human fractures. The mechanism is due to DOX-induced increase in mitochondrial ROS levels, causing mROS to oxidize the transient receptor potential mucin 1(TRPML1) in lysosomes, leading to nuclear localization of TFEB, increasing autophagy levels and OCs differentiation.114
Regulation of Protein Molecules on Autophagy of OBs and OCs
The tumor suppressor M receptor (OMSR) is expressed on a variety of cells, including OBs and OCs. It has been reported that Osmr-/- OBs are defective in differentiation and lead to reduced bone formation.115 Zhou et al reported the direct role and mechanism of OMSR in controlling osteogenic differentiation. OSMR inhibits extracellular regulated kinase (ERK) signaling and autophagy, while silencing OSMR gene can promote OB differentiation, reduce fat accumulation and inhibit OBs differentiation.116 Taurine up-regulated gene 1 (TUG1) is one of the earliest discovered lncRNAs related to human diseases. Hypoxia can up-regulate the expression of TUG1, and knockout of TUG1 can promote SIRT1-induced mitophagy.117,118 In addition, TUG1 plays an important role in regulating OBs and improving OP. The expression of TUG1 increased during the differentiation of BMSCs into OBs, while in TUG1-silenced BMSCs, the expression of AMPK and P53 decreased and the number of autophagosomes decreased, demonstrating the potential of TUG1 to promote the differentiation of BMSCs into OBs by regulating the AMPK/mTOR/autophagy axis.119 Non-imprinted in Prader-Willi/Angelman syndrome region protein 2 (NIPA2) is a highly selective magnesium transporter. The overexpression and silencing of NIPA2 gene indicated that NIPA2 regulates PINK1/Parkin-mediated mitophagy through the PGC-1/FoxO3a pathway, affecting the function of OBs.73
c-Fos is a key regulatory molecule in the receptor activator of RANKL-induced osteoclastogenesis. Inhibition of PI3K expression and Akt phosphorylation can reduce c-Fos expression in RANKL-induced OCs differentiation.120 Overexpression of c-Fos reverses OPG-mediated inhibition of osteoclastogenesis by activating Beclin1-induced autophagy, and sustained activation of c-Fos regulates OCs autophagy and differentiation by activating PI3K/Akt and TAK1/S6 signaling pathways.121 Kruppel-like factor 2 (KLF2) is a subclass of the zinc finger family of transcription factors involved in the regulation of cell growth and differentiation.122 KLf2 has a specific regulatory effect on Beclin-1-mediated autophagy during OCs formation, the mechanism is that KLF2 negatively regulates Beclin-1, thereby inhibiting autophagy flow and suppressing OCs differentiation.123 JNK1 plays a key role in OCs formation after RANKL induction, and JNK1 is recognized as an autophagy regulator under stress conditions. Beclin-1/Bcl-2 complex dissociates and then activates autophagy,124 activation of JNK1 does not affect the expression of Beclin-1, but promotes the entry of Beclin-1 into the autophagosome formation process. Inhibition of JNK1 can reverse RANKL promoted release of Beclin1 from Beclin1/Bcl-2 complex, reduce autophagy, and affect the production of OCs.125 Tet methylcytosine dioxygenase 2 (Tet2) is a DNA demethylase that regulates cell function and differentiation potential. Knockdown of Tet2 increases the expression of Bcl-2, increases the binding of Bcl-2 to Beclin-1, and negatively regulates autophagy and affects OCs differentiation.126 The purinergic receptor P2X7 receptor (P2X7R) is encoded by the P2RX7 gene, a subunit of which functions as an ion channel and supports Ca2+ inward flow mediated by ATP, and the P2X7-dependent signalling pathway involves NFκB, HIF-1α, etc.127 in addition, P2X7R expression levels and intracellular Ca2+ concentrations are very high in mature OBs, and P2X7 overexpression can increase the number of multinucleated OBs. Knockout of P2X7R inhibits OCs differentiation by inhibiting Ca2+/calcineurin/NFATc1 signaling pathway and autophagy.128 Transient receptor potential channel subfamily V member 4 (TRPV4) is a calcium channel. And likewise, TRPV4 overexpression activated Ca2+/calcineurin/NFATc1 signaling and autophagy during OCs differentiation, and knockdown of TRPV4 significantly reduces the number of OBs and alleviates OP in mice.129 Here we summarize a variety of molecules that regulate autophagy in OBs and OCs (Table 1).
 |
Table 1 Regulatory Molecules of Autophagy in Osteoblast and Osteoclast |
Natural Compounds Regulate OBs and OCs Autophagy Signaling Pathways to Produce Anti-OP
Natural Compounds Regulate OBs Autophagy Signaling Pathway
Paeoniflorin is a compound extracted from traditional Chinese medicine with anti-tumor, anti-inflammatory and other pharmacological effects. It can promote autophagy through the AKT/mTOR signaling pathway to reduce dexamethasone-induced OBs apoptosis and promote bone formation.130 Leonurine is a natural compound extracted from Motherwort that has been shown to have anti-inflammatory and antioxidant effects and is effective in the treatment of many diseases including cardiovascular disease and ischaemic stroke.131 It can promote the differentiation of BMSCs into OBs by increasing autophagy through the PI3K/Akt/mTOR signaling pathway. Leonurine may become a new method for the treatment of OP.132 Resveratrol is a polyphenolic hexene compound found in plants such as grapes, mulberry, peanuts, and rhubarb. It can reduce oxidative stress and inflammation and promote autophagy.133,134 When resveratrol inhibits the PI3K/Akt/mTOR pathway to enhance autophagy, PI3K inhibitors or SIRT1 inhibitors can eliminate the effects of resveratrol-induced mitophagy in OBs. The expression of SIRT1 also plays an important role in resveratrol-induced autophagy to protect OBs in osteoporotic rats.135 Geniposide is a natural compound. It can not only inhibit some inflammatory pathways such as NF-κB, MAPK, etc., but also increase some antioxidant enzymes such as superoxide dismutase and lipid peroxidase.136 In OBs, gardenia glycosides can alleviate glucocorticoid-induced endoplasmic reticulum stress and OBs apoptosis by inhibiting the PI3K/Akt/mTOR signaling pathway to activate autophagy and reduce cell damage and apoptosis.137 β-ecdysterone is a polyhydroxysteroid hormone, known as phytoestrogen. Studies have shown that β-ecdysterone reversed the effects of dexamethasone-treated BMSCs apoptosis and autophagy in a dose-dependent manner in vitro.138 In addition, Tang et al found that β-ecdysterone can activate autophagy by inhibiting PI3K/Akt/mTOR signaling pathway to promote OBs differentiation.139 Alpinetin, a flavonoid compound, is an important active ingredient in Alpinia katsumadai. It has been found to have anti-tumour, antioxidant and anti-inflammatory pharmacological effects. In Alpinetin-treated OBs, the expression of p-PKA was most affected, while the expression levels of p-JNK, p-AKT, p-p38, p-β-catenin, p-ERK, BMP2 or p-Smad were weakly affected, regulating autophagy through the PKA/mTOR/ULK1 signalling pathway. Therefore, Alpinetin may become a potential drug for the treatment of OP by targeting the PKA-autophagy pathway.140
Timosaponin BII is a steroidal saponin extracted from Anemarrhena asphodeloides Bge, which has the pharmacological activity in regulating glucose and lipid metabolism.141 And oxidative stress in osteoblasts under high glucose conditions can cause autophagy defects, which may be the pathogenesis of diabetic osteoporosis, Wang et al reported that Timosaponin BII can inhibit the phosphorylation of mTOR and downstream factor NF-κB, activate autophagy through mTOR/NF-κB signaling pathway, reduce oxidative stress and OBs apoptosis induced by high glucose, and alleviate the deterioration of tibial microstructure in diabetic rats.142 Cladrin are the main bioactive components found in the stem bark of purple rivet, which can promote the proliferation and differentiation of OBs,143,144 Peng et al showed that the apoptosis rate of OBs treated with Cladrin in dexamethasone environment was significantly lower than that of OBs without Cladrin treatment, and the level of p-AMPK/T-AMPK was significantly up-regulated, the AMPK/mTOR signaling pathway was significantly involved in Cladrin-induced autophagy in OBs under dexamethasone.144 Aucubin, an iridoid glycoside mainly found in Chinese herbal medicine, can enhance OBs autophagy through the AMPK signaling pathway to counteract steroid-induced apoptosis.145 Trehalose, a naturally occurring non-reducing disaccharide found in several plants and algae, has been reported to enhance cellular antioxidant capacity by increasing the expression of Nrf2 in cells,146,147 Trehalose can also protect mitochondrial function by activating transcription factor EB (TFEB)-mediated autophagy,148 moreover, Cao et al found that trehalose alleviated OBs apoptosis induced by palmitic acid by enhancing OBs autophagy through AMPK/mTOR/ULK1 signaling pathway and up-regulating SIRT3 expression.149 Tetramethylpyrazine (TMP) is extracted from Ligusticum chuanxiong Hort, which has anti-apoptotic effect. During the study of TMP protecting BMSCs from Dex-induced apoptosis, it was found that TMP can activate AMPK/mTOR signaling pathway to promote autophagy of BMSCs exposed to dexamethasone.150 Cistanche Deserticola is usually used in the treatment of kidney deficiency in traditional Chinese medicine. Based on the theory of “kidney dominate bone”, Cistanche Deserticola can be used as an alternative drug for the intervention of osteoporosis.151 Cistanoside A (Cis A) is a phenylethanoid glycoside extracted from Cistanche Deserticola, which has the antioxidant effect of inhibiting ROS activity to inhibit apoptosis.152 The expression of Wnt, β-catenin protein and autophagy were enhanced in OBs treated with Cis A. When Dickkopf-1 (DDK-1), an inhibitor of Wnt/β-catenin pathway, was used, it showed a significant decrease in LC3 fluorescence and a decrease in autophagosomes. Cis A induces autophagy by activating the Wnt/β-catenin signaling pathway and promotes primary OBs mineralization and osteogenesis153 (Figure 3).
Natural Compounds Regulate OCs Autophagy Signaling Pathway
Kaempferol, a flavonoid compound renowned for its anti-inflammatory and antioxidant properties, has garnered considerable attention. Notably, literature reports have elucidated its potent capacity to inhibit the expression of p62/SQSTM1 protein in OCs, thereby suppressing autophagy and attenuating OCs differentiation and bone resorption.154 Curcumin, derived from the turmeric root, has shown promising potential in ameliorating glucocorticoid-induced OP.155 It has a dual regulatory effect on pre-OCs. It can not only stimulate the autophagy of pre-OCs alone, but also inhibit the expression of Beclin-1 in pre-OCs induced by RANKL, and reduce the level of autophagy, resulting in a decrease in OCs formation. This action positions curcumin as a prospective candidate for targeted drug development in the realm of OP treatment.156 Orcinol glucoside (OG), a naturally occurring phenolic glycoside derived from Curculigo curculigo, exhibits a multitude of beneficial properties, including antioxidative, anxiolytic, neuroprotective, and anti-osteoporotic activities.157,158 Senile OP is associated with oxidative stress, Gong et al have reported that OG holds the potential to mitigate oxidative stress and curb OCs autophagy by activating the Nrf2/Keap1 and mTOR signaling pathways. Additionally, OG exerts a down-regulatory effect on OCs differentiation and function, thereby presenting a preventive approach against senile OP.158 Lycorine is an alkaloid isolated from Lycoraceae plants, which has anti-inflammatory and antioxidant effects.159 Notably, lycorine exhibits the ability to diminish the production of mROS, modulate the triggering signal for autophagy via the mROS/TRPML1/TFEB axis, and mitigate lipopolysaccharide-induced OCs autophagy, as well as suppress OCs activity and abundance.160 Epigallocatechin-3-gallate (EGCG) is a polyphenolic compound extracted from the leaves of Camellia sinensis. EGCG has attracted much attention because of its antioxidant, antiviral, anti-inflammatory, anti-aging and effects, and effectiveness for many diseases.161 In the mechanism of inhibiting OCs differentiation, EGCG not only blocks the binding of Rank and Rankl, but also reduces ROS and mitochondrial membrane potential, inhibits the expression of mitophagy-related molecules, and regulates mitophagy through AKT and p38MAPK pathways.162 Here we summarize the details of the possible link between natural compounds regulating autophagy in OBs and OCs (Table 2).
 |
Table 2 Structure, Dose, Cell Lines and Mechanism of Natural Products Regulating Autophagy of Osteoblasts in vitro |
Conclusion
OP is a disease of bone metabolism imbalance, which is caused by the imbalance of OBs and OCs metabolism in bone homeostasis. Autophagy can regulate cell proliferation, differentiation, maturation and metabolic processes, and can resist cell damage caused by various reasons such as aging and stress, and provide energy and material basis for cell homeostasis and survival. Autophagy plays an important role in maintaining cell function and dynamic balance of OBs and OCs. In addition, there are a variety of autophagy signaling molecules that activate OBs and OCs. Researchers have found that a large number of protein and natural compounds molecules can play an anti-OP role by regulating the autophagy signaling pathway to affect the differentiation of OBs and OCs, showing the potential of autophagy in bone remodeling. Therefore, regulating autophagy-related signaling pathways may be a target for understanding the pathogenesis of OP and future treatment of OP, providing new methods and ideas for the treatment of OP.
Funding
National Natural Science Foundation of China (82260181), Key project of Natural Science Basic Research Plan of Shaanxi Province (2022JZ-43) and the National Key Research and Development Program of China (No. 2022YFC2407503).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Song S, Guo Y, Yang Y, et al. Advances in pathogenesis and therapeutic strategies for osteoporosis. Pharmacol Ther. 2022;237:108168.
2. Ma Y, Ran D, Zhao H, et al. Cadmium exposure triggers osteoporosis in duck via P2X7/PI3K/AKT-mediated osteoblast and osteoclast differentiation. Sci Total Environ. 2021;750:141638. doi:10.1016/j.scitotenv.2020.141638
3. Lane NE. Epidemiology, etiology, and diagnosis of osteoporosis. Am J Clin Exp Obstet Gynecol. 2006;194(2 Suppl):S3–S11. doi:10.1016/j.ajog.2005.08.047
4. Li J, Chen X, Lu L, et al. The relationship between bone marrow adipose tissue and bone metabolism in postmenopausal osteoporosis. Cytokine Growth Factor Rev. 2020;52:88–98. doi:10.1016/j.cytogfr.2020.02.003
5. Fleming A, Bourdenx M, Fujimaki M, et al. The different autophagy degradation pathways and neurodegeneration. Neuron. 2022;110(6):935–966. doi:10.1016/j.neuron.2022.01.017
6. Mauvezin C, Neufeld TP. Bafilomycin A1 disrupts autophagic flux by inhibiting both V-ATPase-dependent acidification and Ca-P60A/SERCA-dependent autophagosome-lysosome fusion. Autophagy. 2015;11(8):1437–1438. doi:10.1080/15548627.2015.1066957
7. Yuan N, Song L, Zhang S, et al. Bafilomycin A1 targets both autophagy and apoptosis pathways in pediatric B-cell acute lymphoblastic leukemia. Haematologica. 2015;100(3):345–356. doi:10.3324/haematol.2014.113324
8. Mizushima N, Komatsu M. Autophagy: renovation of cells and tissues. Cell. 2011;147(4):728–741. doi:10.1016/j.cell.2011.10.026
9. Wang L, Heckmann BL, Yang X, et al. Osteoblast autophagy in glucocorticoid-induced osteoporosis. J Cell Physiol. 2019;234(4):3207–3215. doi:10.1002/jcp.27335
10. Zhu C, Shen S, Zhang S, et al. Autophagy in bone remodeling: a regulator of oxidative stress. Front Endocrinol. 2022;13:898634. doi:10.3389/fendo.2022.898634
11. Harvey AL, Edrada-Ebel R, Quinn RJ. The re-emergence of natural products for drug discovery in the genomics era. Nat Rev Drug Discov. 2015;14(2):111–129. doi:10.1038/nrd4510
12. Efferth T, Oesch F. The immunosuppressive activity of artemisinin-type drugs towards inflammatory and autoimmune diseases. Med Res Rev. 2021;41(6):3023–3061. doi:10.1002/med.21842
13. Mizushima N, Levine B, Longo DL. Autophagy in human diseases. N Engl J Med. 2020;383(16):1564–1576. doi:10.1056/NEJMra2022774
14. Montaseri A, Giampietri C, Rossi M, et al. The role of autophagy in osteoclast differentiation and bone resorption function. Biomolecules. 2020;10(10):1398. doi:10.3390/biom10101398
15. Klionsky DJ, Petroni G, Amaravadi RK, et al. Autophagy in major human diseases. EMBO J. 2021;40(19):e108863. doi:10.15252/embj.2021108863
16. Guo YF, Su T, Yang M, et al. The role of autophagy in bone homeostasis. J Cell Physiol. 2021;236(6):4152–4173. doi:10.1002/jcp.30111
17. Levine B, Kroemer G. Biological functions of autophagy genes: a disease perspective. Cell. 2019;176(1–2):11–42. doi:10.1016/j.cell.2018.09.048
18. Gatica D, Lahiri V, Klionsky DJ. Cargo recognition and degradation by selective autophagy. Nat Cell Biol. 2018;20(3):233–242. doi:10.1038/s41556-018-0037-z
19. Lamark T, Johansen T. Mechanisms of selective autophagy. Annu Rev Cell Dev Biol. 2021;37(1):143–169. doi:10.1146/annurev-cellbio-120219-035530
20. Licheva M, Raman B, Kraft C, et al. Phosphoregulation of the autophagy machinery by kinases and phosphatases. Autophagy. 2022;18(1):104–123. doi:10.1080/15548627.2021.1909407
21. Kim J, Kundu M, Viollet B, et al. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 2011;13(2):132–141. doi:10.1038/ncb2152
22. Hu Y, Reggiori F. Molecular regulation of autophagosome formation. Biochem Soc Trans. 2022;50(1):55–69. doi:10.1042/BST20210819
23. Zhou C, Qian X, Hu M, et al. STYK1 promotes autophagy through enhancing the assembly of autophagy-specific class III phosphatidylinositol 3-kinase complex I. Autophagy. 2020;16(10):1786–1806. doi:10.1080/15548627.2019.1687212
24. Tremel S, Ohashi Y, Morado DR, et al. Structural basis for VPS34 kinase activation by Rab1 and Rab5 on membranes. Nat Commun. 2021;12(1):1564. doi:10.1038/s41467-021-21695-2
25. Wang Y, Ramos M, Jefferson M, et al. Control of infection by LC3-associated phagocytosis, CASM, and detection of raised vacuolar pH by the V-ATPase-ATG16L1 axis. Sci Adv. 2022;8(43):eabn3298. doi:10.1126/sciadv.abn3298
26. Glick D, Barth S, Macleod KF. Autophagy: cellular and molecular mechanisms. J Pathol. 2010;221(1):3–12. doi:10.1002/path.2697
27. Tian X, Teng J, Chen J. New insights regarding SNARE proteins in autophagosome-lysosome fusion. Autophagy. 2021;17(10):2680–2688. doi:10.1080/15548627.2020.1823124
28. Morgan NE, Cutrona MB, Simpson JC. Multitasking rab proteins in autophagy and membrane trafficking: a focus on Rab33b. Int J Mol Sci. 2019;20(16):3916. doi:10.3390/ijms20163916
29. Maeda K, Kobayashi Y, Koide M, et al. The regulation of bone metabolism and disorders by Wnt signaling. Int J Mol Sci. 2019;20(22):5525. doi:10.3390/ijms20225525
30. Zhang M, Bian YQ, Tao HM, et al. Simvastatin induces osteogenic differentiation of MSCs via Wnt/β-catenin pathway to promote fracture healing. Eur Rev Med Pharmacol Sci. 2018;22(9):2896–2905. doi:10.26355/eurrev_201805_14992
31. Chen X, Sun K, Zhao S, et al. Irisin promotes osteogenic differentiation of bone marrow mesenchymal stem cells by activating autophagy via the Wnt/β-catenin signal pathway. Cytokine. 2020;136:155292. doi:10.1016/j.cyto.2020.155292
32. Hou Z, Wang Z, Tao Y, et al. KLF2 regulates osteoblast differentiation by targeting of Runx2. Lab Invest. 2019;99(2):271–280. doi:10.1038/s41374-018-0149-x
33. Narayanan A, Srinaath N, Rohini M, et al. Regulation of Runx2 by MicroRNAs in osteoblast differentiation. Life Sci. 2019;232:116676. doi:10.1016/j.lfs.2019.116676
34. Ren C, Xu Y, Liu H, et al. Effects of runt-related transcription factor 2 (RUNX2) on the autophagy of rapamycin-treated osteoblasts. Bioengineered. 2022;13(3):5262–5276. doi:10.1080/21655979.2022.2037881
35. Xing L, Li Y, Li W, et al. Expression of RUNX2/LAPTM5 in the induction of MC3T3-e1 mineralization and its possible relationship with autophagy. Tissue Eng Regen Med. 2022;19(6):1223–1235. doi:10.1007/s13770-022-00477-x
36. Li H, Li D, Ma Z, et al. Defective autophagy in osteoblasts induces endoplasmic reticulum stress and causes remarkable bone loss. Autophagy. 2018;14(10):1726–1741. doi:10.1080/15548627.2018.1483807
37. Gao J, Feng Z, Wang X, et al. SIRT3/SOD2 maintains osteoblast differentiation and bone formation by regulating mitochondrial stress. Cell Death Differ. 2018;25(2):229–240. doi:10.1038/cdd.2017.144
38. Lee SY, An HJ, Kim JM, et al. PINK1 deficiency impairs osteoblast differentiation through aberrant mitochondrial homeostasis. Stem Cell Res Ther. 2021;12(1):589. doi:10.1186/s13287-021-02656-4
39. Ma S, Li S, Zhang Y, et al. BMSC-derived exosomal CircHIPK3 promotes osteogenic differentiation of MC3T3-E1 cells via mitophagy. Int J Mol Sci. 2023;24(3);1.
40. Maity J, Deb M, Greene C, et al. KLF2 regulates dental pulp-derived stem cell differentiation through the induction of mitophagy and altering mitochondrial metabolism. Redox Biol. 2020;36:101622. doi:10.1016/j.redox.2020.101622
41. Chou HC, Lin SY, Chou LY, et al. Ablation of discoidin domain receptor 1 provokes an osteopenic phenotype by regulating osteoblast/osteocyte autophagy and apoptosis. Biomedicines. 2022;10(9):2173. doi:10.3390/biomedicines10092173
42. Lian WS, Ko JY, Chen YS, et al. Chaperonin 60 sustains osteoblast autophagy and counteracts glucocorticoid aggravation of osteoporosis by chaperoning RPTOR. Cell Death Dis. 2018;9(10):938. doi:10.1038/s41419-018-0970-6
43. Su W, Lv C, Huang L, et al. Glucosamine delays the progression of osteoporosis in senile mice by promoting osteoblast autophagy. Nutr Metab. 2022;19(1):75. doi:10.1186/s12986-022-00688-y
44. Zheng J, Zhu X, He Y, et al. CircCDK8 regulates osteogenic differentiation and apoptosis of PDLSCs by inducing ER stress/autophagy during hypoxia. Ann N Y Acad Sci. 2021;1485(1):56–70. doi:10.1111/nyas.14483
45. Wang Q, Wang H, Yan H, et al. Suppression of osteoclast multinucleation via a posttranscriptional regulation-based spatiotemporally selective delivery system. Sci Adv. 2022;8(26):eabn3333. doi:10.1126/sciadv.abn3333
46. Song C, Yang X, Lei Y, et al. Evaluation of efficacy on RANKL induced osteoclast from RAW264.7 cells. J Cell Physiol. 2019;234(7):11969–11975. doi:10.1002/jcp.27852
47. Kodama J, Kaito T. Osteoclast multinucleation: review of current literature. Int J Mol Sci. 2020;21(16). doi:10.3390/ijms21165685
48. Kim J-M, Lin C, Stavre Z, et al. Osteoblast-osteoclast communication and bone homeostasis. Cells. 2020;9(9):2073. doi:10.3390/cells9092073
49. DeSelm CJ, Miller BC, Zou W, et al. Autophagy proteins regulate the secretory component of osteoclastic bone resorption. Dev Cell. 2011;21(5):966–974. doi:10.1016/j.devcel.2011.08.016
50. Lee BS. Myosins in osteoclast formation and function. Biomolecules. 2018;8(4):157. doi:10.3390/biom8040157
51. Zhang Y, Cui Y, Wang L, et al. Autophagy promotes osteoclast podosome disassembly and cell motility athrough the interaction of kindlin3 with LC3. Cell Signalling. 2020;67:109505. doi:10.1016/j.cellsig.2019.109505
52. Yuan FL, Wu QY, Miao ZN, et al. Osteoclast-derived extracellular vesicles: novel regulators of osteoclastogenesis and osteoclast-osteoblasts communication in bone remodeling. Front Physiol. 2018;9:628. doi:10.3389/fphys.2018.00628
53. Theoleyre S, Wittrant Y, Tat SK, et al. The molecular triad OPG/RANK/RANKL: involvement in the orchestration of pathophysiological bone remodeling. Cytokine Growth Factor Rev. 2004;15(6):457–475. doi:10.1016/j.cytogfr.2004.06.004
54. Xiu Y, Xu H, Zhao C, et al. Chloroquine reduces osteoclastogenesis in murine osteoporosis by preventing TRAF3 degradation. J Clin Invest. 2014;124(1):297–310. doi:10.1172/JCI66947
55. Ke D, Yu Y, Li C, et al. Phosphorylation of BCL2 at the Ser70 site mediates RANKL-induced osteoclast precursor autophagy and osteoclastogenesis. Mol Med. 2022;28(1):22. doi:10.1186/s10020-022-00449-w
56. Ballard A, Zeng R, Zarei A, et al. The tethering function of mitofusin2 controls osteoclast differentiation by modulating the Ca(2+)-NFATc1 axis. J Biol Chem. 2020;295(19):6629–6640. doi:10.1074/jbc.RA119.012023
57. Ling W, Krager K, Richardson KK, et al. Mitochondrial Sirt3 contributes to the bone loss caused by aging or estrogen deficiency. JCI Insight. 2021;6(10). doi:10.1172/jci.insight.146728
58. Zhu L, Wang Z, Sun X, et al. STAT3/mitophagy axis coordinates macrophage NLRP3 inflammasome activation and inflammatory bone loss. J Bone Miner Res. 2023;38(2):335–353. doi:10.1002/jbmr.4756
59. Huang T, Wang Y, Yu Z, et al. Effect of mitophagy in the formation of osteomorphs derived from osteoclasts. iScience. 2023;26(5):106682. doi:10.1016/j.isci.2023.106682
60. Fischer V, Haffner-Luntzer M. Interaction between bone and immune cells: implications for postmenopausal osteoporosis. Semin Cell Dev Biol. 2022;123:14–21. doi:10.1016/j.semcdb.2021.05.014
61. Nagy V, Penninger JM. The RANKL-RANK story. Gerontology. 2015;61(6):534–542. doi:10.1159/000371845
62. Dai W, Jiang L, Lay YA, et al. Prevention of glucocorticoid induced bone changes with beta-ecdysone. Bone. 2015;74:48–57. doi:10.1016/j.bone.2015.01.001
63. Shen G, Ren H, Shang Q, et al. Autophagy as a target for glucocorticoid-induced osteoporosis therapy. Cell Mol Life Sci. 2018;75(15):2683–2693. doi:10.1007/s00018-018-2776-1
64. Shi J, Wang L, Zhang H, et al. Glucocorticoids: dose-related effects on osteoclast formation and function via reactive oxygen species and autophagy. Bone. 2015;79:222–232. doi:10.1016/j.bone.2015.06.014
65. Zhu SY, Zhuang JS, Wu Q, et al. Advanced oxidation protein products induce pre-osteoblast apoptosis through a nicotinamide adenine dinucleotide phosphate oxidase-dependent, mitogen-activated protein kinases-mediated intrinsic apoptosis pathway. Aging Cell. 2018;17(4):e12764. doi:10.1111/acel.12764
66. Yang YH, Chen K, Li B, et al. Estradiol inhibits osteoblast apoptosis via promotion of autophagy through the ER-ERK-mTOR pathway. Apoptosis. 2013;18(11):1363–1375. doi:10.1007/s10495-013-0867-x
67. Sun X, Yang X, Zhao Y, et al. Effects of 17β-estradiol on mitophagy in the murine MC3T3-E1 osteoblast cell line is mediated via G protein-coupled estrogen receptor and the ERK1/2 signaling pathway. Med Sci Monit. 2018;24:903–911. doi:10.12659/MSM.908705
68. Gavali S, Gupta MK, Daswani B, et al. Estrogen enhances human osteoblast survival and function via promotion of autophagy. Biochim Biophys Acta. 2019;1866(9):1498–1507. doi:10.1016/j.bbamcr.2019.06.014
69. Cheng L, Zhu Y, Xie D, et al. Oestrogen-activated autophagy has a negative effect on the anti-osteoclastogenic function of oestrogen. Cell Prolif. 2020;53(4):e12789. doi:10.1111/cpr.12789
70. Ebeling PR, Nguyen HH, Aleksova J, et al. Secondary osteoporosis. Endocr Rev. 2022;43(2):240–313. doi:10.1210/endrev/bnab028
71. Wongdee K, Charoenphandhu N. Update on type 2 diabetes-related osteoporosis. World J Diabetes. 2015;6(5):673–678. doi:10.4239/wjd.v6.i5.673
72. Zhang P, Liao J, Wang X, et al. High glucose promotes apoptosis and autophagy of MC3T3-E1 osteoblasts. Arch Med Sci. 2023;19(1):138–150. doi:10.5114/aoms.2020.101307
73. Zhao W, Zhang W, Ma H, et al. NIPA2 regulates osteoblast function by modulating mitophagy in type 2 diabetes osteoporosis. Sci Rep. 2020;10(1):3078. doi:10.1038/s41598-020-59743-4
74. Steinberg GR, Hardie DG. New insights into activation and function of the AMPK. Nat Rev Mol Cell Biol. 2023;24(4):255–272. doi:10.1038/s41580-022-00547-x
75. Zhang S, Xie Y, Yan F, et al. Negative pressure wound therapy improves bone regeneration by promoting osteogenic differentiation via the AMPK-ULK1-autophagy axis. Autophagy. 2022;18(9):2229–2245. doi:10.1080/15548627.2021.2016231
76. Li Y, Chen Y. AMPK and autophagy. Adv Exp Med Biol. 2019;1206:85–108.
77. Ge Y, Zhou M, Chen C, et al. Role of AMPK mediated pathways in autophagy and aging. Biochimie. 2022;195:100–113. doi:10.1016/j.biochi.2021.11.008
78. Li Y, Su J, Sun W, et al. AMP-activated protein kinase stimulates osteoblast differentiation and mineralization through autophagy induction. Int J Mol Med. 2018;41(5):2535–2544. doi:10.3892/ijmm.2018.3498
79. Guo X, Liang M. Metformin alleviates dexamethasone-induced apoptosis by regulating autophagy via AMPK/mTOR/p70S6K in osteoblasts. Exp Cell Res. 2022;415(1):113120. doi:10.1016/j.yexcr.2022.113120
80. Cai ZY, Yang B, Shi YX, et al. High glucose downregulates the effects of autophagy on osteoclastogenesis via the AMPK/mTOR/ULK1 pathway. Biochem Biophys Res Commun. 2018;503(2):428–435. doi:10.1016/j.bbrc.2018.04.052
81. Yao Z, Getting SJ, Locke IC. Regulation of TNF-induced osteoclast differentiation. Cells. 2021;11(1):132. doi:10.3390/cells11010132
82. Tong X, Gu J, Song R, et al. Osteoprotegerin inhibit osteoclast differentiation and bone resorption by enhancing autophagy via AMPK/mTOR/p70S6K signaling pathway in vitro. J Cell Biochem. 2019;120(2):1630–1642. doi:10.1002/jcb.27468
83. Tong X, Zhang C, Wang D, et al. Suppression of AMP-activated protein kinase reverses osteoprotegerin-induced inhibition of osteoclast differentiation by reducing autophagy. Cell Prolif. 2020;53(1):e12714. doi:10.1111/cpr.12714
84. Zhu K, Wu Y, He P, et al. PI3K/AKT/mTOR-targeted therapy for breast cancer. Cells. 2022;11(16):2508. doi:10.3390/cells11162508
85. Li H, Prever L, Hirsch E, et al. Targeting PI3K/AKT/mTOR signaling pathway in breast cancer. Cancers. 2021;13(14):1.
86. Leyane TS, Jere SW, Houreld NN. Cellular signalling and photobiomodulation in chronic wound repair. Int J Mol Sci. 2021;22(20):11223. doi:10.3390/ijms222011223
87. Zuo X, Wei X, Ju C, et al. Protective effect of photobiomodulation against hydrogen peroxide-induced oxidative damage by promoting autophagy through inhibition of PI3K/AKT/mTOR pathway in MC3T3-E1 cells. Oxid Med Cell Longev. 2022;2022:7223353. doi:10.1155/2022/7223353
88. Liu Y, Zhao L, He X, et al. Jintiange proteins promote osteogenesis and inhibit apoptosis of osteoblasts by enhancing autophagy via PI3K/AKT and ER stress pathways. J Ethnopharmacol. 2023;311:116399. doi:10.1016/j.jep.2023.116399
89. Jiang C, Wang Y, Zhang M, et al. Cholesterol inhibits autophagy in RANKL-induced osteoclast differentiation through activating the PI3K/AKT/mTOR signaling pathway. Mol Biol Rep. 2022;49(10):9217–9229. doi:10.1007/s11033-022-07747-w
90. Fu L, Wu W, Sun X, et al. Glucocorticoids enhanced osteoclast autophagy through the PI3K/Akt/mTOR signaling pathway. Calcif Tissue Int. 2020;107(1):60–71. doi:10.1007/s00223-020-00687-2
91. Zhao H, Sun Z, Ma Y, et al. Antiosteoclastic bone resorption activity of osteoprotegerin via enhanced AKT/mTOR/ULK1-mediated autophagic pathway. J Cell Physiol. 2020;235(3):3002–3012. doi:10.1002/jcp.29205
92. Cheng Z. The FoxO-autophagy axis in health and disease. Trends Endocrinol Metab. 2019;30(9):658–671. doi:10.1016/j.tem.2019.07.009
93. Ambrogini E, Almeida M, Martin-Millan M, et al. FoxO-mediated defense against oxidative stress in osteoblasts is indispensable for skeletal homeostasis in mice. Cell Metab. 2010;11(2):136–146. doi:10.1016/j.cmet.2009.12.009
94. Lian WS, Wu RW, Chen YS, et al. MicroRNA-29a mitigates osteoblast senescence and counteracts bone loss through oxidation resistance-1 control of FoxO3 methylation. Antioxidants. 2021;10(8):1248. doi:10.3390/antiox10081248
95. Jiang Y, Luo W, Wang B, et al. 1α,25-Dihydroxyvitamin D3 ameliorates diabetes-induced bone loss by attenuating FoxO1-mediated autophagy. J Biol Chem. 2021;296:100287. doi:10.1016/j.jbc.2021.100287
96. Gómez-Puerto MC, Verhagen LP, Braat AK, et al. Activation of autophagy by FOXO3 regulates redox homeostasis during osteogenic differentiation. Autophagy. 2016;12(10):1804–1816. doi:10.1080/15548627.2016.1203484
97. Yu XH, Xu XM, Zhang SX. Low-dose dexamethasone promotes osteoblast viability by activating autophagy via the SGK1/FOXO3a signaling pathway. Cell Biol Int. 2023;47(3):669–678. doi:10.1002/cbin.11971
98. Carafa V, Rotili D, Forgione M, et al. Sirtuin functions and modulation: from chemistry to the clinic. Clinical Epigenetics. 2016;8(1):61. doi:10.1186/s13148-016-0224-3
99. Dai H, Sinclair DA, Ellis JL, et al. Sirtuin activators and inhibitors: promises, achievements, and challenges. Pharmacol Ther. 2018;188:140–154. doi:10.1016/j.pharmthera.2018.03.004
100. Kuno A, Hosoda R, Tsukamoto M, et al. SIRT1 in the cardiomyocyte counteracts doxorubicin-induced cardiotoxicity via regulating histone H2AX. Cardiovasc Res. 2023;118(17):3360–3373. doi:10.1093/cvr/cvac026
101. Wang Y, Mei R, Hao S, et al. Up-regulation of SIRT1 induced by 17beta-estradiol promotes autophagy and inhibits apoptosis in osteoblasts. Aging. 2021;13(20):23652–23671. doi:10.18632/aging.203639
102. Denu RA. SIRT3 enhances mesenchymal stem cell longevity and differentiation. Oxid Med Cell Longev. 2017;2017:5841716. doi:10.1155/2017/5841716
103. Wang S, Yang J, Lin T, et al. Excessive production of mitochondrion‑derived reactive oxygen species induced by titanium ions leads to autophagic cell death of osteoblasts via the SIRT3/SOD2 pathway. Mol Med Rep. 2020;22(1):257–264. doi:10.3892/mmr.2020.11094
104. Li W, Jiang WS, Su YR, et al. PINK1/Parkin-mediated mitophagy inhibits osteoblast apoptosis induced by advanced oxidation protein products. Cell Death Dis. 2023;14(2):88. doi:10.1038/s41419-023-05595-5
105. Cui Y, Li B, Du J, et al. Dibutyl phthalate causes MC3T3-E1 cell damage by increasing ROS to promote the PINK1/Parkin-mediated mitophagy. Environ Toxicol. 2022;37(10):2341–2353. doi:10.1002/tox.23600
106. Chen L, Shi X, Weng SJ, et al. Vitamin K2 can rescue the dexamethasone-induced downregulation of osteoblast autophagy and mitophagy thereby restoring osteoblast function in vitro and in vivo. Front Pharmacol. 2020;11:1209. doi:10.3389/fphar.2020.01209
107. Tang H, Zhu S, Chen K, et al. IL-17A regulates autophagy and promotes osteoclast differentiation through the ERK/mTOR/Beclin1 pathway. PLoS One. 2023;18(2):e0281845. doi:10.1371/journal.pone.0281845
108. Chung YH, Jang Y, Choi B, et al. Beclin-1 is required for RANKL-induced osteoclast differentiation. J Cell Physiol. 2014;229(12):1963–1971. doi:10.1002/jcp.24646
109. Arai A, Kim S, Goldshteyn V, et al. Beclin1 modulates bone homeostasis by regulating osteoclast and chondrocyte differentiation. J Bone Miner Res. 2019;34(9):1753–1766. doi:10.1002/jbmr.3756
110. Tong X, Min W, Li S, et al. Beclin 1 positively regulates osteoprotegerin-induced inhibition of osteoclastogenesis by increasing autophagy in vitro. Differentiation. 2021;121:35–43. doi:10.1016/j.diff.2021.08.003
111. Xie X, Hu L, Mi B, et al. Metformin alleviates bone loss in ovariectomized mice through inhibition of autophagy of osteoclast precursors mediated by E2F1. Cell Commun Signal. 2022;20(1):165. doi:10.1186/s12964-022-00966-5
112. Qu Z, An H, Feng M, et al. Urolithin B suppresses osteoclastogenesis via inhibiting RANKL-induced signalling pathways and attenuating ROS activities. J Cell Mol Med. 2022;26(16):4428–4439. doi:10.1111/jcmm.17467
113. Sul OJ, Li G, Kim JE, et al. 7-ketocholesterol enhances autophagy via the ROS-TFEB signaling pathway in osteoclasts. J Nutr Biochem. 2021;96:108783. doi:10.1016/j.jnutbio.2021.108783
114. Park HJ, Yoon SY, Park JN, et al. Doxorubicin induces bone loss by increasing autophagy through a mitochondrial ROS/TRPML1/TFEB axis in osteoclasts. Antioxidants. 2022;11(8). doi:10.3390/antiox11081476
115. Walker EC, McGregor NE, Poulton IJ, et al. Oncostatin M promotes bone formation independently of resorption when signaling through leukemia inhibitory factor receptor in mice. J Clin Invest. 2010;120(2):582–592. doi:10.1172/JCI40568
116. Zhou J, Yang J, Dong Y, et al. Oncostatin M receptor regulates osteoblast differentiation via extracellular signal-regulated kinase/autophagy signaling. Stem Cell Res Ther. 2022;13(1):278. doi:10.1186/s13287-022-02958-1
117. Xue LX, Chen SF, Xue SX, et al. LncRNA TUG1 compromised neuronal mitophagy in cerebral ischemia/reperfusion injury by targeting sirtuin 1. Cell Biol Toxicol. 2022;38(6):1121–1136. doi:10.1007/s10565-022-09700-w
118. Su Q, Liu Y, Lv XW, et al. LncRNA TUG1 mediates ischemic myocardial injury by targeting miR-132-3p/HDAC3 axis. Am J Physiol Heart Circ Physiol. 2020;318(2):H332–H344. doi:10.1152/ajpheart.00444.2019
119. Lu DG, Lu MJ, Yao SH, et al. Long non-coding RNA TUG1 promotes the osteogenic differentiation of bone marrow mesenchymal stem cells by regulating the AMPK/mTOR/autophagy pathway. Biomed Res. 2021;42(6):239–246. doi:10.2220/biomedres.42.239
120. Jiang T, Gu H, Wei J. Echinacoside Inhibits osteoclast function by down-regulating PI3K/Akt/C-Fos to alleviate osteolysis caused by periprosthetic joint infection. Front Pharmacol. 2022;13:930053. doi:10.3389/fphar.2022.930053
121. Tong X, Chen M, Song R, et al. Overexpression of c-Fos reverses osteoprotegerin-mediated suppression of osteoclastogenesis by increasing the Beclin1-induced autophagy. J Cell Mol Med. 2021;25(2):937–945. doi:10.1111/jcmm.16152
122. Tang X, Wang P, Zhang R, et al. KLF2 regulates neutrophil activation and thrombosis in cardiac hypertrophy and heart failure progression. J Clin Invest. 2022;132(3). doi:10.1172/JCI147191
123. Laha D, Deb M, Das H. KLF2 (kruppel-like factor 2 [lung]) regulates osteoclastogenesis by modulating autophagy. Autophagy. 2019;15(12):2063–2075. doi:10.1080/15548627.2019.1596491
124. Pattingre S, Tassa A, Qu X, et al. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122(6):927–939. doi:10.1016/j.cell.2005.07.002
125. Ke D, Ji L, Wang Y, et al. JNK1 regulates RANKL-induced osteoclastogenesis via activation of a novel Bcl-2-Beclin1-autophagy pathway. FASEB J. 2019;33(10):11082–11095. doi:10.1096/fj.201802597RR
126. Yang C, Tao H, Zhang H, et al. TET2 regulates osteoclastogenesis by modulating autophagy in OVX-induced bone loss. Autophagy. 2022;18(12):2817–2829. doi:10.1080/15548627.2022.2048432
127. Li Z, Huang Z, Zhang H, et al. P2X7 receptor induces pyroptotic inflammation and cartilage degradation in osteoarthritis via NF-κB/NLRP3 crosstalk. Oxid Med Cell Longev. 2021;2021:8868361. doi:10.1155/2021/8868361
128. Ma Y, Di R, Zhao H, et al. P2X7 receptor knockdown suppresses osteoclast differentiation by inhibiting autophagy and Ca 2+ /calcineurin signaling. Mol Med Rep. 2022;25(5). doi:10.3892/mmr.2022.12677
129. Cao B, Dai X, Wang W. Knockdown of TRPV4 suppresses osteoclast differentiation and osteoporosis by inhibiting autophagy through Ca(2+) -calcineurin-NFATc1 pathway. J Cell Physiol. 2019;234(5):6831–6841. doi:10.1002/jcp.27432
130. Yang L, Liu S, Mu S, et al. Paeoniflorin attenuates dexamethasone-induced apoptosis of osteoblast cells and promotes bone formation via regulating AKT/mTOR/autophagy signaling pathway. Evid Based Complement Alternat Med. 2021;2021:6623464. doi:10.1155/2021/6623464
131. Zhao B, Peng Q, Wang D, et al. Leonurine protects bone mesenchymal stem cells from oxidative stress by activating mitophagy through PI3K/Akt/mTOR pathway. Cells. 2022;11(11):1724. doi:10.3390/cells11111724
132. Zhao B, Peng Q, Poon EHL, et al. Leonurine promotes the osteoblast differentiation of rat BMSCs by activation of autophagy via the PI3K/Akt/mTOR pathway. Front Bioeng Biotechnol. 2021;9:615191. doi:10.3389/fbioe.2021.615191
133. Malaguarnera L. Influence of resveratrol on the immune response. Nutrients. 2019;11(5). doi:10.3390/nu11050946
134. Breuss JM, Atanasov AG, Uhrin P. Resveratrol and its effects on the vascular system. Int J Mol Sci. 2019;20(7):1523. doi:10.3390/ijms20071523
135. Yang X, Jiang T, Wang Y, et al. The role and mechanism of SIRT1 in resveratrol-regulated osteoblast autophagy in osteoporosis rats. Sci Rep. 2019;9(1):18424. doi:10.1038/s41598-019-44766-3
136. Gao S, Feng Q. The beneficial effects of geniposide on glucose and lipid metabolism: a review. Drug Des Devel Ther. 2022;16:3365–3383. doi:10.2147/DDDT.S378976
137. Huang J, Ye Y, Xiao Y, et al. Geniposide ameliorates glucocorticoid-induced osteoblast apoptosis by activating autophagy. Biomed Pharmacother. 2022;155:113829. doi:10.1016/j.biopha.2022.113829
138. Tang YH, Yue ZS, Li GS, et al. Effect of β‑ecdysterone on glucocorticoid‑induced apoptosis and autophagy in osteoblasts. Mol Med Rep. 2018;17(1):158–164. doi:10.3892/mmr.2017.7840
139. Tang Y, Mo Y, Xin D, et al. Regulation of osteoblast autophagy based on PI3K/AKT/mTOR signaling pathway study on the effect of β-ecdysterone on fracture healing. J Orthop Surg Res. 2021;16(1):719. doi:10.1186/s13018-021-02862-z
140. Zeng C, Wang S, Chen F, et al. Alpinetin alleviates osteoporosis by promoting osteogenic differentiation in BMSCs by triggering autophagy via PKA/mTOR/ULK1 signaling. Phytother Res. 2023;37(1):252–270. doi:10.1002/ptr.7610
141. Dong GM, Yu H, Pan LB, et al. Biotransformation of timosaponin BII into seven characteristic metabolites by the gut microbiota. Molecules. 2021;26(13):3861. doi:10.3390/molecules26133861
142. Wang N, Xu P, Wu R, et al. Timosaponin BII improved osteoporosis caused by hyperglycemia through promoting autophagy of osteoblasts via suppressing the mTOR/NFκB signaling pathway. Free Radic Biol Med. 2021;171:112–123. doi:10.1016/j.freeradbiomed.2021.05.014
143. Rashid M, Singh SK, Malik MY, et al. Development and validation of UPLC-MS/MS assay for quantification of cladrin: absolute bioavailability and dose proportionality study in rats. J Pharm Biomed Anal. 2018;152:289–297. doi:10.1016/j.jpba.2018.01.044
144. Rai R, Singh KB, Khanka S, et al. Cladrin alleviates dexamethasone-induced apoptosis of osteoblasts and promotes bone formation through autophagy induction via AMPK/mTOR signaling. Free Radic Biol Med. 2022;190:339–350. doi:10.1016/j.freeradbiomed.2022.08.028
145. Yue C, Jin H, Zhang X, et al. Aucubin prevents steroid-induced osteoblast apoptosis by enhancing autophagy via AMPK activation. J Cell Mol Med. 2021;25(21):10175–10184. doi:10.1111/jcmm.16954
146. Mizunoe Y, Kobayashi M, Sudo Y, et al. Trehalose protects against oxidative stress by regulating the Keap1-Nrf2 and autophagy pathways. Redox Biol. 2018;15:115–124. doi:10.1016/j.redox.2017.09.007
147. Pupyshev AB, Klyushnik TP, Akopyan AA, et al. Disaccharide trehalose in experimental therapies for neurodegenerative disorders: molecular targets and translational potential. Pharmacol Res. 2022;183:106373. doi:10.1016/j.phrs.2022.106373
148. Zhu L, Yuan Y, Yuan L, et al. Activation of TFEB-mediated autophagy by trehalose attenuates mitochondrial dysfunction in cisplatin-induced acute kidney injury. Theranostics. 2020;10(13):5829–5844. doi:10.7150/thno.44051
149. Cao L, Zhou S, Qiu X, et al. Trehalose improves palmitic acid-induced apoptosis of osteoblasts by regulating SIRT3 -medicated autophagy via the AMPK/mTOR / ULK1 pathway. FASEB J. 2022;36(9):e22491. doi:10.1096/fj.202200608RR
150. Wang L, Zhang HY, Gao B, et al. Tetramethylpyrazine protects against glucocorticoid-induced apoptosis by promoting autophagy in mesenchymal stem cells and improves bone mass in glucocorticoid-induced osteoporosis rats. Stem Cells Dev. 2017;26(6):419–430. doi:10.1089/scd.2016.0233
151. Zhang B, Yang LL, Ding SQ, et al. Anti-osteoporotic activity of an edible traditional Chinese medicine cistanche deserticola on bone metabolism of ovariectomized rats through RANKL/RANK/TRAF6-mediated signaling pathways. Front Pharmacol. 2019;10:1412. doi:10.3389/fphar.2019.01412
152. Xu X, Zhang Z, Wang W, et al. Therapeutic effect of cistanoside A on bone metabolism of ovariectomized mice. Molecules. 2017;22(2):1.
153. Chen T, Gao F, Luo D, et al. Cistanoside A promotes osteogenesis of primary osteoblasts by alleviating apoptosis and activating autophagy through involvement of the Wnt/β-catenin signal pathway. Ann Transl Med. 2022;10(2):64. doi:10.21037/atm-21-6742
154. Kim CJ, Shin SH, Kim BJ, et al. The effects of Kaempferol-inhibited autophagy on osteoclast formation. Int J Mol Sci. 2018;19(1):1.
155. Chen Z, Xue J, Shen T, et al. Curcumin alleviates glucocorticoid-induced osteoporosis through the regulation of the Wnt signaling pathway. Int J Mol Med. 2016;37(2):329–338. doi:10.3892/ijmm.2015.2432
156. Ke D, Wang Y, Yu Y, et al. Curcumin-activated autophagy plays a negative role in its anti-osteoclastogenic effect. Mol Cell Endocrinol. 2020;500:110637. doi:10.1016/j.mce.2019.110637
157. Nahak P, Gajbhiye RL, Karmakar G, et al. Orcinol glucoside loaded polymer - lipid hybrid nanostructured lipid carriers: potential cytotoxic agents against gastric, colon and hepatoma carcinoma cell lines. Pharm Res. 2018;35(10):198. doi:10.1007/s11095-018-2469-3
158. Gong W, Liu M, Zhang Q, et al. Orcinol glucoside improves senile osteoporosis through attenuating oxidative stress and autophagy of osteoclast via activating Nrf2/Keap1 and mTOR signaling pathway. Oxid Med Cell Longev. 2022;2022:5410377. doi:10.1155/2022/5410377
159. Yuan Q, Zhang X, Wei W, et al. Lycorine improves peripheral nerve function by promoting Schwann cell autophagy via AMPK pathway activation and MMP9 downregulation in diabetic peripheral neuropathy. Pharmacol Res. 2022;175:105985. doi:10.1016/j.phrs.2021.105985
160. Park HJ, Gholam-Zadeh M, Suh JH, et al. Lycorine attenuates autophagy in osteoclasts via an axis of mROS/TRPML1/TFEB to reduce LPS-induced bone loss. Oxid Med Cell Longev. 2019;2019:8982147. doi:10.1155/2019/8982147
161. Xu J, Xu Z, Zheng W. A review of the antiviral role of green tea catechins. Molecules. 2017;22(8). doi:10.3390/molecules22081337
162. Sarkar J, Das M, Howlader MSI, et al. Epigallocatechin-3-gallate inhibits osteoclastic differentiation by modulating mitophagy and mitochondrial functions. Cell Death Dis. 2022;13(10):908. doi:10.1038/s41419-022-05343-1
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.