Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 17
The Global Prevalence of Methicillin-Resistant Staphylococcus Aureus in Patients with Diabetic Foot Ulcers: A Systematic Review and Meta-Analysis
Authors Zhou S, Hu X, Wang Y, Fei W, Sheng Y, Que H
Received 31 October 2023
Accepted for publication 16 January 2024
Published 3 February 2024 Volume 2024:17 Pages 563—574
DOI https://doi.org/10.2147/DMSO.S446911
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Konstantinos Tziomalos
Siyuan Zhou,1,2 Xiaojie Hu,1,2 Yunfei Wang,1,2 Wenting Fei,2 Yuqin Sheng,2 Huafa Que1,2
1Department of Traditional Chinese Surgery, Longhua Hospital Affiliated to Shanghai University of Traditional Chinese Medicine, Shanghai, People’s Republic of China; 2Longhua Clinical Medical College, Shanghai University of Traditional Chinese Medicine, Shanghai, People’s Republic of China
Correspondence: Huafa Que, Department of Traditional Chinese Surgery, Longhua Hospital affiliated to Shanghai University of Traditional Chinese Medicine, No. 725, Wanping South Road, Xuhui District, Shanghai, 200032, People’s Republic of China, Tel +86 18917763260, Email [email protected]
Objective: Diabetic foot ulcer (DFU) frequently leads to infections, with infected DFUs being a common cause of amputation. Infection by methicillin-resistant Staphylococcus aureus (MRSA) notably increases the necessity for amputation and surgical debridement in affected individuals. Consequently, determining the prevalence and trends of MRSA in patients with DFU is of critical importance. This study aimed to assess the global prevalence and to identify trends in the occurrence of MRSA in tissue or wound swab samples from DFU patients.
Methods: We conducted a comprehensive literature search across PubMed, Embase, Scopus, and Ovid, spanning from the inception of these databases to July 2023, imposing no language restrictions. The inclusion criteria required that the studies report on 30 or more patients with DFU. Additionally, we categorized our analysis based on geographic region, publication date, and the economic status of the patient’s domicile. Our primary endpoint was to ascertain the prevalence of MRSA in DFUs. This systematic review has been registered at (https://www.crd.york.ac.uk/prospero/), with the identifier CRD 42023444360.
Results: Our analysis encompassed 40 studies involving 12,924 patients across 20 countries. We found that the overall prevalence of MRSA in DFU was 17% (95% Confidence Interval [CI] 0.14– 0.20). Regional prevalence varied significantly: in South America, it was 61% (95% CI 0.46– 0.76), in North America 20% (95% CI 0.12– 0.27), in Europe 19% (95% CI 0.14– 0.25), in Africa 13% (95% CI 0.06– 0.20), and in other subgroups 11% (95% CI 0.08– 0.15). The prevalence of MRSA in DFUs also differed according to the economic status of the countries: 19% (95% CI 0.15– 0.23) in high-income countries, 24% (95% CI 0.1– 0.37) in upper-middle-income countries, 11% (95% CI 0.07– 0.15) in lower-middle-income countries, and 20% (95% CI 0.13– 0.27) in low-income countries. Notably, there has been a decline in MRSA prevalence, from 25% before 2010 to 9% thereafter.
Conclusion: This meta-analysis reveals a decreasing yet still significant global prevalence of MRSA in DFUs. This trend has important implications for antimicrobial resistance and underscores the need for developing targeted programs focusing on infection prevention and exploring alternative therapeutic strategies.
Keywords: diabetic foot ulcers, global prevalence, epidemiological trends, systematic review, meta-analysis
Introduction
Diabetes mellitus represents a critical global health concern, with its incidence rising annually. It is projected that by 2035, approximately 592 million individuals will be affected.1 DFUs constitute a frequent complication of diabetes, affecting about 15% of individuals with this condition during their lifetime.2,3 Diabetic foot infection (DFI) is clinically characterized by signs of inflammation in any tissue below the malleoli in individuals with diabetes mellitus. It ranks among the most severe complications of diabetes, significantly contributing to diminished quality of life for patients and substantial economic losses.4 Patients with DFU exhibit a dismal prognosis, as evidenced by a comprehensive prospective study. One year following diagnosis, a mere 46% of patients achieved ulcer healing, with a subsequent 10% experiencing recurrence. Additionally, a noteworthy 15% of patients succumbed to the condition, underscoring its life-threatening nature. Furthermore, a substantial 17% of patients necessitated lower limb amputation.5 Presently, the therapeutic approach for DFU encompasses a multifaceted strategy, which comprises the following key principles: Treatment of foot infections, Restoration of tissue perfusion, Pressure offloading and ulcer protection, Local ulcer care, and Person-centered care. These core principles constitute the fundamental pillars of DFU management, reflecting a holistic and patient-centric approach to address the complex challenges associated with this condition.6 Following the aforementioned therapeutic principles, the majority of patients with DFU experience successful wound healing. However, a subset of patients may encounter severe complications, some of which pose life-threatening risks. Furthermore, the financial burden associated with DFUs is substantial, with annual costs estimated at nearly £1 billion in the United Kingdom, amounting to approximately 1% of the National Health Service (NHS) budget.7 In contrast, the proportion of healthcare resources dedicated to DFUs is considerably higher in developing countries, underscoring the urgent need for targeted interventions and resource allocation to address this pressing issue.8
Staphylococcus aureus, a bacterium, is the predominant microorganism found in diabetic foot infections. These infections can be further classified into two main categories: MRSA and methicillin-sensitive Staphylococcus aureus (MSSA).9 A study conducted in the United Kingdom revealed that DFUs testing positive for MRSA exhibit a prolonged time to ulcer healing compared to DFUs testing positive for MSSA.10 In addition, there has been an increase in the requirement for amputation and surgical wound debridement among patients infected with MRSA.6 The occurrence of MRSA infection in wounds imposes a substantial economic and clinical burden, characterized by elevated hospitalization expenses and an augmented likelihood of patient mortality.11,12 Factors such as diabetes mellitus, previous exposure to antimicrobial agents, recent hospitalization within the preceding 12 months, the presence of skin or soft tissue infections upon admission, and HIV infection have all been identified as significant contributors to an increased susceptibility to MRSA infection.13
Presently, the incidence of diabetes mellitus continues to escalate annually, while the worldwide prevalence of antimicrobial drug resistance exhibits a corresponding upward trajectory. Consequently, it becomes imperative to comprehend the prevalence and epidemiological patterns of drug-resistant pathogens, such as MRSA, within the context of DFU. Despite numerous antecedent investigations documenting MRSA prevalence in DFU, a comprehensive global assessment of MRSA prevalence and its epidemiological trends within this specific clinical context remains conspicuously absent. To address this critical knowledge gap, we conducted a systematic review and meta-analysis aimed at elucidating the global prevalence of MRSA in DFU and its temporal prevalence trends.
Methods
Registration
The research endeavor adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for its execution and subsequent reporting. Additionally, it was duly registered with PROSPERO under the registration identifier CRD42023444360.14
Search Strategy and Selection Criteria
Two authors conducted independent searches across four databases (PubMed, Embase, Scopus, Ovid) from their inception to July 2023, without imposing any language restrictions. In the aforementioned databases, the following search terms were employed: [(“diabetic foot” OR “Diabetic Feet” OR “Diabetic foot infection” OR “diabetic foot ulcer”) AND (“Methicillin-Resistant Staphylococcus aureus” OR “MRSA”) AND (“Prevalence” OR “incidence” OR “epidemiology” OR “occurrence” OR “rate”)] (See Appendix 1).
The articles incorporated in our analysis were observational and focused on documenting the prevalence of MRSA in patients with DFUs in the absence of specific interventions. The studies included in our analysis required minimum recruitment of 30 participants and the utilization of established diagnostic techniques for assessing exudates from ulcer surfaces. In the case of clinical trials, we exclusively extracted baseline data. Articles that could not be accessed in full text and those that exhibited redundancy with the datasets already included, as well as systematic reviews, editorials, case reports, and case series, were excluded from our study.
Data Extraction and Quality Assessment
Data extraction was performed by a single author utilizing a standardized template within an Excel spreadsheet. This extraction was subsequently cross-verified by another author, with any ambiguities or discrepancies resolved through collaborative discussion. The extracted information encompassed details such as the publication year, primary author, publication country, geographical setting, study design, sample size, study duration, source of the study sample, and the reported prevalence.
Our analysis was stratified based on the regions defined by the World Health Organization (WHO), which include Africa, the Eastern Mediterranean, Europe, Southeast Asia, the Americas, and the Western Pacific, as well as the income categories designated by the World Bank. We employed the Joanna Briggs Institute (JBI) Prevalence Essential Assessment Tool to evaluate the quality of the studies included in our analysis.15 The checklist was divided into three categories of risk of bias, depending on the number of met criteria: high (0–3 items), moderate (4–7 items), and low (8–10 items). The process of data extraction and quality assessment was executed meticulously, with any discrepancies resolved through consensus.
Statistical Analyses
We conducted a meta-analysis of the compiled data employing STATA 16.0 software. To assess inter-study heterogeneity, Cochran’s Q test and the I2 index were utilized.16 When P > 0.10 and I2 ≤ 50%, it indicated a lack of statistical heterogeneity among study results, and a fixed-effects model was employed for analysis. Conversely, if P ≤ 0.1 and I2 > 50%, a random-effects model was utilized for the meta-analysis.17 Additionally, subgroup analyses were performed based on publication year, patient origin, World Health Organization (WHO)-defined region, income level, and diagnostic method type. Prevalence rates were calculated for each subgroup, followed by comparisons of prevalence rates among subgroups using the χ2 test. It is noteworthy that publication bias was not assessed in this study, as it was deemed unrelated to prevalence.18
Results
Study Characteristics
A total of 860 records were retrieved from the four databases. Following the removal of 314 duplicates, the initial screening encompassed 546 documents. Subsequently, after a meticulous evaluation of titles and abstracts, a comprehensive review was conducted on 164 documents, culminating in the inclusion of 40 original articles for analysis (Figure 1).9,10,19–56 These selected studies involved a collective cohort of 12,924 patients with Diabetic Foot Ulcers (DFU) spanning the years 1999 through 2021 (refer to Table 1). Among the 40 studies, 26 (65%) were conducted on inpatients, six focused on outpatients (15%), and eight did not differentiate between outpatient and inpatient populations (20%). All studies uniformly utilized samples of exudates from ulcer surfaces for bacterial culture. Geographically, the distribution of these studies was as follows: 13 (32.5%) from Europe, 12 (30%) from Asia, 10 (25%) from North America, 4 (10%) from Africa, and 1 (2.5%) from South America, collectively representing 20 different countries in the study design.
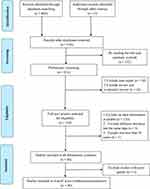 |
Figure 1 Study selection. |
 |
Table 1 Basic Information |
Meta-Analysis Results
We conducted a comprehensive meta-analysis utilizing data extracted from 40 studies that met the stipulated inclusion criteria. The analysis estimated the global prevalence of MRSA among patients with DFU to be 17.0% (95% CI 0.14–0.27) (Figure 2). Interestingly, the risk of MRSA contraction from DFUs has exhibited a declining trend over the past two decades. Before 2010, the prevalence was 25% (95% CI 0.13–0.37), whereas it reduced to 9% (95% CI 0.05–0.13) after the year 2021 (Figure 3). The prevalence of MRSA in DFUs exhibited geographical variations, with South America recording the highest prevalence at 61% (95% CI 0.46–0.76), followed by North America (20%, 95% CI 0.12–0.27), Europe (19%, 95% CI 0.14–0.25), Africa (13%, 95% CI 0.06–0.20), and the lowest prevalence observed in the subgroup (11%, 95% CI 0.08–0.15) (Figure 4). Furthermore, we categorized our analysis according to the latest World Bank classification (https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups), which segregated countries into low-income, middle-income, and high-income groups. This revealed that the prevalence of MRSA in diabetic foot ulcers was 19% (95% CI 0.15–0.23) in high-income countries, 13% (95% CI 0.09–0.17) in middle-income countries, and 20% (95% CI 0.13–0.27) in low-income countries (Figure 5). Importantly, it is worth noting that all 40 studies included in our analysis were assessed as low-risk based on the JBI assessment tool.
 |
Figure 2 Global prevalence of MRSA in patients with diabetic foot ulcers. |
 |
Figure 3 Global prevalence of MRSA in diabetic foot ulcers, by time of study publication. |
 |
Figure 4 Global prevalence of diabetic foot ulcer MRSA, by geographic location. |
 |
Figure 5 Global prevalence of MRSA in diaxbetic foot ulcers, by income. |
Discussion
This marks the inaugural systematic review and meta-analysis to delineate the global prevalence of MRSA in DFU. Within the scope of this investigation, we have furnished a comprehensive meta-analysis, encompassing diverse geographical regions, to ascertain the prevalence of MRSA in DFU. Our findings indicate a worldwide MRSA prevalence of 17%, a figure in alignment with previous reports (18%), thus reinforcing the high incidence of MRSA in this context.9 However, our study further contributes by offering a detailed breakdown of MRSA prevalence across various geographical locations, encompassing a spectrum of economic strata, while also affording insight into the temporal trends of MRSA prevalence.
Furthermore, we identified a paucity of relevant evidence in numerous regions across the globe, with only approximately 20 countries furnishing robust data. This observation underscores the pronounced regional disparities in MRSA prevalence, potentially associated with the respective countries’ economic status and healthcare infrastructure. Notably, our analysis revealed a relatively diminished MRSA prevalence in low-income regions, a trend likely attributed to factors such as delayed access to healthcare services for individuals with Diabetic Foot Ulcers (DFU) or limited availability of antibiotic treatment. These discrepancies are reflected in the limited data contributions from countries characterized by lower economic income. Moreover, our findings indicate a discernible decrease in the prevalence of MRSA in Diabetic Foot Infections (DFI) over time, aligning with the observations made by Moore et al.8 The plausible rationale behind this declining trend could be the implementation of standardized antibiotic protocols.
Long-term DFU and prolonged antibiotic use are associated with MRSA infections.10 Chronic ulcers represent the most significant risk factor for MRSA infection. Additionally, chronic kidney disease presents another risk factor for MRSA isolation.6 One study has indicated an association between MRSA isolated from DFU and nasal MRSA carriage.57 Long-term or inappropriate antibiotic use and previous hospitalizations are contributing factors to MRSA infections. It is crucial to note that MRSA infections not only prolong wound healing times but also elevate the risk of amputation in cases of DFI.10,58 Moreover, MRSA escalates the likelihood of osteomyelitis development in DFUs.52 Consequently, understanding the prevalence and temporal trends of MRSA in DFU is paramount for clinical practice. Firstly, it serves as a reminder to healthcare providers to exercise restraint in antibiotic utilization. Secondly, it underscores the importance of healthcare practitioners and policymakers focusing on preventive measures, timely detection, and appropriate management of DFIs, thus providing valuable insights for policy decisions.
Several limitations must be acknowledged in this study. It is crucial to recognize that significant heterogeneity existed among the studies, likely attributed to population diversity and variations in hygienic environments. While certain studies exclusively included patients hospitalized for their initial episode, others might have encompassed recurrent hospitalizations. Such substantial heterogeneity among these studies may potentially limit the interpretability of pooled estimates.
Furthermore, subanalyses conducted by geographical regions, although beneficial for assessing overall trends, may offer relatively lower resolution due to the intricate factors underlying MRSA prevalence. Additionally, the lack of standardized criteria for classifying the degree of ulceration in patients with diabetic foot ulcers, coupled with the varying degrees of ulceration observed across the included studies, introduces an additional layer of complexity.
To address these limitations, further research is warranted, particularly investigations specifically focused on MRSA in patients with diabetic foot ulcers. Such endeavors would bolster the evidence base and facilitate more refined stratification of data in future analyses.
Conclusions
In summary, the findings of this study reveal that while there is a declining trend in MRSA prevalence among patients with DFU, it remains at a relatively elevated level. Our comprehensive assessment has quantified these global trends in MRSA prevalence within the context of DFU, providing essential foundational data for future research and clinical endeavors. These insights can also inform healthcare policymakers in devising programs and interventions aimed at improving hygiene practices and mitigating adverse outcomes. There is a pressing need for concerted efforts within the healthcare sector to educate both clinical personnel and patients afflicted with DFUs about preventive measures, thus reducing the risk of unfavorable prognoses in this patient population. Furthermore, considering the rising levels of antibiotic resistance, it is imperative to prioritize research into alternative therapies, alongside continued efforts in infection prevention.59
Data Sharing Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author Contributions
All authors made significant contributions to the work reported in the conception, study design, execution, acquisition, analysis, and interpretation of data. All authors took part in drafting, revising or critically reviewing the article and gave final approval of the version to be published. All authors have agreed to the approval of the final manuscript for publication in the current journal and to be accountable for all aspects of this work.
Funding
This study was supported by the Construction project for Shanghai Municipal Health Commission key clinical speciality (shslczdzk03801).
Disclosure
All the authors declare no conflicts of interest.
References
1. Kharroubi AT, Darwish HM. Diabetes mellitus: the epidemic of the century. World J Diabetes. 2015;6:850–867. doi:10.4239/wjd.v6.i6.850
2. Armstrong DG, Boulton AJM, Bus SA. Diabetic foot ulcers and their recurrence. N Engl J Med. 2017;376:2367–2375. doi:10.1056/NEJMra1615439
3. Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA. 2005;293:217–228. doi:10.1001/jama.293.2.217
4. Lipsky BA, Senneville É, Abbas ZG, et al. Guidelines on the diagnosis and treatment of foot infection in persons with diabetes (IWGDF 2019 update). Diabetes Metab Res. 2020;36(Suppl 1):e3280. doi:10.1002/dmrr.3280
5. Ndosi M, Wright-Hughes A, Brown S, et al. Prognosis of the infected diabetic foot ulcer: a 12-month prospective observational study. Diabet Med. 2018;35:78–88. doi:10.1111/dme.13537
6. Eleftheriadou I, Tentolouris N, Argiana V, et al. Methicillin-resistant Staphylococcus aureus in diabetic foot infections. Drugs. 2010;70(14):1785–1797. doi:10.2165/11538070-000000000-00000
7. Kerr M, Barron E, Chadwick P, et al. The cost of diabetic foot ulcers and amputations to the national health service in England. Diabet Med. 2019;36(8):995–1002. doi:10.1111/dme.13973
8. Macdonald KE, Boeckh S, Stacey HJ, et al. The microbiology of diabetic foot infections: a meta-analysis. BMC Infect Dis. 2021;21(1):770. doi:10.1186/s12879-021-06516-7
9. Moore J, Gooday C, Soliman R, et al. Reduction in the prevalence of methicillin-resistant Staphylococcus aureus in tissue and wound swab samples taken from outpatients attending a specialist diabetic foot clinic 2005–2021. Diabet Med. 2023;40(10):e15081. doi:10.1111/dme.15081
10. Tentolouris N, Jude EB, Smirnof I, et al. Methicillin-resistant Staphylococcus aureus: an increasing problem in a diabetic foot clinic. Diabet Med. 1999;16(9):767–771. doi:10.1046/j.1464-5491.1999.00132.x
11. Cosgrove SE, Sakoulas G, Perencevich EN, et al. Comparison of mortality associated with methicillin-resistant and methicillin-susceptible Staphylococcus aureus Bacteremia: a meta-analysis. Clin Infect Dis. 2003;36(1):53–59. doi:10.1086/345476
12. Filice GA, Nyman JA, Lexau C, et al. Excess costs and utilization associated with methicillin resistance for patients with Staphylococcus aureus infection. Infect Control Hosp Epidemiol. 2010;31(4):365–373. doi:10.1086/651094
13. Hidron AI, Kourbatova EV, Halvosa JS, et al. Risk factors for colonization with Methicillin-Resistant Staphylococcus aureus (MRSA) in patients admitted to an urban hospital: emergence of community-associated MRSA nasal carriage. Clin Infect Dis. 2005;41(2):159–166. doi:10.1086/430910
14. Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. doi:10.1186/2046-4053-4-1
15. Munn Z, Moola S, Riitano D, et al. The development of a critical appraisal tool for use in systematic reviews addressing questions of prevalence. Int J Health Policy Manag. 2014;3(3):123–128. doi:10.15171/ijhpm.2014.71
16. Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi:10.1136/bmj.327.7414.557
17. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi:10.1002/sim.1186
18. Hunter JP, Saratzis A, Sutton AJ, et al. In meta-analyses of proportion studies, funnel plots were found to be an inaccurate method of assessing publication bias. J Clin Epidemiol. 2014;67:897–903. doi:10.1016/j.jclinepi.2014.03.003
19. Ahmed TE. Bacteriology of diabetic foot infections. Saudi Med J. 2000;21:344–347.
20. Dang CN, Prasad YD, Boulton AJ, et al. Methicillin-resistant Staphylococcus aureus in the diabetic foot clinic: a worsening problem. Diabet Med. 2003;20(2):159–161. doi:10.1046/j.1464-5491.2003.00860.x
21. Shankar EM, Mohan V, Premalatha G, et al. Bacterial etiology of diabetic foot infections in South India. Eur J Intern Med. 2005;16(8):567–570. doi:10.1016/j.ejim.2005.06.016
22. Lipsky BA, Armstrong DG, Citron DM, et al. Ertapenem versus piperacillin/tazobactam for diabetic foot infections (SIDESTEP): prospective, randomised, controlled, double-blinded, multicentre trial. Lancet. 2005;366(9498):1695–1703. doi:10.1016/S0140-6736(05)67694-5
23. Tentolouris N, Petrikkos G, Vallianou N, et al. Prevalence of methicillin-resistant Staphylococcus aureus in infected and uninfected diabetic foot ulcers. Clin Microbiol Infect. 2006;12(2):186–189. doi:10.1111/j.1469-0691.2005.01279.x
24. Martínez-Gómez Dde A, Ramírez-Almagro C, Campillo-Soto A, et al. Infecciones del pie diabético. Prevalencia de los distintos microorganismos y sensibilidad a los antimicrobianos [Diabetic foot infections. Prevalence and antibiotic sensitivity of the causative microorganisms]. Enferm Infecc Microbiol Clin. 2009;27:317–321. Spanish. doi:10.1016/j.eimc.2008.07.004
25. Lagacé-Wiens PR, Ormiston D, Nicolle LE, et al. The diabetic foot clinic: not a significant source for acquisition of methicillin-resistant Staphylococcus aureus. Am J Infect Control. 2009;37:587–589. doi:10.1016/j.ajic.2008.11.006
26. Mendes JJ, Marques-Costa A, Vilela C, et al. Clinical and bacteriological survey of diabetic foot infections in Lisbon. Diabet Res Clin Pract. 2012;95:153–161. doi:10.1016/j.diabres.2011.10.001
27. Lipsky BA, Itani KM, Weigelt JA, et al. The role of diabetes mellitus in the treatment of skin and skin structure infections caused by methicillin-resistant Staphylococcus aureus: results from three randomized controlled trials. Int J Infect Dis. 2011;15:e140–e146. doi:10.1016/j.ijid.2010.10.003
28. Feng SH, Chu YJ, Wang PH, et al. Risk factors and gene type for infections of MRSA in diabetic foot patients in Tianjin, China. Int J Low Extrem Wounds. 2013;12:106–112. doi:10.1177/1534734613489991
29. Djahmi N, Messad N, Nedjai S, et al. Molecular epidemiology of Staphylococcus aureus strains isolated from inpatients with infected diabetic foot ulcers in an Algerian University Hospital. Clin Microbiol Infect. 2013;19:E398–E404. doi:10.1111/1469-0691.12199
30. Sugandhi P, Prasanth DA. Microbiological profile of bacterial pathogens from diabetic foot infections in tertiary care hospitals, Salem. Diabetes Metab Syndr. 2014;8:129–132. doi:10.1016/j.dsx.2014.07.004
31. Senneville E, Brière M, Neut C, et al. First report of the predominance of clonal complex 398 Staphylococcus aureus strains in osteomyelitis complicating diabetic foot ulcers: a national French study. Clin Microbiol Infect. 2014;20:O274–O277. doi:10.1111/1469-0691.12375
32. Małecki R, Rosiński K, Adamiec R. Etiological factors of infections in diabetic foot syndrome - attempt to define optimal empirical therapy. Adv Clin Exp Med. 2014;23:39–48. doi:10.17219/acem/37020
33. Lavery LA, Fontaine JL, Bhavan K, et al. Risk factors for methicillin-resistant Staphylococcus aureus in diabetic foot infections. Diabet Foot Ankle. 2014;5. doi:10.3402/dfa.v5.23575
34. Ahmed EF, Gad GF, Abdalla AM, et al. Prevalence of methicillin resistant Staphylococcus aureus among Egyptian patients after surgical interventions. Surg Infect. 2014;15:404–411. doi:10.1089/sur.2013.212
35. Perim MC, Borges JC, Celeste SR, et al. Aerobic bacterial profile and antibiotic resistance in patients with diabetic foot infections. Rev Soc Bras Med Tro. 2015;48:546–554. doi:10.1590/0037-8682-0146-2015
36. Parsa H, Samani S. Microbiological features and risk factors in patients with diabetic foot ulcers. Wounds. 2015;27:308–312.
37. Commons RJ, Robinson CH, Gawler D, et al. High burden of diabetic foot infections in the top end of Australia: an emerging health crisis (DEFINE study). Diabet Res Clin Pract. 2015;110(2):147–157. doi:10.1016/j.diabres.2015.09.016
38. Reveles KR, Duhon BM, Moore RJ, et al. Epidemiology of methicillin-resistant Staphylococcus aureus diabetic foot infections in a large academic hospital: implications for antimicrobial stewardship. PLoS One. 2016;11(8):e0161658. doi:10.1371/journal.pone.0161658
39. Pobiega M, Myjak I, Pomorska-Wesolowska M, et al. Virulence potential of Staphylococcus aureus strains isolated from diabetic foot ulcers among patients from Southern Poland. Curr Vasc Pharmacol. 2016;14(6):547–551. doi:10.2174/1570161114666160625083742
40. Wu WX, Liu D, Wang YW, et al. Empirical antibiotic treatment in diabetic foot infection: a study focusing on the culture and antibiotic sensitivity in a population from Southern China. Int J Low Extrem Wounds. 2017;16:173–182. doi:10.1177/1534734617725410
41. Dunyach-Remy C, Courtais-Coulon C, DeMattei C, et al. Link between nasal carriage of Staphylococcus aureus and infected diabetic foot ulcers. Diabetes Metab. 2017;43(2):167–171. doi:10.1016/j.diabet.2016.09.003
42. van Asten SAV, Mithani M, Peters EJG, et al. Complications during the treatment of diabetic foot osteomyelitis. Diabet Res Clin Pract. 2018;135:58–64. doi:10.1016/j.diabres.2017.06.002
43. Obeid M, Moughames E, Aboulhosn P, et al. Epidemiology and susceptibility profiles of diabetic foot infections in five hospitals in Lebanon. J Infect Dev Ctries. 2018;12(05):347–351. doi:10.3855/jidc.10063
44. Neves JM, Duarte B, Pinto M, et al. Diabetic foot infection: causative pathogens and empiric antibiotherapy considerations-the experience of a tertiary center. Int J Low Extrem Wounds. 2019;18(2):122–128. doi:10.1177/1534734619839815
45. Kananizadeh P, Ohadian Moghadam S, Sadeghi Y, et al. Molecular characteristics of Methicillin-Resistant Staphylococcus aureus (MRSA) isolated from diabetic foot infection. Iran J Pathol. 2019;14:329–337. doi:10.30699/ijp.2019.101092.2035
46. Ullah I, Ali SS, Ahmed I, et al. Bacteriological profile and antibiotic susceptibility patterns in diabetic foot infections, at lady reading hospital, Peshawar. J Ayub Med Coll Abbottabad. 2020;32(3):382–388.
47. Lin SY, Lin NY, Huang YY, et al. Methicillin-resistant Staphylococcus aureus nasal carriage and infection among patients with diabetic foot ulcer. J Microbiol Immunol Infect. 2020;53:292–299. doi:10.1016/j.jmii.2018.03.005
48. Kim JJ, Lydecker A, Davé R, et al. Diabetic foot infections: local prevalence of and case-control study of risk factors for methicillin-resistant Staphylococcus aureus and Pseudomonas aeruginosa. Open Forum Infect Dis. 2020;7:ofaa412. doi:10.1093/ofid/ofaa412
49. Jouhar L, Jaafar RF, Nasreddine R, et al. Microbiological profile and antimicrobial resistance among diabetic foot infections in Lebanon. Int Wound J. 2020;17:1764–1773. doi:10.1111/iwj.13465
50. Anafo RB, Atiase Y, Dayie NTKD, et al. Methicillin-Resistant Staphylococcus aureus (MRSA) infection of diabetic foot ulcers at a tertiary care hospital in Accra, Ghana. Pathogens. 2021;10(8):937. doi:10.3390/pathogens10080937
51. Woldeteklie AA, Kebede HB, Abdela AA, et al. Prevalence of extended-spectrum β-lactamase and carbapenemase producers of gram-negative bacteria, and methicillin-resistant Staphylococcus aureus in isolates from diabetic foot ulcer patients in Ethiopia. Infect Drug Resist. 2022;15:4435–4441. doi:10.2147/IDR.S371431
52. Stańkowska M, Garbacz K, Korzon-Burakowska A, et al. Microbiological, clinical and radiological aspects of diabetic foot ulcers infected with methicillin-resistant and -sensitive Staphylococcus aureus. Pathogens. 2022;11:701. doi:10.3390/pathogens11060701
53. Pany S, Sharma BM, Sen SK, et al. Association of PVL gene in MSSA and MRSA strains among diabetic ulcer patients from Odisha, India. Int J Low Extrem Wounds. 2022:15347346221091355. doi:10.1177/15347346221091355
54. Hockney SM, Steker D, Bhasin A, et al. Role of bone biopsy and deep tissue culture for antibiotic stewardship in diabetic foot osteomyelitis. J Antimicrob Chemother. 2022;77(12):3482–3486. doi:10.1093/jac/dkac345
55. Brondo J, Morneau K, Hopkins T, et al. Correlation between patients with Methicillin-Resistant Staphylococcus aureus (MRSA) nares colonization and MRSA diabetic foot infections. Int J Low Extrem Wounds. 2022;21:502–505. doi:10.1177/1534734620963570
56. Arfaoui A, Sallem RB, Fernández-Fernández R, et al. Methicillin-resistant Staphylococcus aureus from diabetic foot infections in a Tunisian hospital with the first detection of MSSA CC398-t571. Antibiotics. 2022;11(12):1755. doi:10.3390/antibiotics11121755
57. Stanaway S, Johnson D, Moulik P, et al. Methicillin-resistant Staphylococcus aureus (MRSA) isolation from diabetic foot ulcers correlates with nasal MRSA carriage. Diabetes Res Clin Pract. 2006;75:47–50. doi:10.1016/j.diabres.2006.05.021
58. Moulik PK, Mtonga R, Gill GV. Amputation and mortality in new-onset diabetic foot ulcers stratified by etiology. Diabetes Care. 2003;26:491–494. doi:10.2337/diacare.26.2.491
59. Fish R, Kutter E, Wheat G, et al. Bacteriophage treatment of intransigent diabetic toe ulcers: a case series. J WOUND CARE. 2016;25:S27–S33. doi:10.12968/jowc.2016.25.7.s27
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
