Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 16
The Metabolic Characteristics of Patients at the Risk for Diabetic Foot Ulcer: A Comparative Study of Diabetic Patients with and without Diabetic Foot
Authors Li X, Wen S , Dong M , Yuan Y, Gong M, Wang C, Yuan X, Jin J, Zhou M, Zhou L
Received 13 July 2023
Accepted for publication 27 September 2023
Published 17 October 2023 Volume 2023:16 Pages 3197—3211
DOI https://doi.org/10.2147/DMSO.S430426
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Konstantinos Tziomalos
Xiucai Li,1,* Song Wen,1,* Meiyuan Dong,1,2 Yue Yuan,1 Min Gong,1 Congcong Wang,1 Xinlu Yuan,1 Jianlan Jin,1 Mingyue Zhou,3 Ligang Zhou1,2,4
1Department of Endocrinology, Shanghai Pudong Hospital, Fudan University, Shanghai, 201399, People’s Republic of China; 2Hebei Medical University, Shijiazhuang, 050013, People’s Republic of China; 3Clinical Research OB/GYN REI Division, University of California, San Francisco, CA, USA; 4Shanghai Key Laboratory of Vascular Lesions Regulation and Remodeling, Shanghai Pudong Hospital, Shanghai, 201399, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Ligang Zhou, Department of Endocrinology, Shanghai Pudong Hospital, Fudan University, Shanghai, 201399, People’s Republic of China, Tel +8613611927616, Email [email protected]
Backgrounds and Objective: Diabetic foot is a relatively severe complication in patients with type 2 diabetes (T2D), with peripheral neuropathy and angiopathy frequently serving as risk factors. However, it is unknown how the other major systemic metabolic factors impacted the profile of these patients, besides glucose management. Thus, we investigated the distinct characteristics of patients with diabetic foot ulcers and their relationships with angiopathy.
Materials and Methods: We obtained the laboratory data of 334 diabetic patients at Shanghai Pudong Hospital from 2020 to 2023. The comparisons were performed between the groups with or without diabetic foot, including glucose metabolism, lipids profile, liver and kidney function, thyroid function, and serum iron. The association between metabolic factors and lower extremity computed tomography angiography (CTA) was analyzed.
Results: We found significant disparities between groups in relation to age, serum protein content, liver transferase, serum creatinine, estimated glomerular filtration rate (eGFR), serum uric acid (UA), small dense low-density lipoprotein (sdLDL), lipoprotein A (LP(a)), apolipoprotein A1 (APOA1), thyroid function, serum iron, and hemoglobin (Hb) (p< 0.05). The Spearman correlational analyses showed that the severity of CTA, categorized by the unilateral or bilateral plaque or occlusion, was positively significantly correlated with UA (r=0.499), triglyceride (TG) (r=0.751), whereas inversely correlated with serum albumin (r=− 0.510), alanine aminotransferase (r=− 0.523), direct bilirubin (DBil) (r=− 0.494), total bilirubin (TBil) (r=− 0.550), Hb (r=− 0.646).
Conclusion: This cross-section investigation showed that compared to T2D only, the patients with diabetic foot ulcer (DFU) might display similar glucose metabolic control context but adverse metabolic profiles, and this profile is associated with macrovascular angiopathy characteristics and their severity.
Keywords: diabetic foot, macrovascular angiopathy, CTA, thyroid function, serum iron, serum bilirubin
Introduction
Diabetic foot is the infection, ulceration or destruction of tissues of the foot associated with neuropathy and/or peripheral artery disease in the lower extremity of a person with (a history of) diabetes mellitus. Diabetic foot ulcer (DFU) is a break of the skin of the foot that includes minimally the epidermis and part of the dermis in a diabetic patient.1 To date, the summarized current investigations showed that the incidence of DFU was inconsistently due to the variations in study plot, different study cohorts, as well as the study time. It was also reported that from the year of 2010, the annual incidence of DFU could be expected from 0.2% to 11% in diabetic background or account for about 0.1–8% originated from community- and population-source.2 According to the survey from the International Diabetes Foundation, approximately 40 million to 60 million patients are complicated with DFU, and this data changed remarkably from 9 million to 26 million in 2015.3 In China, the DFU in the constitution of the cause of chronic wound rises sharply in recent years from 4.9% in 1996 to the 33% in 2007–2008 estimated in 17 Chinese tertiary hospitals. According to a recent study, the incidence of newly onset DFU within 1 year in diabetic adults aged above 50 years was 8.1%, and 31.6% recurrent in cured DFU.4 Intriguingly, a previous epidemic study reported the there were differences in risk factors distribution in DFU between the south and north Chinese patients: age, patient income, coronary heart disease, retinopathy, HbA1c, kidney function, and amputation rate seemed to pave for this divergency.5
The current standard care of DFU includes early recognition of risk factors such as poor glycemic control, peripheral neuropathy (DPN) or arterial diseases (PAD), foot deformities, pre-ulcerative abnormalities, smoking, prior amputation, retinopathy and nephropathy; evaluation of protective sensation, the surveillance of early foot problem, follow-up based on the severity of DPN, PAD and vigilant for other pre-ulcerative or higher risks. In addition, the treatment of DFU is based on the amplitude of risks for DFU, where the approaches regarding patients at higher risk include negative-pressure treatment, administer growth factors, bioengineered tissue, acellular matrix tissue, stem cell therapy, hyperbaric oxygen therapy, and, most recently, topical oxygen therapy. It is recommended that the initial treatment and evaluation encompass the following principles: offloading of plantar ulcerations, debridement of necrotic, nonviable tissue, revascularization of ischemic wounds, when necessary, management of infection: soft tissue or bone, and use of physiologic, topical dressings.6
However, the issue of slowly healing with higher recurrence above 20% remains unsolved in the past 15–20 years in spite of the updating in techniques in treatment of DFU.7 The mean risk for DFU in lifetime has been reported to be within 12% to 25%, although it is proclaimed higher according to the study by Armstrong et al about 19% and 34%, which could be possibly traced to the elongation of lifespan.8,9 Foot ulcers or even lower extremity amputations are predisposed by diabetic neuropathy and/or PAD,10,11 which significantly account for the major causes of morbidity and mortality in diabetes.12,13 In a survey of 2010 from Chinese tertiary hospital, DFU accounts for 27.3% of the all causes of amputation of lower extremity, and 56.5% in non-traumatic amputation. The data shows in 2012–2013, the total amputation rate had been dropped to 19.03%, where the major amputation was 2.14%, and 16.88% of minor amputation. The current annual mortality of DFU without amputation is 14.4%, whereas it escalates to the 40% in patients received amputation regardless of major or minor.4 Therefore, identification of feet with higher risks for DFU, pre-ulcerative alterations, and immediately address the mild ulcerations, as well as improve the complications of lower-extremity as soon as possible could postpone the incident of adverse consequences.
The presence of multiple risk factors, including age, duration of diabetes, poor glucose management, peripheral neuropathy, peripheral arterial diseases (PAD), and foot deformities such as hammertoe or Charcot arthropathy, increases the probability of developing DFU.2,14 Moreover, in the advanced stages of type 2 diabetes (T2D), systemic features such as nutritional status, obesity, renal function, and residual pancreatic function of the patient may influence the occurrence and outcome of DFU.15–17 In recent years, there emerged multiple studies identifying the critical factors contributed to the DFU, which reached to the consensus were listed as: age, gender, duration of DM, previous DFU, location of ulcer, smoking, prior amputation, foot deformity, DPN, PAD, other metabolic factors such as hypertension, lipids, glycated hemoglobin A1C (HbA1C), body mass index (BMI), kidney function and others.18–20 Nonetheless, few clinical investigations identified a comprehensive scenario for patients at risk for DFU, and fail to recognize the systemic factors such as hepatic function, thyroid function, nutrients status, and their relationship with the macro-or micro-vasculopathy.
Therefore, this study seeks to provide a more thorough picture of the clinical profile of DFU-at-risk individuals, including systemic parameters such as lipid parameters, thyroid function, and hepatic and renal function. By comparing diabetes individuals with and without DFUs, we can uncover more significant underlying risk factors and related comorbidities that contribute to DFU development. Endocrinologists will be able to implement targeted interventions, such as nutrition education, cholesterol control techniques, and early diagnosis of peripheral neuropathy and vascular disease, to reduce the incidence of DFU as a result of these findings.
Materials and Methods
Source of In-Patient Data
This comparison study will compare the diabetic patients with foot ulcers (DFU) against those without diabetic foot ulcers (non DFU). A total of 334 patients were recruited from Pudong Hospital over the period of 2020–2023. Using a review of medical records, the demographic features, duration of diabetes, glycemic control, peripheral neuropathy, peripheral vascular disease, foot deformities, time of insulin use, serum lipid, hepatic or renal function, as well as thyroid function will be investigated. The information on the patients with DFU and non DFU at admission of wards was collected from Shanghai Pudong Hospital’s in-patient information system from 2020 to 2023. The inclusion criteria for DFU were in conform with the definition of DFU acclaimed by the International Working Group on the Diabetic Foot (IWGDF), who were neither with amputation history nor evidence of gangrene or severe ulcer determined by the risk stratification of Wagner (0-III level). The consciousness status of all included patients was evaluated with 15 scores by Glasgow Coma Scale (GCS). The initial screen at admission examined the status of the lower extremity circuitry and peripheral nerve function via lower vascular ultrasound examination, electromyography, sensation threshold tests, which supported the diagnoses of PAD or DPN; The condition of these patients was stable at admission demonstrated by pertinent laboratory tests, which supported exclusion of the possibilities of acute myocardial infarction, stroke, pulmonary embolism, acute pancreatitis, acute diabetic complication such as diabetic hypoglycemic coma, diabetic ketoacidosis, hyperosmolar hyperglycemic state and other severe conditions. We randomly included the appropriate amount non-DFU patients (n=250) corresponding (3:1) to the DFU patients (n=84) at the same time, where duration of DM, fasting or postprandial plasma glucose value, and HbA1C were matched between two groups. All of data were collected at the beginning of hospital admission. The subjects with T1DM, hypothyroidism, and hyperthyroidism were excluded from the study. We also excluded extreme conditions such as diabetic ketoacidosis, hyperglycemic hyperosmolar state, etc. The T2D diagnosis was established using World Health Organization (WHO) guidelines published in 1999.21 The characteristics of the DFU and non-DFU patients are listed in Table 1.
 |
Table 1 The Clinical and Demographic Characteristics of the Included DFU and Non-DFU Patients |
Blood Sampling and Evaluation of Laboratory Data
The laboratory data including blood sampling were recorded at admission of ward of every T2D patients on the diabetes status, thyroid function, and trace micronutrients. All parameters, including biochemical indicators: fasting blood glucose, lipid parameters, hepatic function, and kidney function indicators: estimated glomerular filtration rate (eGFR), creatinine, and serum iron were analyzed via full-automatic biochemical analyzer (ADVIA chemistry XPT, SIEMENS, USA). Hemoglobin A1C (HbA1C) was analyzed by an HbA1C analyzer (TOSOH G8), which reflects the level of glycemic control in the past 3 months. C-peptide and thyroid function indicators: thyroid stimulating hormone (TSH), free triiodothyronine (FT3), free thyroxine (FT4), total triiodothyronine (TT3), total thyroxine (TT4), and anti-thyroid peroxidase antibody (TPOAb), anti-thyroid globin antibody (TgAb), two markers of chronic lymphatic thyroiditis, were processed via chemiluminescence methods, in a full-automatic chemiluminescence immunoassay analyzer (ADIVA Centaur XPT, SIEMENS, USA); Procalcitonin via methods of immunofluorescent assay (Pylon, ETHealthcare, USA). The circulation status of lower extremity was assessed via computed tomography angiography (CTA) (128 slice, OMATOM DefinitionFlash, SIEMENS) following blood sample.
Established HOMA-IR and HOMA-β Models
In estimating and compare the insulin resistance and β-cell function in the groups with and without DFU, we performed homeostasis model evaluation using fasting glucose and fasting C-peptide. This analysis was carried out in preparation for the start of insulin therapy. The HOMA-IR and HOMA-β were executed using a calculator that was downloaded from the University of Oxford. http://www.dtu.ox.ac.uk/.
The Severity Score of Arterial Disease is Evaluated Using Lower Extremity Computed CT Angiography
The feature of computed tomography angiography (CTA) of the lower extremities, which is highly related to systemic parameters, reveals the location, severity of narrowing, perfusion status, and compensation of the lower limb’s local circulation. We classified the severity of CTA according to five scores: Score=0 indicates that there are no plaque or narrowing; Score=1 indicates that there are unilateral plaque or narrowing; Score=2 indicates that there is unilateral occlusion; Score=3 indicates that there are bilateral plaque or narrowing; and Score=4 indicates that there is bilateral partially or completely occlusion.
Statistical Analyses
We initiated the data analyses via software of SPSS (IBM, version 26.0) and Prism (GraphPad, version 9.0). Either nonparametric analyses or two-way ANOVA were applicated for purposes of comparing distinction in levels of plasma C-peptide, HbA1c, serum iron, and thyroid function in this study. The non parametric analyses, such as Spearman correlational analyses, revealed the association between the age, gender, multiple metabolic variables with the severity of DFU in CTA. Multilinear regression model was used to discern the critical independent factors related to the change in thyroid hormone FT3. For the statistic results interpretation in the current study, p<0.05 was set as significance.
Results
The Difference in Age, Duration, Smoking History, Glucose Metabolic Context, Blood Pressure, and Lipids Profile
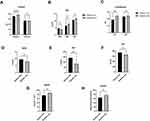
After matching for the duration, fasting plasma glucose (FPG), HbA1c, HOMA-IR, HOMA-β, and systolic and diastolic blood pressure (SBP and DBP), there is a significant difference in age between patients with and without DFU in the age (DFU vs T2D: male: 70.32±14.04 years vs 64.04±13.21 years, p=0.0069; female: 75.88±12.99 years vs 67.15±12.21 years, p=0.0017), smoking history (DFU vs T2D: 8.29±8.31 years vs 4.48±6.59 years, p<0.0001), small dense low-density lipoprotein (sdLDL, DFU vs T2D: 0.65±0.39 mmol/L vs 0.89±0.44 mmol/L, p=0.01), lipoprotein (LPa, DFU vs T2D: 349.18±278.14 mmol/L vs 228.41±258.58 mmol/L, p=0.01) and apoA1 (DFU vs T2D: 0.96±0.22 g/L vs 1.13±0.20 g/L, p<0.001). The total cholesterol (TC), total triglyceride (TG), high-density lipoprotein (HDL), low-density lipoprotein (LDL), apoA2, and apoE levels showed no significant differences (Figure 1).
The Distinction in Liver and Kidney Function Between Patients with Diabetic Foot and Non DFU
We continuedly to compare the difference in liver and kidney function between such two groups and found that the indices of proteins content, including serum albumin (DFU vs T2D: 35.27±5.21 g/L vs 38.07±4.28 g/L, p<0.0001), showed substantially significant between the two groups, as well as ALT (DFU vs T2D: 18.38±13.24 U/L vs 24.09±12.36 U/L, p=0.003), whereas serum globulin (p=0.86), AST (p=0.87), bilirubin (DBiL p=0.43; SBiL: p=0.0529; TBiL: p=0.15) demonstrated no difference.
Then, when we aimed to delineate the profile of kidney function between two groups, we only found that blood urea nitrogen (BUN) (p=0.08) and urinary albumin to creatinine ratio (UACR) (p=0.39) were generally comparable, though significant differences were found in levels of serum creatinine (SCr) (DFU vs T2D: 141.28±188.51 μmol/L vs 82.86±69.84 μmol/L, p=0.01), serum uric acid (UA) (DFU vs T2D: 370.64±144.87 μmol/L vs 330.43±118.51 μmol/L, p=0.03) and estimated glomerular filtration rate (eGFR) (DFU vs T2D: 75.68±35.48 mL/min*1.73m2 vs 86.88±26.49 mL/min*1.73m2, p=0.01) (Figure 2).
Thyroid Function, Procalcitonin, Serum Iron, and Hemoglobin Comparisons Between Patients with and without DFU
In a stepwise analysis, we evaluated the disparity of thyroid function between two groups, which may be decreased during extreme conditions such as diabetic ketoacidosis (DKA), severe infection or diabetic foot ulcers, and may predicted the severity of the underlying disease. We found that the decreased level of FT3 (DFU vs T2D: 3.73±1.28 pmol/L vs 4.08±0.91 pmol/L, p=0.046), decreased level of FT4 (DFU vs T2D: 14.16±3.23 pmol/L vs 15.05±2.32 pmol/L, p=0.04), and decreased level of TT3 (DFU vs T2D: 1.11±0.47 nmol/L vs 1.28±0.37 nmol/L, p=0.01) in the group with DFU (p<0.05), but not that of TT4 (p=0.24) and TPOAb (p=0.36). Although the levels of procalcitonin were elevated in the diabetic foot with high variation, this dissimilarity did not reach significance (p=0.19). In addition, the level of serum iron, which is related with thyroid function (DFU versus T2D: 9.06 5.24 mol/L vs 11.83 4.68 mol/L, p=0.0001), was significantly lower in patients with DFU compared to those without, as did hemoglobin (Hb) (DFU vs T2D: 115.88±21.67 g/L vs 130.87±19.57 g/L, p<0.0001) (Figure 3).
The Relationship Between the Severity Score of Lower Extremity CTA and the Multiple Metabolic Parameters in Patients with Diabetic Foot
We performed the Spearman correlational relationship between the CTA severity and aforementioned metabolic parameters and found that it positively significantly correlated with UA (r=0.499; p=0.043), triglyceride (TG) (r=0.751; p=0.002), whereas it inversely correlated with serum albumin (r=−0.510; p=0.031), ALT (r=−0.523; p=0.026), DBil (r=−0.494; p=0.037), total bilirubin (TBil) (r=−0.550; p=0.018), Hb (r=−0.646; p=0.004) (Figure 4).
On the other hand, we also found a relationship between other metabolic parameters. Since iron is essential to the proper function of the thyroid and multiple organs, we also found that iron was significantly decreased in DFU; therefore, we observed that serum iron had significant relation with the serum protein content, including albumin (r=0.44, p=0.001), globulin (r=−0.56; p<0.001), ALT (r=0.27; p=0.049), indirect bilirubin (r=0.53; p<0.001), total bilirubin (r=0.41; p=0.002), triglyceride (r=0.26; p=0.01), LDL (r=0.28; p=0.046), sdLDL (r=0.72; p<0.001), APOA1 (r=0.41; p=0.008), FT3 (r=0.56; p<0.001), FT4 (r=0.29; p=0.044), TT3 (r=0.50; p=0.002). We found FT3 is associated with Age (r=−0.248; p=0.046), FPG (r=−0.614; p=0.004), albumin (r=0.457; p<0.001), globulin (r=−0.281; p=0.029), SBiL (r=0.461; p<0.001), TBil (r=0.381; p=0.002), iron (r=0.564; p<0.001), SCr (r=−0.439; p<0.001), BUN (r=−0.489; p<0.001), UA (r=−0.289; p=0.023), eGFR (r=0.549; p<0.001), TC (r=0.280; p=0.029); HDL (r=0.296; p=0.021), LDL (r=0.297, p=0.02), sdLDL (r=0.436, p=0.029), APOA1 (r=0.523; p<0.001), PCT (r=−0.648, p=0.001) (Figure 4).
The Multilinear Regression Analyses on the Independent Predictor of FT3 in Metabolic Parameters in the Patients with DFU
Since serum iron level is significantly associated with thyroid function, especially we found FT3 was reduced considerably in DFU. We, thus, performed multilinear regression analyses on the independent variables to the change of the FT3. We found that the levels of APOA1, SBiL, HOMA-β, and eGFR were the significant independent predictors of the change in FT3 levels in patients with DFU (Table 2) (Figure 5A and B).
 |
Table 2 The Multilinear Regression Analyses on FT3 and Its Independent Predictors |
Discussion
The patients with diabetic foot were characterized by poor circulation, nerve damage, and a compromised ability to fight local and/or systemic infections.22 Diabetic foot is one of the severe diabetic complications, if not properly managed, could result in foot ulcers, infections, and even amputations. Patients with diabetes are at increased risk to develop diabetic foot if they have uncontrolled hyperglycemia, which can cause peripheral neuropathy or angiopathy in the feet. Common diabetic foot symptoms include foot discomfort or tingling, redness or swelling, open sores or non-healing wounds, and alterations in the morphology or color of the feet.23,24 For the prevention and management of diabetic foot, appropriate foot care, including daily sanitation, regular checkups, wearing properly fitted shoes, and regulating glucose levels, is essential.25 Multiple variables, including hyperglycemia, neuropathy, and peripheral artery disease, etc., contribute to the development of diabetic foot.17 A combination of reduced sensation, poor circulation, and local infection of the wound can lead to foot ulcers or slow-healing wounds. Additionally, the presence of hyperglycemia can contribute to bacterial infections that further worsen the condition.26 However, systemic factors besides glycemic control also affect and facilitate the healing or worsening of DFU27 (Figure 6).
There were also suggestions that other parameters connected to the severity of DFU, as defined by the Wagner scale, Texas University stage scale, Foster classification, PEDIS and IWGDF.28 In the present study, we evaluated the complete metabolic profile of patients with DFU and compared to the patients with non DFU. After matching the duration of diabetes, FPG, 2hPPG, and HbA1C, we identified a worsening condition in patients with DFU than in groups with non DFU; these characteristics may be connected with the severity of CTA in DFU. These parameters may serve as biomarkers for the injury to the lower extremities. This could assist clinicians decide not just to initiate anti-hyperglycemia therapy, but also a complete strategy that could postpone the progression of diabetic foot ulcer.29–32
Firstly, after adjusting for the duration of diabetes, blood glucose, and HbA1c, we found an aging tendency of DFU in both genders’ distributions, suggesting that aging is an independent risk factor in the DFU group; we also revealed that sdLDL, a component of LDL and atherogenic particle, was significantly decreased in patients with DFU, but not TC, TG, HDL, or LDL levels. Furthermore, as a major independent risk factor for cardiovascular disorders, LP(a) was shown to be higher in the DFU group, suggesting that LP(a) may be a metabolic risk for cardiovascular disease in DFU patients.33,34 In addition, we found that APOA1, a component of HDL, decreased significantly in DFU; whereas the levels of APOA2 and APOE, which are associated with TG level, did not differ significantly, indicating that decreased APOA1 may be an early marker for cardiovascular lesion in the patients with DFU.35
In addition, as an admission requirement, we examined the level of hepatic and renal function between two groups. Interestingly, albumin, which reflects liver synthesis ability, and ALT, a liver injury biomarker, were shown to decrease in the patients with DFU when compared with those of diabetes alone. Multiple risk factors may be underlying this status, such as aging, malabsorption, overconsumption status, loss of protein, etc. could not be excluded as critical factors.36,37 As for renal function, we consistently observed substantial changes in Scr, UA, and eGFR levels (for SCr and eGFR are only mildly impaired in the group without DFU), suggesting a decline in renal filtration function in the patients with DFU attributed possibly to the advanced diabetic nephropathy, in that the level of UACR exhibited as the urinary microalbumin stage (UACR: 30–300mg/g), as well as overconsumption status in the group with DFU.38,39 However, no significant differences in BUN and UACR were found between the two groups.
Changes in thyroid function, procalcitonin, serum iron, and hemoglobin were studied using the method of stepwise analysis. The DFU group had significantly lower levels of FT3, FT4, and TT3 compared to the control group. Despite the fact that the thyroid function was generally within the normal range, this difference showed that, as a result of the poor metabolic situation in the DFU group, the thyroid function may demonstrate a reduction in systemic metabolism. The decline of the body’s mechanisms prompted the decrease in thyroid function.40 Concurrently, the level of TPOAb was moderately elevated in the normal range and did not differ substantially between the two groups; consequently, the etiology of autoimmune thyroiditis, such as Hashimoto disease, could be excluded. This decreased thyroid function also indicates the poor health condition of DFU patients. The comparisons of procalcitonin, an infection biomarker, did not reveal a major difference because we did not observe that all DFU patients had severe circulatory infection. Due to the decrease in serum iron (reference level: Male: 11.6–31.3 mmol/L; Female: 9.0–30.4 mmol/L) and the moderate anemia in DFU (Hb>90g/L), the results suggest that the systemic status of Hb and iron may be deficient, and it is associated with the severity of diabetes in DFU.41
In furthermore, Spearman correlation analyses were conducted on DFU patients to evaluate the potential association between systemic factors and the imaging severity score of the lower extremities. Additionally, we discovered that the metabolic variables UA and TG were harmful, whereas albumin, ALT, DBiL, and Hb were related with a positive outcome in DFU. Previous research indicates that bilirubin within the normal range may have antioxidant effects and may be a factor in DFU recovery.42 In addition, analysis of serum iron revealed that it may be a nutrition indicator in DFU and is associated to thyroid function; this effect has been established by a number of clinical studies.43–45 As a sensitive sign of the metabolic health of the body, FT3 revealed many relationships with various parameters in this study, and its level is favorably associated with a favorable prognosis, showing it is a crucial protective indicator in DFU.46
Finally, we conducted a multilinear regression analysis to identify influential independent predictors of FT3 change. After adjusting for the other thyroid hormone indices, the analytic results showed that APOA1, SBiL, FPG, HOMA-β, and eGFR could account for approximately 40–45% of the causes of FT3 dynamic change. This may suggest to endocrinologists that FT3 is a sensitive systemic indicator for evaluating the metabolic condition of DFU patients.46 APOA1, SBiL, HOMA-β, and eGFR were the primary determinants of the change in FT3 levels, as determined by this investigation (Table 2).
Emerging evidence have proclaimed the protective property of bilirubin in diabetic individuals with lower extremities angiopathy, and endocrinologists are beginning to accept this.47,48 Multiple clinical studies have proved that there is a reverse relationship between the levels of blood bilirubin and the severity or markers of lower extremity lesions, such as ankle brachial index, atherosclerotic plaque, and arterial stiffness, etc.49,50 It has been hypothesized that bilirubin may modulate oxidative stress.51 As indicated by a previous large-sample clinical trial, serum bilirubin levels are inversely correlated with C-response protein (CRP) levels in diabetic patients with peripheral arterial disease (PAD).52 Some studies concluded that serum bilirubin, especially indirect bilirubin (SBiL), could have a direct impact upon serum lipids due to its hydrophobic properties, whereas DBiL poses lower anti-lipids effects owing to its hydrophilic attributes.52,53 Another human study confirmed the observation in rodent researches that serum bilirubin concentration was negatively correlated with BMI and adiposity in obese participants, especially that urobilin is closely linear correlated with the BMI and insulin resistance represented by HOMA-IR in female participants.54 Even though this phenomenon was not evident in our study, we also found increased HOMA-IR and reduced bilirubin levels (Figure 5A and B). One possible explanation to this is that bilirubin could act as a hormonal signal binding to PPAR-α nuclear receptor, while cytochrome P450 or glucuronyl UGT1A1 enzymes could promote the elimination of bilirubin from the circulation, which result in overweight or obesity.55,56 The metabolite of serum bilirubin is urobilin which could reabsorb from the intestine to the liver through hepatic portal vein, and its level is associated with visceral fat area in obese. It was reported recently the level of serum bilirubin were extraordinarily increased in elite athlete population, which indicates that higher normal bilirubin could predispose a better physical performance, and the effect of regular physical activity could induce mildly higher of serum bilirubin concentrations.57 Other clinical explorations have found that bilirubin is associated with the renal function, macrovascular or microvascular complications such as urinary albumin excretion.58–60 Therefore, these findings could support that mildly increased serum bilirubin, especially SBiL could be have a protective consequence on DFU, thus, maintenance of the levels of SBiL or the antioxidant ability maybe a critical target for DFU.
On the other hand, we observed a decline in serum iron level in DFU, which was closely associated with lower euthyroid function. Few clinical trials have investigated iron deficiency and iron supplementation in DFU; hence, the relationship between the concentration of serum iron and diabetes severity as well as its therapeutic effects in DFU patients remains unclear.61 However, the iron is intimately associated with the antioxidant function of bilirubin and synthesis of hemoglobin.62 The existence of overwhelmed oxidative stress, chronic inflammation, the disequilibrium immune components, repairment of epithelium latency, and attenuated angiogenesis locally aggravate wound healing in diabetic patients.63 Heme oxygenase-1 (HO-1) determines the catabolic speed of heme, led to the generation of biliverdin, and the metabolites of which was comprised of bilirubin and iron. Antioxidant therapy utilizing iron and other enzymes may play a role on anti-inflammatory, proliferative, angiogenesis-promoting, and cytoprotection. In addition, a study on rodents investigated the effects of various iron meal compositions on neuropathy and found that a low quantity of iron added to the diet could prevent peripheral nerve inflammation and degeneration.64 Therefore, iron supplementation may be a helpful strategy for DFU rehabilitation.
For thyroid function, we have already understood its essential role in metabolic regulation, and the tendency of decline in multiple severe systemic diseases, known as non-thyroidal illness syndrome. We previously have reported the significant change of thyroid function in metabolic disorder such as diabetic ketoacidosis (DKA), and its relationship with bone turnover activity.65,66 To date, there is no guideline for thyroid hormone replacement therapy (thyroxin) in the patients with DFU, due to its possible adverse influence on cardiovascular outcomes in elderly people. In severe acute complications such as DKA and HHS, according to one study, there is a trend toward restoration of thyroid function, and T4 treatment may not be necessary.67 Therefore, we proposed that the administration of thyroxin to a patient with DFU may be dependent on clinical manifestation, cardiovascular tolerance, and thyroid function.
In our clinical contact with DFU patients, evaluate and address the systemic conditions could be as well important as the target of glycemic control, which includes better handling the metabolic risk factors like hypertension, dyslipidemia, cardiovascular or cerebral vessel disorders prevention by anti-platelet or anti-coagulation therapy, improving the extremity circulation and DPN status by some antioxidant therapy, as well dispose the hypothyroidism, antibiotic medication use in case of infection, rectify the malnutrition, organ protection like ameliorate liver or kidney insufficiency, foot ulcer care by sanitation, implement specific care by consultation of other department like surgery when it is necessary. For the medical care givers or professionals, it is crucial to maintain updating of their care knowledge on DFU, enhance their foot care practice, promote effective communication skills, and disseminate education on patients, which ensures the quality of foot care delivered to the patients.68,69 As well it is essential for medical practitioner to recognize the systemic conditions and rise to the occasion to the patients’ DFU needs. However, due to the multi-facet problems in foot care of DFU, a multi-discipline team (MDT) work will be effective and benefit more for DFU treatment than single department disposals.70
Limitations
The conclusion was supported by research conducted at one research center. In addition, further data on renal tubular damage markers, tumor indicators, and microbial culture data should be included. Therefore, further research should be conducted to corroborate the recent results.
Conclusion
In the present study, a comprehensive status of DFU patients was observed. Compared to patients with non DFU, the patients with DFU had a worse metabolic profile. In addition, we discovered both protective and harmful systemic factors for DFU. To promote the rehabilitation of DFU, it is essential to consider not only glycemic control, as well as predisposed neuropathy and angiopathy, but also the amelioration of the reversible systemic factors, such as nutrition status, liver and renal function, lipid control, and antioxidative therapy, etc. Iron supplementation may be required for patients with DFU, although the necessity of thyroxine and biliverdin supplementation is questionable. Therefore, in the future, results of these interventions will be complemented with more sample data examined by different institutions. The relationships between DFU systemic conditions and renal microvascular indicators, tumor biomarkers, as well as microorganism culture data will be deserved to be identified for the individualized rehabilitation.
Ethical Statement
The study, including sampling, examinations, and access or utilization of the raw data for this study, obtained ethical approval from the Shanghai Pudong Hospital. Before the study, informed written consent was obtained. The guidelines outlined and whole procedures were conducted in accordance with the Declaration of Helsinki. All the data used in this study were anonymized before their use.
Acknowledgment
We should especially thank to the staff of the Department of Clinical Laboratory, Radiology, and other staff from Shanghai Pudong Hospital who provided massive contributions to this work.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Fudan Zhangjiang Clinical Medicine Innovation Fund Project (KP0202118), Talents Training Program of Shanghai Pudong Hospital (YQ202101), the Project of Key Medical Discipline of Pudong Hospital of Fudan University (Zdxk2020-11), Project of Key Medical Specialty and Treatment Center of Pudong Hospital of Fudan University (Zdzk2020-24), Special Department Fund of the Pudong New Area Health Planning Commission (PWZzk2017-03), Outstanding Leaders Training Program of Pudong Health Bureau of Shanghai (PWR12014-06), Pudong New Area Clinical Plateau Discipline Project (PWYgy-2021-03), the Natural Science Foundation of China (21675034), National Natural Science Foundation of China (81370932), and Shanghai Natural Science Foundation (19ZR1447500), the Project of Key Medical Specialty of Pudong Hospital of Fudan University (No Tszb2023-14), Pudong New Area Clinical Characteristic Discipline Project (PWYts2021-01), Wenzhou Medical University Education Grant (JG2021197).
Disclosure
The authors declare that there is no potential conflict of interest in this work.
References
1. van Netten JJ, Bus SA, Apelqvist J, et al. Definitions and criteria for diabetic foot disease. Diabetes Metab Res Rev. 2020;36(Suppl 1):e3268. doi:10.1002/dmrr.3268
2. McDermott K, Fang M, Boulton AJM, Selvin E, Hicks CW. Etiology, epidemiology, and disparities in the burden of diabetic foot ulcers. Diabetes Care. 2023;46(1):209–221. doi:10.2337/dci22-0043
3. International Diabetes Federation. The Diabetic Foot. Brussels, Belgium: International Diabetes Federation; 2020.
4. Society CD. Chinese guidelines for the prevention and treatment of type 2 diabetes (2020). Chin J Pract Inter Med. 2021;41(09):668–784.
5. Wang YZ, Wang AH, Zhao S, et al. 中国南方与北方地区糖尿病足病危险因素分析 [Differences in risk factors of diabetic foot in the patients in South and North China]. Zhonghua Yi Xue Za Zhi. 2007;87(26):1817–1820. Chinese.
6. ElSayed NA, Aleppo G, Aroda VR, et al. 12. Retinopathy, neuropathy, and foot care: standards of care in diabetes-2023. Diabetes Care. 2023;46(Suppl 1):S203–S215. doi:10.2337/dc23-S012
7. Fu XL, Ding H, Miao WW, Mao CX, Zhan MQ, Chen HL. Global recurrence rates in diabetic foot ulcers: a systematic review and meta-analysis. Diabetes Metab Res Rev. 2019;35(6):e3160. doi:10.1002/dmrr.3160
8. Zhang P, Lu J, Jing Y, Tang S, Zhu D, Bi Y. Global epidemiology of diabetic foot ulceration: a systematic review and meta-analysis (†). Ann Med. 2017;49(2):106–116. doi:10.1080/07853890.2016.1231932
9. Armstrong DG, Boulton AJM, Bus SA, Ingelfinger JR. Diabetic foot ulcers and their recurrence. N Engl J Med. 2017;376(24):2367–2375. doi:10.1056/NEJMra1615439
10. Soyoye DO, Abiodun OO, Ikem RT, Kolawole BA, Akintomide AO. Diabetes and peripheral artery disease: a review. World J Diabetes. 2021;12(6):827–838. doi:10.4239/wjd.v12.i6.827
11. Lechleitner M, Abrahamian H, Francesconi C, Kofler M, Sturm W, Köhler G. Diabetische Neuropathie und diabetischer Fuß (Update 2019) [Diabetic neuropathy and diabetic foot syndrome (Update 2019)]. Wien Klin Wochenschr. 2019;131(Suppl 1):141–150. German. doi:10.1007/s00508-019-1487-4
12. Lazzarini PA, Cramb SM, Golledge J, Morton JI, Magliano DJ, Van Netten JJ. Global trends in the incidence of hospital admissions for diabetes-related foot disease and amputations: a review of national rates in the 21st century. Diabetologia. 2023;66(2):267–287. doi:10.1007/s00125-022-05845-9
13. Ezzatvar Y, García-Hermoso A. Global estimates of diabetes-related amputations incidence in 2010–2020: a systematic review and meta-analysis. Diabetes Res Clin Pract. 2023;195:110194. doi:10.1016/j.diabres.2022.110194
14. Armstrong DG, Tan TW, Boulton AJM, Bus SA. Diabetic Foot Ulcers: a Review. JAMA. 2023;330(1):62–75. doi:10.1001/jama.2023.10578
15. Yang L, Rong GC, Wu QN. Diabetic foot ulcer: challenges and future. World J Diabetes. 2022;13(12):1014–1034. doi:10.4239/wjd.v13.i12.1014
16. Naemi R, Balasubramanian G, Darvel T, Chockalingam N. Predicting diabetic foot ulceration using routinely collected data in a foot clinic. What level of prognostic accuracy can be achieved? Diabetes Metab Res Rev. 2023;39(6):e3674. doi:10.1002/dmrr.3674
17. Kaka AS, Landsteiner A, Ensrud KE, et al. Risk prediction models for diabetic foot ulcer development or amputation: a review of reviews. J Foot Ankle Res. 2023;16(1):13. doi:10.1186/s13047-023-00610-6
18. Wang M, Chen D, Fu H, et al. Development and validation of a risk prediction model for the recurrence of foot ulcer in type 2 diabetes in China: a longitudinal cohort study based on a systematic review and meta-analysis. Diabetes Metab Res Rev. 2023;39(4):e3616. doi:10.1002/dmrr.3616
19. Peng B, Min R. Development of predictive nomograms clinical use to quantify the risk of diabetic foot in patients with type 2 diabetes mellitus. Front Endocrinol. 2023;14:1186992. doi:10.3389/fendo.2023.1186992
20. Shao Z, Wang Z, Bi S, Zhang J. Establishment and validation of a nomogram for progression to diabetic foot ulcers in elderly diabetic patients. Front Endocrinol. 2023;14:1107830. doi:10.3389/fendo.2023.1107830
21. ElSayed NA, Aleppo G, Aroda VR, et al. 2. Classification and diagnosis of diabetes: standards of care in diabetes-2023. Diabetes Care. 2023;46(Suppl 1):S19–S40. doi:10.2337/dc23-S002
22. Das SK, Roy P, Singh P, et al. Diabetic foot ulcer identification: a review. Diagnostics. 2023;13(12). doi:10.3390/diagnostics13121998
23. Smith S, Normahani P, Lane T, Hohenschurz-Schmidt D, Oliver N, Davies AH. Prevention and management strategies for diabetic neuropathy. Life. 2022;12(8):1185.
24. Joseph S, Munshi B, Agarini R, Kwok RCH, Green DJ, Jansen S. Near infrared spectroscopy in peripheral artery disease and the diabetic foot: a systematic review. Diabetes Metab Res Rev. 2022;38(7):e3571. doi:10.1002/dmrr.3571
25. Wang X, Yuan CX, Xu B, Yu Z. Diabetic foot ulcers: classification, risk factors and management. World J Diabetes. 2022;13(12):1049–1065. doi:10.4239/wjd.v13.i12.1049
26. Rajab AAH, Hegazy WAH. What’s old is new again: insights into diabetic foot microbiome. World J Diabetes. 2023;14(6):680–704. doi:10.4239/wjd.v14.i6.680
27. Kurze C, Farn CJ, Siow J. The interdisciplinary approach: preventive and therapeutic strategies for diabetic foot ulcers. Foot Ankle Clin. 2022;27(3):529–543. doi:10.1016/j.fcl.2022.03.001
28. Ramsey DJ, Kwan JT, Sharma A. Keeping an eye on the diabetic foot: the connection between diabetic eye disease and wound healing in the lower extremity. World J Diabetes. 2022;13(12):1035–1048. doi:10.4239/wjd.v13.i12.1035
29. Werkman NCC, Driessen JHM, Stehouwer CDA, et al. The use of sodium-glucose co-transporter-2 inhibitors or glucagon-like peptide-1 receptor agonists versus sulfonylureas and the risk of lower limb amputations: a nation-wide cohort study. Cardiovasc Diabetol. 2023;22(1):160. doi:10.1186/s12933-023-01897-2
30. Lu Y, Guo C. Risk of lower limb amputation in diabetic patients using SGLT2 inhibitors versus DPP4 inhibitors or GLP-1 agonists: a meta-analysis of 2 million patients. Ther Adv Drug Saf. 2023;14:20420986231178126. doi:10.1177/20420986231178126
31. Caruso P, Maiorino MI, Bellastella G, Esposito K, Giugliano D. Pleiotropic effects of GLP-1 receptor agonists on peripheral artery disease: is there any hope? Diabetes Metab Res Rev. 2023;39(7):e3627. doi:10.1002/dmrr.3627
32. Shao S, Zhang X, Xu Q, Pan R, Chen Y. Emerging roles of Glucagon like peptide-1 in the management of autoimmune diseases and diabetes-associated comorbidities. Pharmacol Ther. 2022;239:108270. doi:10.1016/j.pharmthera.2022.108270
33. Nurmohamed NS, Kraaijenhof JM, Stroes ESG. Lp(a): a new pathway to target? Curr Atheroscler Rep. 2022;24(11):831–838. doi:10.1007/s11883-022-01060-4
34. Berberich AJ, Hegele RA. A modern approach to dyslipidemia. Endocr Rev. 2022;43(4):611–653. doi:10.1210/endrev/bnab037
35. Ulloque-Badaracco JR, Mosquera-Rojas MD, Hernandez-Bustamante EA, et al. Association between lipid profile and apolipoproteins with risk of diabetic foot ulcer: a systematic review and meta-analysis. Int J Clin Pract. 2022;2022:5450173. doi:10.1155/2022/5450173
36. Vlad LG, Grosser JA, Dodenhoff KA, Peoples AE, Aguilo-Seara G, Molnar JA. Examining albumin as a bioindicator of healing capability in patients with diabetic foot ulcers: a retrospective review. Wounds. 2023;35(6):E193–e196. doi:10.25270/wnds/23012
37. Wang Y, Liu B, Pi Y, et al. Risk factors for diabetic foot ulcers mortality and novel negative pressure combined with platelet-rich plasma therapy in the treatment of diabetic foot ulcers. Front Pharmacol. 2022;13:1051299. doi:10.3389/fphar.2022.1051299
38. Lewis S, Raj D, Guzman NJ. Renal failure: implications of chronic kidney disease in the management of the diabetic foot. Semin Vasc Surg. 2012;25(2):82–88. doi:10.1053/j.semvascsurg.2012.04.007
39. Kaminski MR, Raspovic A, McMahon LP, et al. Risk factors for foot ulceration and lower extremity amputation in adults with end-stage renal disease on dialysis: a systematic review and meta-analysis. Nephrol Dial Transplant. 2015;30(10):1747–1766. doi:10.1093/ndt/gfv114
40. Tran SK, Carr JB, Hall MJ, Park JS, Cooper MT. Incidence of thyroid disease in patients with forefoot deformity. Foot Ankle Surg. 2020;26(4):445–448. doi:10.1016/j.fas.2019.05.014
41. Yammine K, Hayek F, Assi C. Is there an association between anemia and diabetic foot ulcers? A systematic review and meta-analysis. Wound Repair Regen. 2021;29(3):432–442. doi:10.1111/wrr.12902
42. Yan P, Zhang Z, Miao Y, Xu Y, Zhu J, Wan Q. Physiological serum total bilirubin concentrations were inversely associated with diabetic peripheral neuropathy in Chinese patients with type 2 diabetes: a cross-sectional study. Diabetol Metab Syndr. 2019;11(1):100. doi:10.1186/s13098-019-0498-7
43. Alqahtani N, Ghazwani EY, Al-Qahtani AM, Elmahboub RA. Correlation of iron levels with glycemia and microvascular complications among type II diabetes mellitus patients in Najran university hospital. J Fam Med Prim Care. 2022;11(6):2690–2694. doi:10.4103/jfmpc.jfmpc_545_21
44. Reddy S, Anoop S, Jebasingh FK, et al. Differentials in dietary intake of macro and micronutrients in patients with type 2 diabetes and foot ulcers: observations from a pilot study. Clin Nutr ESPEN. 2022;47:170–176. doi:10.1016/j.clnesp.2021.12.023
45. Kulprachakarn K, Ounjaijean S, Wungrath J, Mani R, Rerkasem K. Micronutrients and natural compounds status and their effects on wound healing in the diabetic foot ulcer. Int J Low Extrem Wounds. 2017;16(4):244–250. doi:10.1177/1534734617737659
46. Hong J, Liu WY, Hu X, et al. Free triiodothyronine and free triiodothyronine to free thyroxine ratio predict all-cause mortality in patients with diabetic foot ulcers. Diabetes Metab Syndr Obes. 2022;15:467–476. doi:10.2147/DMSO.S354754
47. Perlstein TS, Pande RL, Beckman JA, Creager MA. Serum total bilirubin level and prevalent lower-extremity peripheral arterial disease: national Health and Nutrition Examination Survey (NHANES) 1999 to 2004. Arterioscler Thromb Vasc Biol. 2008;28(1):166–172. doi:10.1161/ATVBAHA.107.153262
48. Lan Y, Liu H, Liu J, Zhao H, Wang H. The relationship between serum bilirubin levels and peripheral arterial disease and gender difference in patients with hypertension: BEST study. Angiology. 2020;71(4):340–348. doi:10.1177/0003319719900734
49. Ozeki M, Morita H, Miyamura M, et al. High serum bilirubin is associated with lower prevalence of peripheral arterial disease among cardiac patients. Clin Chim Acta. 2018;476:60–66. doi:10.1016/j.cca.2017.11.013
50. Kim ES, Mo EY, Moon SD, Han JH, Catapano A. Inverse association between serum bilirubin levels and arterial stiffness in Korean women with type 2 diabetes. PLoS One. 2014;9(10):e109251. doi:10.1371/journal.pone.0109251
51. Inoguchi T, Sonoda N, Maeda Y. Bilirubin as an important physiological modulator of oxidative stress and chronic inflammation in metabolic syndrome and diabetes: a new aspect on old molecule. Diabetol Int. 2016;7(4):338–341. doi:10.1007/s13340-016-0288-5
52. Zhao CC, Wang JW, Chen MY, Ke JF, Li MF, Li LX. High-normal serum bilirubin decreased the risk of lower limb atherosclerosis in type 2 diabetes: a real-world study. Diabetol Metab Syndr. 2023;15(1):105. doi:10.1186/s13098-023-01088-9
53. Mayer M. Association of serum bilirubin concentration with risk of coronary artery disease. Clin Chem. 2000;46(11):1723–1727. doi:10.1093/clinchem/46.11.1723
54. Kipp ZA, Xu M, Bates EA, Lee WH, Kern PA, Hinds TD. Bilirubin levels are negatively correlated with adiposity in obese men and women, and its catabolized product, urobilin, is positively associated with insulin resistance. Antioxidants. 2023;12(1):170. doi:10.3390/antiox12010170
55. Wang P, Shao X, Bao Y, et al. Impact of obese levels on the hepatic expression of nuclear receptors and drug-metabolizing enzymes in adult and offspring mice. Acta Pharm Sin B. 2020;10(1):171–185. doi:10.1016/j.apsb.2019.10.009
56. Stec DE, John K, Trabbic CJ, et al. Bilirubin binding to PPARα inhibits lipid accumulation. PLoS One. 2016;11(4):e0153427. doi:10.1371/journal.pone.0153427
57. Woronyczová J, Nováková M, Leníček M, et al. Serum bilirubin concentrations and the prevalence of gilbert syndrome in elite athletes. Sports Med. 2022;8(1):84.
58. Xu MR, Jin CH, Lu JX, Li MF, Li LX. High-normal unconjugated bilirubin is associated with decreased risk of chronic kidney disease in type 2 diabetes: a real-world study. Diabetes Metab Res Rev. 2023;39(6):e3672. doi:10.1002/dmrr.3672
59. Tafese R, Genet S, Addisu S. Association of serum total bilirubin and uric acid with low glomerular filtration rate diabetic kidney disease in type 2 diabetic patients. Diabetes Metab Syndr Obes. 2022;15:3993–3999. doi:10.2147/DMSO.S391777
60. Li S, Li N, Li L, et al. Association of serum bilirubin levels with macro- and microvascular complications in Chinese people with type 2 diabetes mellitus: new insight on gender differences. Diabetes Metab Syndr Obes. 2023;16:597–606. doi:10.2147/DMSO.S403483
61. Kurian SJ, Baral T, Unnikrishnan MK, et al. The association between micronutrient levels and diabetic foot ulcer: a systematic review with meta-analysis. Front Endocrinol. 2023;14:1152854. doi:10.3389/fendo.2023.1152854
62. Leal EC, Carvalho E. Heme Oxygenase-1 as therapeutic target for diabetic foot ulcers. Int J Mol Sci. 2022;23(19). doi:10.3390/ijms231912043
63. Deng H, Li B, Shen Q, et al. Mechanisms of diabetic foot ulceration: a review. J Diabetes. 2023;15(4):299–312. doi:10.1111/1753-0407.13372
64. Kosacka J, Woidt K, Toyka KV, et al. The role of dietary non-heme iron load and peripheral nerve inflammation in the development of peripheral neuropathy (PN) in obese non-diabetic leptin-deficient ob/ob mice. Neurol Res. 2019;41(4):341–353. doi:10.1080/01616412.2018.1564191
65. Xu C, Gong M, Wen S, Zhou M, Li Y, Zhou L. The comparative study on the status of bone metabolism and thyroid function in diabetic patients with or without ketosis or ketoacidosis. Diabetes Metab Syndr Obes. 2022;15:779–797. doi:10.2147/DMSO.S349769
66. Gong M, Xu C, Wen S, et al. The effects of obesity on bone turnover markers in diabetic patients with diabetic ketosis or ketoacidosis. Endocr Metab Immune Disord Drug Targets. 2023. doi:10.2174/1871530323666230509101203
67. Iwamoto Y, Kimura T, Tatsumi F, et al. Effect of hyperglycemia-related acute metabolic disturbance on thyroid function parameters in adults. Front Endocrinol. 2022;13:869869. doi:10.3389/fendo.2022.869869
68. Ranuve MS, Mohammadnezhad M. Healthcare workers’ perceptions on diabetic foot ulcers (DFU) and foot care in Fiji: a qualitative study. BMJ open. 2022;12(8):e060896. doi:10.1136/bmjopen-2022-060896
69. Kuhnke JL, Keast D, Rosenthal S, Evans RJ. Health professionals’ perspectives on delivering patient-focused wound management: a qualitative study. J Wound Care. 2019;28(Sup7):S4–S13. doi:10.12968/jowc.2019.28.Sup7.S4
70. Lo ZJ, Tan E, Chandrasekar S, et al. Diabetic foot in primary and tertiary (DEFINITE) Care: a health services innovation in coordination of diabetic foot ulcer (DFU) Care within a healthcare cluster - 18-month results from an observational population health cohort study. Int Wound J. 2023;20(5):1609–1621. doi:10.1111/iwj.14016
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.