Back to Journals » Neuropsychiatric Disease and Treatment » Volume 20
The Impact of Integrating a Low-Lectin Diet with Traditional ADHD Treatments on Gut Microbiota Composition and Symptom Improvement in Children - A Cohort Study
Authors Long L, Peng H, Chen X, Wang F, Long W, Cheng M, Ma J
Received 10 November 2023
Accepted for publication 23 February 2024
Published 9 March 2024 Volume 2024:20 Pages 535—549
DOI https://doi.org/10.2147/NDT.S449186
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Roger Pinder
Liying Long,1,2,* Haiyan Peng,1,2,* Xi Chen,1,2 Fei Wang,1,2 Wenjie Long,1,2 Ming Cheng,1,2 Jing Ma1,2
1Department of Psychiatry, The School of Clinical Medicine, Hunan University of Chinese Medicine, Changsha, Hunan, People’s Republic of China; 2Department of Psychiatry, Brain Hospital of Hunan Province (The Second People’s Hospital of Hunan Province), Changsha, Hunan, 410007, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jing Ma, Department of Psychiatry, Brain Hospital of Hunan Province (The Second People’s Hospital of Hunan Province), No. 427, Section 3, Furong Middle Road, Yuhua District, Changsha, Hunan, 410007, People’s Republic of China, Tel/Fax +86-731-85232224, Email [email protected]
Background and Purpose: This study aimed to investigate the impact of implementing a low-lectin diet on gut microbiota composition and symptom amelioration in pediatric patients diagnosed with Attention Deficit Hyperactivity Disorder (ADHD).
Methods: A total of 58 children (ages 7– 15 years), meeting the criteria for ADHD were recruited. In addition to standard medication treatment, participants in the experimental group with a low-lectin diet, while those in the control group received standard medication treatment alone. Clinical outcomes were assessed through evaluations conducted by physicians and teachers, implementation of the Conners Parent Rating Scales, and analysis of gut microbiota composition.
Results: The results revealed significant improvements in symptom reduction and attention allocation rate within the experimental group, surpassing those observed in the control group. Specifically, the experimental group exhibited lower physician ratings, teacher ratings, and attention allocation rate compared to the control group. Moreover, analysis of gut microbiota composition identified notable distinctions between the two groups.
Conclusion: These findings provide compelling evidence and valuable guidance supporting the integration of a low-lectin diet as an adjunctive intervention for managing ADHD.
Keywords: ADHD, gut microbiota, low-lectin diet, integrative intervention, children
Introduction
Attention deficit hyperactivity disorder (ADHD) is a prevalent neurodevelopmental disorder characterized by persistent symptoms of inattention and/or hyperactivity-impulsivity. Its global prevalence in children is approximately 7.2%,1 with a specific prevalence of 6.4% observed in Chinese children,2 and these numbers are increasing. Children with ADHD face an elevated risk of accidents, including a higher likelihood of traffic accidents, engagement in criminal behavior, and substance abuse.3 Additionally, ADHD commonly co-occurs with other mental disorders such as depression, anxiety disorders, oppositional defiant disorder, conduct disorder, and substance use, further impacting individuals’ quality of life and functioning and complicating the treatment process. Therefore, timely diagnosis and appropriate intervention are critical for enhancing the prognosis of individuals with ADHD.
Currently, combination therapy, which involves the use of medication along with cognitive-behavioral therapy, is widely recommended as the most effective approach for the treatment of ADHD, aiming to achieve an ideal treatment success rate of 80%-90%.4,5 However, the actual treatment success rate in clinical practice ranges from 60% to 70%,6 indicating the influence of various factors on the effectiveness of treatment. These factors include poor adherence to medication, high rates of self-discontinuation of medication, medication side effects, as well as low adherence and high dropout rates in cognitive-behavioral and family-based therapies. Moreover, the high costs associated with these treatment modalities pose additional challenges in attaining optimal treatment outcomes for individuals with ADHD. Consequently, a significant proportion of patients continue to experience symptoms into adulthood.
To improve the treatment success rate of ADHD, many researchers have proposed dietary interventions as adjunctive treatment for ADHD. Research on the gut-brain axis related to ADHD etiology suggests that the gut microbiota plays a significant role in the pathogenesis of ADHD.7 Neurotransmitters associated with ADHD symptoms are primarily produced in the gut, and there is a close relationship between neurotransmitters and the composition and quantity of the gut microbiota, indicating that changes in the gut microbiota may be related to the occurrence and development of ADHD.8,9 Alterations in the composition of gut microbiota have been found to correlate with ADHD symptoms, suggesting a potential role in its pathogenesis.3–5 Short-Chain Fatty Acids (SCFAs): Short-chain fatty acids, including acetate, butyrate, and propionate, exhibit the capacity to traverse the blood-brain barrier, thereby influencing brain function.5–8 ADHD may be associated with reduced GABA levels, potentially linked to specific gut bacteria.4,6,9 Perturbations in dopamine levels are frequently observed in ADHD and may be connected to specific gut microbiota.10,11 Dietary patterns, particularly the Western diet, are implicated in potentially exacerbating ADHD symptoms.2 Thus, it is feasible to target the gut microbiota as an adjunctive treatment for ADHD through dietary interventions. Several studies have demonstrated the therapeutic effects of dietary interventions in improving ADHD symptoms.10–12 Two major dietary interventions for ADHD have shown benefits: one excludes specific food components like artificial additives, sugars, and certain foods (the “few-foods diet”), while the other increases intake of specific nutrients such as amino acids, essential fatty acids, vitamins, and minerals. However, the most common interventions involve food supplementation and dietary modifications.13 Restricted diets may offer clinical benefit in managing ADHD in a subset of cases with clinical significance.14 But insufficient evidence supports the elimination diet, which may lead to nutritional deficiencies, warranting caution.15 Overall, ADHD symptoms can be alleviated by supplementing or restricting specific nutrients or foods. This is attributed to the varying effects of different dietary structures on the gut microbiota, which, in turn, regulate the behavior and psychology of individuals with ADHD. However more scientific evidence is needed.
Lectins, known as a group of proteins or sugar molecules, are found in various foods, particularly in seeds and tubers such as grains, potatoes, and legumes.16 Research indicates that consuming lectins may affect gut absorption and overall health in animals. Lectins exhibit resistance to heat, requiring prolonged exposure above 100 °C to deactivate fully.17 It has been established that many lectins exhibit toxicity, inflammation-inducing properties, as well as the ability to induce platelet aggregation and adhesion.18 These mechanisms interact synergistically, engendering far-reaching consequences encompassing selective microbiota effects, elicitation of inflammatory responses, and modulation of metabolic byproducts. Notably, lectins have the potential to disrupt the polysaccharide structure of the intestinal mucosa, thereby impeding bacterial adhesion and proliferation.12 Additionally, lectins may impede enzyme activity at the small intestine’s brush border, culminating in compromised nutrient digestion and absorption.13,14 Furthermore, lectins may downregulate the secretion of immunoglobulin A (IgA), a pivotal factor in constraining bacterial overgrowth.15
Research has revealed a potential connection between lectin consumption and the development of certain diseases and inflammatory reactions, including intestinal inflammation and autoimmune disorders. Consequently, lectins might impact the gut microbiota, thereby influencing the onset and progression of ADHD.19,20 Therefore, the objective of this study is to implement a low-lectin diet, aiming to reduce lectin intake, modulate the abundance and composition of the gut microbiota, and alleviate symptoms associated with ADHD.
Methods
Participants
This study is a single-center prospective cohort study. The subjects recruited for this study were children with ADHD aged 7–15 years old. All participants underwent screening and recruitment at the Outpatient Department of the Psychiatry Department at Hunan Brain Hospital between June 2022 and March 2023. A total of 58 children with ADHD were enrolled, with 30 in the control group and 28 in the experimental group. This study complies with the principles of the Declaration of Helsinki. All participants and their parents were provided with a written informed consent form after a thorough explanation of the study’s content to ensure the ethicality of the study and protect the rights of participants. This study was approved by the Ethics Committee of Hunan Brain Hospital (Approval No.: 2023K008).
Inclusion criteria: a) Clinical symptoms of hyperactivity, inattention, and other symptoms consistent with the diagnostic criteria for ADHD in the fifth edition of the American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders (DSM-5), supported by detailed clinical data and medical records. b) Demonstrated cooperation and active involvement of family members in the study. c) Age ranging from 7 to 15 years. d) Parents or guardians are required to sign a safety information sheet. Exclusion criteria were as follows: a) Existence of severe physical illness, intellectual developmental disorders, or the presence of comorbidities with other psychiatric disorders. b) Inadequate compliance that may impede the successful completion of the treatment. Dropout criteria: a) Inability to adhere to the study protocol or receive treatment for a duration less than 2 months following enrollment. b) Occurrence of severe adverse reactions during treatment, such as a weight loss exceeding 30% of the initial weight or hypersensitivity reactions to stimulant medications. c) Voluntary withdrawal during the course of the study.
Experimental Procedures
Study Methods
Throughout the study duration, the control group and the experimental group underwent standard medication treatment, maintaining their original dietary habits. The participants in the experimental group adhered to a specific food list and dietary plan (Supplementary Tables 1 and 2).
Evaluation Indicators
Severity Rating (Physician, Teacher): The assessment covered multiple aspects and categorizes the severity based on scores. (Score 1: No clinical symptoms, no impulsive or hyperactive behavior, focused attention in class, independently completes assignments, gets along well with classmates. Score 2: No impulsive or hyperactive behavior, can attentively listen for more than 30 minutes, completes assignments independently but takes longer, gets along well with peers. Score 3: Occasional unwarranted outbursts, moderate attention span, minimal disruptive behavior in class, lengthy and supervised assignment completion, average interpersonal relationships. Score 4: Impulsive behavior, easily gets into arguments with parents and friends, short attention span in class, difficulty completing assignments, poor interpersonal relationships. Score 5: Impulsive behavior (self-harm, suicide), nearly unable to complete assignments and exams, unable to focus attention in class, unstable relationships with peers, frequent arguments.) It is important to note that physician ratings were conducted independently by two psychiatry psychiatrists with over 3 years of clinical experience who were unaware of the patient grouping. In cases where significant discrepancies existed between the ratings, a third expert with over 5 years of clinical experience in psychiatry reassessed the scores to ensure accuracy and reliability of the assessments.
Conners Parent Rating Scales (CPRS): The parent-completed evaluation assessed different aspects of the child’s behavior, including conduct problems, learning problems, anxiety, impulsivity-hyperactivity, somatic complaints, and the hyperactivity index.
Attentional Allocation Test: The traditional attention test of number cancellation test was used to assess and compare the subjects’ perceptual speed, accuracy in recognition, attention, intellectual lag, fatigue, and efficiency in proofreading tasks. The results of this test demonstrated an inverse correlation with attentional focus, meaning that a higher percentage indicates lower attentional concentration.
Gut Microbiota: Changes in gut microbiota were observed by analyzing pre- and post-study fecal samples from the experimental and control groups using 16S rDNA sequencing. The methods included sampling, PCR amplification, library construction, sequencing, and species analysis.
Statistical Analysis
Statistical analysis was performed using SPSS 26.0 software. Normality tests were performed on the pre- and post-test data to determine the appropriate methods for comparative analysis between groups. Continuous variables were presented as medians with interquartile range or means ± standard deviations. We performed repeated-measures ANOVA satisfying the normal distribution, which included conduct problems, learning problems, anxiety, hyperactivity factor, and attention allocation rate. Simple effects analysis was performed using a paired-samples t-test and independent samples’ t-test. The rank sum test was conducted for repeated measurement data that did not meet the normal distribution, such as psychosomatic disorder, impulsivity-hyperactivity, doctor’s rating, and teacher’s rating. Statistical significance was set at P < 0.05.
QIIME software (version 2-2021.2) was used to process the high-quality sequences, calculate species abundance, and calculate alpha diversity indices, including Shannon, Chao1, and Simpson indices. Anosim analysis was performed to assess whether the dissimilarities between groups were significantly larger than the dissimilarities within groups, thus determining the significance of the groupings. Additionally, the Euclidean distance was calculated as a measure of beta diversity, followed by principal coordinate analysis (PCoA). To identify the most significantly differentially abundant taxa at the genus and species levels between the two groups, the LDA Effect Size (LEfSe) method was employed. Wilcoxon tests were conducted to evaluate differences in various indices across different groups.
Results
Changes in Clinical Symptoms
A total of 58 ADHD patients were included in this study, and the general demographic statistics are shown in Table 1. After treatment, 1 participants dropped out from the experimental group (1 voluntarily withdrew due to inability to adhere to the low-lectin diet). In the control group, 4 participants dropped out (During the course of the experiment, a subset of participants exhibited changes in their prescribed treatment medication or declined to provide the second stool sample and complete the questionnaire). The comparison results of pre-treatment in the experimental group (EXP1) and pre-treatment in the control group (CRTL1) were shown in Table 2, there was no statistical difference between the variables of the CRTL1 and EXP1. In Table 3, both the doctor’s rating and teacher’s rating in the experimental and control groups showed a significant decrease after treatment (P<0.05), and the results of post-treatment in the experimental group (EXP2) had a greater reduction in ratings compared to post-treatment in the control group (CRTL2) (p<0.05). The attention allocation rate in both groups also showed a significant decrease after treatment (P<0.05), but without a greater reduction in the EXP2 compared to the CRTL2. The post-treatment results of the Conners Parent Rating Scales indicated a significant improvement in the experimental group (P<0.05), particularly in the impulsivity-hyperactivity, learning problems, hyperactivity and anxiety indices. The control group also showed significant improvement in the learning problems, hyperactivity and anxiety indices (P<0.05). However, there were no significant differences in any of the Conners Parent Rating Scales indices between the EXP2 and CRTL2 (P>0.05).
 |
Table 1 Characterization of Participants in the Study |
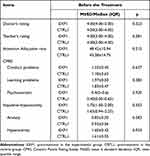 |
Table 2 The Severity Scores Before the Treatment |
 |
Table 3 The Severity Scores Before and After the Treatment |
Alpha Diversity Analysis
To observe the gut microbiota communities, 16S rDNA sequencing was performed on fecal samples from both the experimental and control groups before and after the study. Due to the substantial variation in the number of reads corresponding to different samples, in order to avoid biases during analysis caused by differences in sequencing data size, we randomly subsampled each sample after reaching sufficient sequencing depth. Subsequently, the samples were clustered into operational taxonomic units (OTUs) at a 97% similarity threshold. Figure 1 depicts the species accumulation curve, which shows a rapid increase followed by a more gradual rise, indicating sufficient sampling. The diversity indices of the gut microbiota community, including Shannon and Simpson diversity indices, as well as the estimated number of OTUs (Chao1), are presented in Table 4. There were no statistically significant differences in any of these indices between different groups at different levels (p>0.05), suggesting no significant differences in species diversity among the different groups.
 |
Table 4 Chao1, Shannon and Impson Indexes in Different Groups |
 |
Figure 1 Specaccum was utilized to evaluate the adequacy of the sampling procedure. |
Beta Diversity Analysis
Beta diversity analysis was employed to evaluate the variations in the composition of gut microbiota across different groups. An Anosim analysis was executed, and the corresponding outcomes are visually represented in Figure 2. The analysis revealed that the dissimilarities between all groups were greater than the dissimilarities within each group, indicating significant grouping (R > 0, p < 0.05).
To visualize the dissimilarities in species diversity, Principal Coordinates Analysis (PCoA) and Permutational multivariate analysis of variance analysis (PERMANOVA) were employed to illustrate the distinctions among the samples. Figure 3 portrayed the PCoA plots based on the Euclidean distance, which exhibited noteworthy independent clustering of microbiota composition at various taxonomic levels. Specifically, at the phylum level, significant variations in gut microbiota composition were observed between CRTL1 and CRTL2 (R2=0.218, p<0.05), as well as between EXP1 and EXP2 (R2=0.394, p<0.05). There were significant differences in the gut microbiota composition between CRTL1 and CRTL2 on family level (R2=0.416, p<0.05). However, due to significant individual variations, PERMANOVA analysis could not distinguish between the remaining different groups at other taxonomic levels. These results demonstrate significant differences in the gut microbiota composition between different groups, indicating that the interventions and treatments have influenced the microbial community structure at various taxonomic levels.
LEfSe Analysis
We utilized LEfSe to compare the gut microbiota composition between different groups. Figure 4A–C displayed clustering dendrograms representing the evolutionary branching patterns of the major microbial taxa in each group. These branching patterns clearly indicate notable differences in gut microbiota composition among the various groups. Figure 4D–F exhibit the LDA scores obtained from identifying microbial taxa that significantly contributed to the observed differences between the groups. The following patterns were observed: In EXP1, Bifidobacteriaceae and Bifidobacteriales were found to be more abundant in the gut microbiota of participants. Bacteroidia and Bacteroidales were found to be prominent in the gut microbiota of participants in EXP2. In CRTL1, Actinobacteria, Actinobacteridae Actinomyces, Bifidobacterium, and Bifidobacteriaceae were overrepresented. Bacteroides and Bacteroidaceae were identified as the major microbial taxa in CRTL2. Furthermore, comparing EXP2 with CRTL2, Lactobacillaceae, Pasteurellaceae, and Pasteurellales dominated as the prominent microbial taxa in EXP2, while Odoribacteraceae and Escherichia-Shigella were notable in CRTL2.
Discussion
The present study conducted clinical observations and gut microbiota analysis on children with ADHD using a low-lectin diet as a treatment. The statistical analysis results revealed significant improvements in the severity of clinical symptoms in the experimental group compared to the control group after treatment. These improvements were observed in terms of doctor’s ratings, teacher’s ratings, and attention allocation rates, indicating a positive impact of the low-lectin diet on improving ADHD symptoms. Additionally, the low-lectin diet also demonstrated positive effects on improving impulsivity, hyperactivity, and anxiety symptoms. Nevertheless, there were no significant changes observed in other indicators. The gut microbiota analysis revealed that both the control and experimental groups showed dominance of Bacteroides and Bacteroidaceae, or Bacteroidia and Bacteroidales, after treatment. However, comparing EXP2 with CRTL2, CRTL2 showed dominance of Odoribacteraceae and Escherichia-Shigella. The low-lectin diet had some significant effects on the gut microbiota of the experimental group after treatment, including an increase in Lactobacillaceae, Pasteurellales and Pasteurellaceae.
Previous studies have indicated that the onset of ADHD is associated with abnormal expression of neurotransmitter levels.21,22 Specifically, individuals with ADHD often exhibit reduced functioning of central nervous system neurotransmitters, such as dopamine (DA) and norepinephrine (NE), as well as impaired synthesis of gamma-aminobutyric acid (GABA) and increased activity of serotonin (5-HT).23,24 The gut-brain axis theory proposes a bidirectional regulatory mechanism between the gut microbiota and the brain. Notably, ADHD patients demonstrate alterations in gut microbiota composition, and these microbial communities and their metabolites can impact the onset, progression, and treatment of ADHD through neural, immune, and neurotransmitter modulation.8 Comparisons between the microbiota of individuals with ADHD and the general population have revealed specific differences. At the family level, ADHD patients showed increased abundance of Peptostreptococcaceae, Moraxellaceae, Xanthomonadaceae, and Peptococcaceae, while Alcaligenaceae was decreased.25 At the species level, Faecalibacterium prausnitzii, Lachnospiraceae bacterium, and Ruminococcus gnavus showed significant reductions in the ADHD group, whereas Bacteroides caccae, Odoribacter splanchnicus, Paraprevotella xylaniphila, and Veillonella parvula were increased.26 The gut microbiota and its metabolites play a role in the etiology, development, and treatment of ADHD through neural, immune, and neurotransmitter modulation. For instance, approximately 50% of dopamine (DA) and over 90% of serotonin (5-HT) are produced in the gut. Moreover, the gut microbiota influences the central nervous system by secreting and absorbing gamma-aminobutyric acid (GABA).27 Additionally, the gut microbiota can generate precursor substances of monoamine neurotransmitters, such as phenylalanine, tyrosine, and tryptophan, which impact neurotransmitter synthesis in the brain.27,28 A recent study29 investigating the impact of dietary patterns on the gut microbiota of Korean elementary school students with ADHD revealed that ADHD children who consumed processed foods over an extended period displayed significantly higher abundances of harmful bacteria, including Escherichia, Enterobacter, and Clostridium strains, and markedly lower abundances of beneficial bacteria, such as Bifidobacterium and Ruminococcus strains. Furthermore, the alpha-diversity of the gut microbiota in the processed food group was significantly lower than that of the control group. Different dietary patterns can exert distinct effects on the gut microbiota, and imbalanced diets have the potential to disrupt the microbial equilibrium, thereby serving as potential risk factors for ADHD.
16S rDNA testing results revealed differences in the composition of gut microbiota between the experimental and control groups. Both groups showed an abundance of Bacteroides and Bacteroidaceae, or Bacteroidia and Bacteroidales, after treatment. They are generally beneficial to the gut microbiota, produce essential vitamins and co-factors in the intestines and process components such as fiber to promote the normal functioning of the immune system.30,31 However, several studies have indicated that individuals with ADHD, in comparison to the general population, exhibit significantly higher levels of Bacteroidetes in their gut microbiota. Furthermore, it has been found that the relative abundance of Bacteroidetes is positively associated with hyperactivity and impulsivity levels.26 This correlation could be attributed to the production of various abnormal substances by Bacteroidetes, including amyloid proteins, lipopolysaccharides, enterotoxins, and neurotoxins, which may impact the structure of the blood-brain barrier and the central nervous system.32 Nevertheless, further research is necessary to explore the relationship between Bacteroidetes and ADHD, as well as to elucidate the specific mechanisms involved.
In the comparison between EXP2 and CTRL2, Odoribacteraceae and Escherichia-Shigella were notable in CTRL2, while Lactobacillaceae, Pasteurellales and Pasteurellaceae were dominant in EXP2. These findings may be related to the treatment of the low-lectin diet. Lactobacillus species can produce GABA and DA,33 which may contribute to the improve symptoms of anxiety and impulsivity-hyperactivity. Additionally, Lactobacillus can produce beneficial metabolites, such as short-chain fatty acids, which have the potential to modulate the function of the intestinal immune system and suppress inflammatory response.34 The elevated abundance of Pasteurellaceae and Pasteurellales in ADHD patients after a low-lectin intervention need more attention in future studies, as they may play distinct roles in the regulation of the immune system.35,36 Future studies should focus on elucidating the specific mechanisms by which these bacterial taxa influence immune function and how their alterations contribute to ADHD pathology. Understanding the interplay between Pasteurellaceae, Pasteurellales, and the immune system may provide valuable insights for the development of novel therapeutic approaches targeting immune dysregulation in ADHD. On the other hand, Odoribacteraceae and Escherichia-Shigella may have different roles in immune system regulation, leading to the observed differences in gut microbiota composition in the control group. Escherichia-Shigella is known to be associated with intestinal infections, where it induces the production of inflammatory cytokines, activates and regulates the host’s immune response, promotes immune cell activation, and triggers inflammation.37 However, it should be noted that our study’s analysis of alpha diversity and PCoA did not yield statistically significant differences, which could be attributed to the small sample size or other factors.
The potential effects of lectins on the gut microbiota can be categorized into these main aspects. Firstly, lectins have the ability to disrupt the microbial balance in the gut. By binding to sugar molecules on the surface of gut bacteria, lectins can interfere with their attachment and growth. If lectins bind to beneficial bacterial strains, it can lead to a reduction in their abundance, thereby disrupting the balance of the microbiota.38,39 This imbalance may favor the proliferation of opportunistic pathogens like Escherichia coli and Lactobacillus lactis.40 A study suggests that when replacing 75% of defatted fishmeal with soybean meal, significant changes occur in the composition of intestinal microbiota. This substitution leads to a decrease in the abundance of Firmicutes, while Proteobacteria, Bacteroidetes, and Planctomycetes increase. At the genus level, soybean lectin significantly reduces the abundance of Lactococcus, Geobacillus, Pseudomonas, Streptococcus, Bacillus, and Acinetobacter. Conversely, it enhances the abundance of Cetobacterium, Planctomyces, Shewanella, Thermomonas, Rubrivivax, and Carnobacterium. These alterations are primarily attributed to soybean lectin.41 Secondly, certain lectins exhibit antibiotic-like properties, which can inhibit the growth of specific bacterial strains. This can result in a decrease in beneficial bacteria while providing a growth advantage to other bacterial strains, leading to dysbiosis of the microbiota.18,20 Thirdly, the interaction between specific lectins and gut bacteria can trigger an inflammatory response. This inflammatory response can have an impact on gut health and the stability of the microbiota.42,43 At the local level, lectins can impact the turnover and loss of gut epithelial cells, harm the luminal membranes of the epithelium, disrupt nutrient digestion and absorption, induce changes in the bacterial flora, and regulate the immune state of the digestive tract.43 For instance, Phaseolus lectin in the small intestine may hinder food digestion and absorption, promoting coliform bacteria growth. Kidney bean and wheat germ lectins reduce stress protein levels in rat gut and Caco-2 cells, compromising cell protection against harmful gut contents.44 When indigestible plant lectins breach the compromised gut barrier, they have the potential to prime enteric glial cells (EGCs) and inflammasomes, thereby triggering a repetitive enteric neuroinflammatory response.45 By comprehensively investigating these mechanisms, we can gain a better understanding of how a low-lectin diet may contribute to the treatment of ADHD.
This study represents the first prospective cohort study that investigates the effects of a low-lectin diet on the gut microbiota and clinical symptoms in children with ADHD. Previous dietary interventions for ADHD have primarily centered around the inclusion or exclusion of certain foods. Several studies have suggested that supplementation with specific nutrients may have benefits for ADHD symptoms. For instance, omega-3 fatty acids are believed to positively impact brain development and function, and supplementation with omega-3 polyunsaturated fatty acids (PUFAs) has shown improvements in ADHD symptoms.46 Additionally, omega-3 PUFAs may enhance the effects of methylphenidate, a common medication used in ADHD treatment.47 Regarding dietary restrictions, some studies have indicated a potential association between certain foods such as artificial additives, food colorings, preservatives, nitrites, gluten, and dairy products with ADHD symptoms. Eliminating these potential allergens or sensitivities from the diet has been found to significantly reduce symptoms in children with ADHD.48–51 However, these highly restrictive diets may pose challenges in terms of implementation and adherence, and they may not adequately meet the nutritional needs of children.52,53 In contrast, the low-lectin diet is specifically designed to reduce lectin intake and offers a more targeted approach compared to other dietary interventions. While focusing on lectins, it also incorporates other dietary adjustments. For example, it promotes increased consumption of fresh fruits, vegetables, and high-protein foods while reducing the intake of processed foods and sugars. These dietary modifications aim to promote overall health and maintain nutritional balance alongside the reduction of lectin intake.
The primary objective of this study was to examine the impact of a low-lectin diet on the gut microbiota and clinical symptoms in children diagnosed with ADHD. The findings of this study aim to provide valuable insights and serve as a foundation for future randomized controlled trials (RCTs) in this area. Our results indicate that the inclusion of a low-lectin diet as an adjunct to standard treatment yielded positive effects on clinical symptoms associated with ADHD in children. Specifically, the low-lectin diet demonstrated improvements in various clinical symptom assessments, including physician ratings, teacher ratings, and attention allocation. These findings suggest that the low-lectin diet may contribute to the overall management and reduction of ADHD symptoms in affected children. Furthermore, the diet showed promising effects in mitigating impulsivity, hyperactivity, and anxiety symptoms, providing additional benefits beyond the core symptoms of ADHD. However, it is worth noting that no significant changes were observed in other indicators of the Conners Parent Rating Scales. It is important to consider that the questionnaire relies on subjective assessments, which can be susceptible to measurement errors and personal biases. Additionally, parental observations and assessments of their child’s behavior may be influenced by their own subjective perspectives and preconceived notions. Moreover, the duration of the treatment period may have been insufficient to fully manifest the therapeutic effects of the low-lectin diet. Extended intervention periods may be necessary to observe the complete range of treatment outcomes and evaluate the long-term effects of the dietary intervention. In conclusion, this study provides valuable evidence supporting the potential benefits of a low-lectin diet as an adjunctive therapy for children with ADHD. Further research, including longer-term randomized controlled trials, is warranted to gain a more comprehensive understanding of the therapeutic effects of the low-lectin diet and its impact on gut microbiota composition and clinical symptoms in children with ADHD.
Despite the significant findings of this study, it is important to acknowledge and consider the limitations that may have influenced the interpretation of the results. These limitations should be taken into account when assessing the generalizability and reliability of the study findings. Firstly, the sample size in this study may have been small, and the participants could have been recruited from specific regions or populations. This limited sample size may restrict the generalizability of the findings to a broader population. Therefore, caution should be exercised when extrapolating the results to other populations or settings. Secondly, the method of random allocation and selection of control groups may have introduced biases and influenced the reliability of the research outcomes. Additionally, the inclusion of a control group allows for comparison and assessment of the specific effects of the low-lectin diet, but it is important to consider the potential placebo effect and its impact on the observed outcomes. Another limitation is the short duration of the study, which may have limited the evaluation of long-term effects and sustained symptom improvements resulting from the low-lectin diet. Longer-term studies are necessary to assess the durability of the observed effects over time and to establish the long-term efficacy of the dietary intervention. Lastly, it is important to note that this study did not involve directly providing food to the study participants. Instead, it focused on examining the impact of a prescribed low-lectin diet on ADHD symptoms. While this approach enhances practicality and applicability, it also means that there may have been variations in participants’ adherence to the prescribed diet, which could have influenced the outcomes. Future research should address these limitations through larger sample sizes, randomization techniques, longer intervention periods, and careful control of confounding factors to strengthen the validity and generalizability of the findings.
For future research directions, several points can be considered. Firstly, conducting multicenter studies with larger sample sizes would enhance the generalizability of the findings. Secondly, longer-term follow-up studies are crucial to assess the sustained effects of a low-lectin diet. Tracking participants’ dietary habits, gut microbiota composition, and changes in ADHD symptoms over an extended period would allow us to examine the long-term health outcomes associated with adhering to a low-lectin diet. Such studies would provide insights into the durability of the observed effects and inform recommendations regarding the duration of dietary interventions. Lastly, exploring the combination of a low-lectin diet with other treatment methods, like behavioral therapy, could be an interesting avenue for future research. Investigating the potential synergistic effects of combining a low-lectin diet with other evidence-based treatments may lead to more comprehensive and effective approaches for managing ADHD symptoms.
Conclusion
In summary, the findings of this study suggest that a low-lectin diet holds promise as a therapeutic intervention for improving clinical symptoms and gut microbiota in children with ADHD. Meanwhile, further research in the form of long-term, randomized controlled trials is necessary to validate these findings and gain a deeper understanding of the therapeutic mechanisms underlying the effects of a low-lectin diet in children with ADHD. Additional studies are needed to comprehensively evaluate the efficacy and applicability of a low-lectin diet as a potential intervention in the treatment of ADHD.
Data Sharing Statement
This study is a single-center prospective cohort study. The subjects recruited for this study were children with ADHD aged 7–15 years old. All participants underwent screening and recruitment at the Outpatient Department of the Psychiatry Department at Hunan Brain Hospital between June 2022 and March 2023. A total of 58 children with ADHD were enrolled, with 30 in the control group and 28 in the experimental group. The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
This study complies with the principles of the Declaration of Helsinki. All participants and their parents were provided with a written informed consent form after a thorough explanation of the study’s content to ensure the ethicality of the study and protect the rights of participants. This study was approved by the Ethics Committee of Hunan Brain Hospital (Approval No.: 2023K008). All methods were carried out in accordance with relevant guidelines and regulations.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by Hunan Provincial Health Commission Research Plan Project (B2019045).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Wolraich ML, Hagan JF, Allan C, et al. Clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics. 2019;144(4). doi:10.1542/peds.2019-2528
2. Li F, Cui Y, Li Y, et al. Prevalence of mental disorders in school children and adolescents in China: diagnostic data from detailed clinical assessments of 17,524 individuals. J Child Psychol Psychiatr. 2022;63(1):34–46. doi:10.1111/jcpp.13445
3. Quinn PD, Chang Z, Hur K, et al. ADHD medication and substance-related problems. Am J Psychiatry. 2017;174(9):877–885. doi:10.1176/appi.ajp.2017.16060686
4. Dopfner M, Hautmann C, Dose C, et al. ESCAschool study: trial protocol of an adaptive treatment approach for school-age children with ADHD including two randomised trials. BMC Psychiatry. 2017;17(1):269. doi:10.1186/s12888-017-1433-9
5. Moriyama TS, Polanczyk G, Caye A, et al. Evidence-based information on the clinical use of neurofeedback for ADHD. Neurotherapeutics. 2012;9(3):588–598. doi:10.1007/s13311-012-0136-7
6. Zinnow T, Banaschewski T, Fallgatter AJ, et al. ESCAlate – adaptive treatment approach for adolescents and adults with ADHD: study protocol for a randomized controlled trial. Trials. 2018;19(1):280. doi:10.1186/s13063-018-2665-9
7. Aarts E, Ederveen THA, Naaijen J, et al. Gut microbiome in ADHD and its relation to neural reward anticipation. PLoS One. 2017;12(9):e0183509. doi:10.1371/journal.pone.0183509
8. Dam SA, Mostert JC, Szopinska-Tokov JW, et al. The role of the gut-brain axis in attention-deficit/hyperactivity disorder. Gastroenterol Clin North Am. 2019;48(3):407–431. doi:10.1016/j.gtc.2019.05.001
9. Mathee K, Cickovski T, Deoraj A, et al. The gut microbiome and neuropsychiatric disorders: implications for attention deficit hyperactivity disorder (ADHD). J Med Microbiol. 2020;69(1):14–24. doi:10.1099/jmm.0.001112
10. Cooper RE, Tye C, Kuntsi J, et al. The effect of omega-3 polyunsaturated fatty acid supplementation on emotional dysregulation, oppositional behaviour and conduct problems in ADHD: a systematic review and meta-analysis. J Affect Disord. 2016;190:474–482. doi:10.1016/j.jad.2015.09.053
11. Rios-Hernandez A, Alda JA, Farran-Codina A, et al. The Mediterranean diet and ADHD in children and adolescents. Pediatrics. 2017;139(2). doi:10.1542/peds.2016-2027
12. Heilskov MJ, Andersen LBB, Houmann T, et al. Diet in the treatment of ADHD in children—a systematic review of the literature. Nord J Psychiatry. 2015;69(1):1–18. doi:10.3109/08039488.2014.921933
13. Cagigal C, Silva T, Jesus M, et al. Does diet affect the symptoms of ADHD? Curr Pharm Biotechnol. 2019;20(2):130–136. doi:10.2174/1389201019666180925140733
14. Nigg JT, Lewis K, Edinger T, et al. Meta-analysis of attention-deficit/hyperactivity disorder or attention-deficit/hyperactivity disorder symptoms, restriction diet, and synthetic food color additives. J Am Acad Child Adolesc Psychiatry. 2012;51(1):86–97.e8. doi:10.1016/j.jaac.2011.10.015
15. Pinto S, Correia-de-Sá T, Sampaio-Maia B, et al. Eating patterns and dietary interventions in ADHD: a narrative review. Nutrients. 2022;14(20):4332. doi:10.3390/nu14204332
16. Singh A, Trumpff C, Genkinger J, et al. Micronutrient dietary intake in latina pregnant adolescents and its association with level of depression, stress, and social support. Nutrients. 2017;9(11):1212. doi:10.3390/nu9111212
17. Panacer K, Whorwell PJ. Dietary lectin exclusion: the next big food trend? World J Gastroenterol. 2019;25(24):2973–2976. doi:10.3748/wjg.v25.i24.2973
18. Miyake K, Tanaka T, McNeil PL, Zimmerberg J. Disruption-induced mucus secretion: repair and protection. PLoS Biol. 2006;4(9):e276. doi:10.1371/journal.pbio.0040276
19. Buehl OB, Gundry SR. The Plant Paradox: The Hidden Dangers in ”Healthy” Foods That Cause Disease and Weight Gain.
20. Miyake K, Tanaka T, McNeil PL, Steinhardt R. Lectin-based food poisoning: a new mechanism of protein toxicity. PLoS One. 2007;2(8):e687. doi:10.1371/journal.pone.0000687
21. Dinan TG, Stanton C, Cryan JF. Psychobiotics: a novel class of psychotropic. Biol Psychiatry. 2013;74(10):720–726. doi:10.1016/j.biopsych.2013.05.001
22. Sandhu KV, Sherwin E, Schellekens H, et al. Feeding the microbiota-gut-brain axis: diet, microbiome, and neuropsychiatry. Transl Res. 2017;179:223–244. doi:10.1016/j.trsl.2016.10.002
23. Bercik P, Denou E, Collins J, et al. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology. 2011;141(2):599–609, 609.e1–3. doi:10.1053/j.gastro.2011.04.052
24. Sarkar A, Lehto SM, Harty S, et al. Psychobiotics and the manipulation of bacteria-gut-brain signals. Trends Neurosci. 2016;39(11):763–781. doi:10.1016/j.tins.2016.09.002
25. Jiang HY, Zhou -Y-Y, Zhou G-L, et al. Gut microbiota profiles in treatment-naive children with attention deficit hyperactivity disorder. Behav Brain Res. 2018;347:408–413. doi:10.1016/j.bbr.2018.03.036
26. Wan L, Ge W-R, Zhang S, et al. Case-control study of the effects of gut microbiota composition on neurotransmitter metabolic pathways in children with attention deficit hyperactivity disorder. Front Neurosci. 2020;14:127. doi:10.3389/fnins.2020.00127
27. Lyte M. Microbial endocrinology in the microbiome-gut-brain axis: how bacterial production and utilization of neurochemicals influence behavior. PLoS Pathog. 2013;9(11):e1003726. doi:10.1371/journal.ppat.1003726
28. Clayton TA. Metabolic differences underlying two distinct rat urinary phenotypes, a suggested role for gut microbial metabolism of phenylalanine and a possible connection to autism. FEBS Lett. 2012;586(7):956–961. doi:10.1016/j.febslet.2012.01.049
29. Jung TH, Hwang HJ, Han KS. Correlation of attention deficit hyperactivity disorder with gut microbiota according to the dietary intake of Korean elementary school students. PLoS One. 2022;17(9):e0275520. doi:10.1371/journal.pone.0275520
30. Sampson TR, Mazmanian SK. Control of brain development, function, and behavior by the microbiome. Cell Host Microbe. 2015;17(5):565–576. doi:10.1016/j.chom.2015.04.011
31. Zhu B, Wang X, Li L. Human gut microbiome: the second genome of human body. Protein Cell. 2010;1(8):718–725. doi:10.1007/s13238-010-0093-z
32. Lukiw WJ. The microbiome, microbial-generated proinflammatory neurotoxins, and Alzheimer’s disease. J Sport Health Sci. 2016;5(4):393–396. doi:10.1016/j.jshs.2016.08.008
33. Barrett E, Ross RP, O’Toole PW, et al. Gamma-aminobutyric acid production by culturable bacteria from the human intestine. J Appl Microbiol. 2012;113(2):411–417. doi:10.1111/j.1365-2672.2012.05344.x
34. Ratajczak W, Rył A, Mizerski A, et al. Immunomodulatory potential of gut microbiome-derived short-chain fatty acids (SCFAs). Acta Biochim Pol. 2019;66(1):1–12. doi:10.18388/abp.2018_2648
35. Walujkar SA, Dhotre DP, Marathe NP, et al. Characterization of bacterial community shift in human ulcerative colitis patients revealed by Illumina based 16S rRNA gene amplicon sequencing. Gut Pathog. 2014;6(1):22. doi:10.1186/1757-4749-6-22
36. Shin NR, Whon TW, Bae JW. Proteobacteria: microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015;33(9):496–503. doi:10.1016/j.tibtech.2015.06.011
37. Carneiro LA, Travassos LH, Soares F, et al. Shigella induces mitochondrial dysfunction and cell death in nonmyleoid cells. Cell Host Microbe. 2009;5(2):123–136. doi:10.1016/j.chom.2008.12.011
38. Rabia Hamid R, Masood A. Dietary lectins as disease causing toxicants. Pak J Nutr. 2009;8(3):293–303. doi:10.3923/pjn.2009.293.303
39. Mishra A, Behura A, Mawatwal S, et al. Structure-function and application of plant lectins in disease biology and immunity. Food Chem Toxicol. 2019;134:110827. doi:10.1016/j.fct.2019.110827
40. Banwell JG, Howard R, Kabir I, et al. Bacterial overgrowth by indigenous microflora in the phytohemagglutinin-fed rat. Can J Microbiol. 1988;34(8):1009–1013. doi:10.1139/m88-177
41. Miao S, Zhao C, Zhu J, et al. Dietary soybean meal affects intestinal homoeostasis by altering the microbiota, morphology and inflammatory cytokine gene expression in northern snakehead. Sci Rep. 2018;8(1):113. doi:10.1038/s41598-017-18430-7
42. Cordain L, Toohey L, Smith MJ, et al. Modulation of immune function by dietary lectins in rheumatoid arthritis. Br J Nutr. 2000;83(3):207–217. doi:10.1017/S0007114500000271
43. Vasconcelos IM, Oliveira JTA. Antinutritional properties of plant lectins. Toxicon. 2004;44(4):385–403. doi:10.1016/j.toxicon.2004.05.005
44. Ovelgonne JH, Koninkx JF, Pusztai A, et al. Decreased levels of heat shock proteins in gut epithelial cells after exposure to plant lectins. Gut. 2000;46(5):680–688. doi:10.1136/gut.46.5.680
45. Gong T, Wang X, Yang Y, et al. Plant lectins activate the NLRP3 inflammasome to promote inflammatory disorders. J Immunol. 2017;198(5):2082–2092. doi:10.4049/jimmunol.1600145
46. Agostoni C, Nobile M, Ciappolino V, et al. The role of omega-3 fatty acids in developmental psychopathology: a systematic review on early psychosis, autism, and ADHD. Int J Mol Sci. 2017;18(12):2608. doi:10.3390/ijms18122608
47. Checa-Ros A, Haro-García A, Seiquer I, et al. Early monitoring of fatty acid profile in children with attention deficit and/or hyperactivity disorder under treatment with omega-3 polyunsaturated fatty acids. Minerva Pediatr. 2019;71(4):313–325. doi:10.23736/S0026-4946.18.04975-7
48. Kanarek RB. Artificial food dyes and attention deficit hyperactivity disorder. Nutr Rev. 2011;69(7):385–391. doi:10.1111/j.1753-4887.2011.00385.x
49. Sonuga-Barke EJS, Brandeis D, Cortese S, et al. Nonpharmacological interventions for ADHD: systematic review and meta-analyses of randomized controlled trials of dietary and psychological treatments. Am J Psychiatry. 2013;170(3):275–289. doi:10.1176/appi.ajp.2012.12070991
50. Nigg JT, Holton K. Restriction and elimination diets in ADHD treatment. Child Adolesc Psychiatr Clin N Am. 2014;23(4):937–953. doi:10.1016/j.chc.2014.05.010
51. Ghanizadeh A, Haddad B. The effect of dietary education on ADHD, a randomized controlled clinical trial. Ann Gen Psychiatry. 2015;14(1):12. doi:10.1186/s12991-015-0050-6
52. Pelsser LMJ, Frankena K, Toorman J, et al. A randomised controlled trial into the effects of food on ADHD. Eur Child Adolesc Psychiatry. 2009;18(1):12–19. doi:10.1007/s00787-008-0695-7
53. Pelsser LM, Frankena K, Toorman J, et al. Diet and ADHD, reviewing the evidence: a systematic review of meta-analyses of double-blind placebo-controlled trials evaluating the efficacy of diet interventions on the behavior of children with ADHD. PLoS One. 2017;12(1):e0169277. doi:10.1371/journal.pone.0169277
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



