Back to Journals » Journal of Pain Research » Volume 12
The Fear of Pain Questionnaire: psychometric properties of a Brazilian version for adolescents and its relationship with brain-derived neurotrophic factor (BDNF)
Authors Berniger Romariz JA , Nonnemacher C , Abreu M , Dickel Segabinazi J , Bandeira JS , Beltran G, Souza A, Torres ILS , Caumo W
Received 21 December 2018
Accepted for publication 22 April 2019
Published 7 August 2019 Volume 2019:12 Pages 2487—2502
DOI https://doi.org/10.2147/JPR.S199120
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Michael A Ueberall
José Ary Berniger Romariz,1,2 Cássio Nonnemacher,2 Mylena Abreu,2 Joice Dickel Segabinazi,2 Janete Shatkoski Bandeira,2 Gerardo Beltran,1–3 Andressa Souza,4 Iraci LS Torres,5 Wolnei Caumo1,2,6–8
1Postgraduate Program in Medical Sciences, Faculdade de Medicina,Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil; 2Laboratory of Pain and Neuromodulation, Hospital de Clinicas de Porto Alegre, Brazil; 3Psychology Department, Cuenca Catholic University, Cuenca, Ecuador; 4Postgraduate Program in Health and Human Development, La Salle Universitary Center, Canoas, Brazil; 5Pharmacology Department, Instituto de Ciências Básicas da Saúde, Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil; 6Pain and Anesthesia,Surgery Department, School of Medicine, Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil; 7Pain and Palliative Care Service, Hospital de Clínicas de Porto Alegre, Porto Alegre, Brazil; 8Laboratory of Pain and Neuromodulation, Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil
Objectives: The primary aim was to assess the psychometric properties (including internal consistency, construct validity, criterion validity, criterion-group validity and responsiveness) of the Fear of Pain Questionnaire (FOPQ) for adolescents (FOPQ-A) and parents (FOPQ-P) translated to Brazilian Portuguese (BrP). The secondary aim was to analyze the factor structures and their ability to identify subjects with chronic pain conditions and identify the relationship of the BrP FOPQ-A with saliva brain-derived neurotrophic-factor (BDNF).
Methods: A cross-sectional study was conducted with 286 adolescents aged 11 to 18 (257 healthy adolescents [157 females] and 29 adolescents with chronic pain [16 females]). Parents and adolescents completed the BrP-FOPQ. A team of experts translated the FOPQ according to international guidelines. Convergent validity and factor analysis were performed. Later, a subsample (n=146) was used to correlate the BrP-FOPQ-A with saliva BDNF.
Results: The BrP-FOPQ for adolescents and parents presented strong psychometric properties (Cronbach’s α equal to 0.92 and 0.91, respectively). BrP-FOPQ-A confirmatory factor analysis yielded a two-factor structure while the factorial analyses of BrP-FOPQ-P demonstrated that the best solution was a three-structure factorial. The BrP-FOPQ-P scores in healthy adolescents and those in chronic pain conditions was 34.13 (16.71) vs 43.14 (18.08), respectively. A generalized mixed model demonstrated that the scores in the BrP-FOPQ-A are higher in those with chronic pain conditions compared to healthy subjects (29.20 [12.77] vs 33.80 [10.76], respectively; Wald χ2= 17.80; df=1, P<0.0001). The model revealed that the BDNF was positively correlated with the score of BrP-FOPQ-A and subjects with chronic pain showed higher levels of BDNF.
Conclusion: The BrP-FOPQ scores for adolescents and parents were found to be psychometrically robust and reliable instruments, with primary evidence of validity. Higher scores on the BrP-FOPQ-A were correlated positively with saliva BDNF and permitted the identification of subjects with chronic pain conditions.
Keywords: chronic pain, BDNF, pain-related fear, adolescents, assessment
Introduction
Pain is a multidimensional experience with sensory-discriminative, cognitive evaluative, affective-motivational and social components.1 Chronic primary pain persists or recurs for longer than three months, and it is associated with significant emotional distress or functional disability (interference with activities of daily life and social roles).2 Pediatric chronic pain estimates that posit 20–35% of children and adolescents worldwide.3 Chronic pain in children and adolescents may interfere with school activities, leisure and their child’s social relations.4 It is common that child suffering from chronic pain to be seen as fragile and victimized. This social response to their’ suffering may increase the feelings of rejection and exclusion from their environment.5 Changes in mood, anxiety, and sleep disorders are part of the response associated with chronic pain. Fear of pain has been implicated in many aspects of illness, including experimentally induced pain intensity,6 pain during dental care,7 chronic pain behavior and pain-related disability.8,9 Another behavioral manifestation that impairs rehabilitation and increases suffering is coping incapacity. Coping strategies are a link between pain perception and child functionality. Children with more capacity to develop coping strategies are less prone to suffer from pain perception. In studies with musculoskeletal pain, the coping mechanisms adopted by children help in determining their quality of life.10–12 Alternately, the absence of pain-related fear leads to rapid confrontation through continued engagement in routine daily activities and resultant recovery.
In chronic pain, the repetitive activation on pain pathways is associated with amplification of neural signaling within the central nervous system that elicits pain hypersensitivity.13 The brain-derived-neurotrophic-factor (BDNF) has an essential role in influencing the ability of development, synaptic activities, survival and cerebral development. Moreover, this neurotrophic factor has central role in the pathophysiology of a range of neurological and psychiatric disorders, including major depressive disorder, post‐traumatic stress disorder (PTSD) and chronic pain.14 According to an previous study on healthy subjects, higher levels of serum BDNF was associated with a higher pain threshold in women, while it was the opposite in men.15 Although the effect of sex hormones on the relationship between BDNF and pain is a complex process, the estrogen likely mediates it. This gonadal hormone regulates the increase in BDNF mRNA in areas associated with nociceptive sensory processing such as the hippocampus, cerebral cortex and spinal cord.16 In the amygdala responsible for the acquisition, storage and expression of learned fear,17 the chronic stress increases the BDNF while decreasing it in the hippocampus.18 A preclinical study demonstrated that BDNF decreased behavioral response (freezing) and reduced activation of the amygdala and fear-processing circuitry19,20 while stress-stimulated the BDNF salivary secretion.21
Pain-related fear is a maladaptive psychobiological interaction that leads to physical deconditioning of the musculoskeletal system.22 Thus, the identification of the avoidant behavior of the child with pain can help in the symptom management to prevent the development of depressive symptoms. However, a limited number of tools have been available to help clinicians and educators identify children and adolescents’ present symptoms that may be related to fear and avoidance related to pain. To give attention for this behavior in the pediatric chronic pain, Simons et al (2011) developed the Fear of Pain Questionnaire as well as child and parent report (FOPQ-C; FOPQ-P) to assess avoidance and fear of pain with pediatric chronic pain patients. The scales were designed to be multidimensional, emphasizing the domains that contribute to fear avoidance of pain.23 Thus, we conduct the present study to examine the psychometric properties of the translated tools according to the COSMIN (Consensus-based Standards for the selection of health Measurement) guideline terminology in order to have a reliable instrument to assess fear of pain for Brazilian population.24 (I) We assessed the content validity and face validity by semantic equivalence, the comparison of items by experts and a subsample of the target population to evaluate the cross-cultural, which was adapted from the English version of the FOPQ-C and FOPQ-P to Brazilian Portuguese. (II) We examined the internal consistency and the construct validity of the FOPQ translated instruments. (III) We assessed the convergence validity by the correlation of the BrP-FOPQ-A with relevant correlates: depressive symptoms, pain catastrophizing, emotion and conducts problems and physical and psychosocial functioning due to their physical health. (IV) We evaluated the criterion-group validity by the ability of BrP-FOPQ-A to discriminate between chronic pain and pain-free healthy control subjects. (V) We assessed the relationship of the BrP-FOPQ-A with saliva BDNF. We tested the hypothesis of the construct validity, evaluating if the BrP-FOPQ-A scores’ responsiveness would identify the severity of fear of pain, according to an adolescent with a pain condition and pain-free healthy control subjects.
Subjects and methods
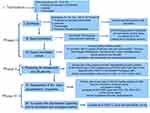
The protocol was reviewed and approved by the Ethics Committee Board of the Hospital de Clínicas de Porto Alegre (protocol n°16-0212). All parents gave their written formal consent for participation on their as well as their child’s behalf. The adolescents gave their assent to participate in the study. Figure 1 presents the flow of the multiple standardized phases of the study.
Phase I. Translation, synthesis and back translation and consensus of experts assessed the content and face validity
The original English version of the FOPQ-C and FOPQ-P were translated into Brazilian Portuguese. The cross-cultural adaptation was conducted by previously published guidelines.25 Three native Brazilian Portuguese speakers (T-1, T-2 and T-3) carried out independent translations of the FOPQ-C and FOPQ-P from English to Brazilian Portuguese. T-1 was a professional translator, T2 was a psychologist and T-3 was a physician with pain specialization. The forward translations were compared with one another as well as the original English version. After discussing any discrepancies, the three versions were combined into one Brazilian Portuguese version.25
Two native English speakers translated the original FOPQ-A for adolescents and FOPQ-P for parents to Brazilian Portuguese and carried out a backtranslation into English. Brazilian Portuguese is their second language. Both back-translators were considered bilingual, but they were not familiar with the subject matter of the questionnaire. A third bilingual person corrected any gross inconsistencies or conceptual errors in the content of the translated versions in preparation for the expert committee meeting.26
An expert committee consisting of clinical researches and experts in the translation of scales, pediatricians, child psychologists and physicians with pain specialization (n=21) were interviewed to collect qualitative data by exploring how the members understood each of the 24 items of BrP-FOCQ-C and 21 items of BrP-FOCQ-P. This expert committee also assessed the comprehension of issues using a 10 cm visual analog scale (VAS; from 0 cm representing unclear to 10 cm representing entirely clear). The task of the experts’ committee was to ensure that the semantics of items and the conceptual content of items (content validity) of the BrP-FOCQ-C and BrP-FOCQ-P.They had an age range of 25–60 years, (n=13 females) higher than 15 formal years of schooling and involvement in the translation of development of the instruments. The committee members and the panel director communicated via chat. The evaluators posted their comments; thus, the panel director processed the information and filtered out the relevant content until they came to a consensus on the item. Rounds coordinated by two clinical research scientists with experience in validating instruments were made until they came to a consensus on the questions. They assessed their understanding of the questions of the BrP-FOCQ-A and BrP-FOCQ-P using 10 cm Visual Analogue Scales (where 0 implied completely unclear and 10 cm indicated completely clear). All items received scores equal or higher than 8.5.
After discussing any discrepancies, the four versions were combined into one Brazilian Portuguese (BrP)-FOPQ-C. Item 3 was changed to “I cannot do everything that healthy people do; they do because it’s so easy to hurt my body”. The children’s scale changed the initial translation “I cannot do everything that normal people do, because I hurt my body easily”. The original wording focused on “normal,” but the translation focuses on “healthy” since the term “normal” is typically employed by Brazilian Portuguese speakers for a “behavior and appearance socially acceptable”. Item 21 had been translated to “If I go to school, my pain gets worse;” we changed it to “I’m not going to school because that makes my pain worse.” The experts judge that changing “I go” to “I’m going” would improve the comprehension of children. All feedback from these subjects was evaluated by the translation workgroup (to assess face validity). Based on the subject feedback, two questions were slightly modified to achieve the final Brazilian Portuguese version of the BrP-FOCQ-A. The final version of the BrP-FOCQ-C is presented. Item 7 of Brazilian Portuguese (BrP)-FOPQ-P was changed [I can’t let my child do things that healthy people do because he gets hurt easily] for the similar reason that we changed item 3 of the BrP-FORQ-A.
Phase II. Pretesting of FOPQ for parents and child in a pilot study
Twenty-two children, including 14 girls (63.22%), whose age median interquartile was 17 (IQ25-75=15.75; 17) and formal years of schooling was 11 (IQ25-75=9.75; 17), participated in this study. We included 20 parents, including 14 women (70%), with the age median interquartile of 43.50 (IQ25-75=37.00; 53.50) and formal years of schooling of 18 (IQ25-75=15.25; 21.25). They were invited to evaluate the meaning of the translated questions and the layout of the pre-final version of the instruments for adolescents and parents. In addition, they were interviewed to explore how the members understood each item. Each adolescent, parent and employee’s self-reported comprehension of the items was assessed by a 10 cm VAS (VAS; from 0 cm representing unclear to 10 cm representing entirely clear). The global median (level of comprehension) of all items in adolescents was 9.66 (IQ25-75=9.11; 10), and for parents, it was 9.61 (IQ25-75=8.90; 10).
Phase III. Assessment of psychometric properties and the validity of the final version of the FOPQ for parents and child
A total of 257 adolescents were recruited from the local community of public schools of the catchment area of Primary Care Unit at Hospital de Clínicas de Porto Alegre between March 2018 and May 2018. For the recruitment processes, we contacted the general coordinator of the municipal education secretary at Porto Alegre. We asked permission to contact the directors of public schools in the area previously mentioned. We reached the directors of five schools and the estimated number of adolescents, where the eligible criteria was 800.
The team of researchers went to school to explain the purposes of research and ask parents to sign a consent form for the agreement of their participation and seek adolescents’ permission for their involvement in the study. They answered a structured questionnaire, which helped us obtain sociodemographic and health state of the adolescents. Inclusion criterion: All adolescents 12 to 17 years of age whose parents had signed the consent form to participate, and if they agree, they were included in the study. One parent of each adolescent also participated. Of a total of 800 adolescents and parents who were approached to participate in the survey, 298 consented and 286 provided enough data at initial evaluation for inclusion in these analyses, resulting in a 14.90% consent rate and 14.45% initial completion rate. The presence of females was predominant in the sample (60.8%), reflecting a similar composition of adolescents’ sample seen at schools. Parents completing the questionnaires were mostly mothers. Exclusion criteria: Adolescents with persistent or recurrent pain in the last six months, according to answers by parents, or those regularly using medications for pain treatment (eg, anti-inflammatory, analgesics, anticonvulsants, antidepressants, etc.).
The clinical convenience subsample underwent a multidisciplinary pain evaluation at Hospital de Clínicas de Porto Alegre, which is a large tertiary teaching hospital in the south of Brazil. Among the clinical subsample, females were predominant (55%), reflecting the composition of adolescents seen at the pain clinic. The mean age was 14.13 years (SD =2.15). Parents completing the questionnaires were predominantly mothers. Pain diagnoses assigned by the physician conducting the medical portion of the clinical evaluation included the following elements: gastroesophageal reflux (eg, complex regional pain syndrome [CRPS], n=11); headache (chronic daily, tension-type, migraine, n=6); musculoskeletal pain (eg, diffuse muscular, one or more joints, n=4); recurrent abdominal pain (n=2); and other pains (eg, chest pain, n=5). All subjects experiencing pain symptoms that recur for longer than three months past the average expected healing time lacks the acute warning function of physiological nociception.2 The pain score was not measured at the time of assessment.
The saliva to measure BDNF was collected in 154 healthy adolescents and all adolescents of the clinical subsample. The dosage was given to 146 adolescents, eight were excluded by insufficient material.
Self-Report Variables and BDNF dosage
Fear of Pain Questionnaire for adolescents (FOPQ-A) is a self-report inventory to evaluate pain-related fears is a 5-point Likert-type scale applied to children and adolescents.4 The FOPQ-C consists of 24 items and two subscales. Each item is rated on from 0 “strongly disagree” to 4 “strongly agree”. Factor 1 labeled avoidance (contains 11 items), whereas factor 2 labeled fear of pain (contains 13 items). Scores range from zero to 96.
Fear of Pain Questionnaire for parents (FOPQ-P) reports their own fear associated with the child’s pain experience. The item’s response format is on a 5-point Likert-type scale ranging from “strongly disagree” to “strongly agree”. It comprises 21 items. The original scale23 consists of four factors. Factor 1 labeled avoidance (contains six items). Factor 2 labeled fear of pain (contains seven items). Factor 3 labeled fear of school (contains four items). Factor 4 labeled fear of movement (contains four items). Additional details regarding the psychometric properties of the FOPQ-P are given in the results section. Total score range zero to 84.
The Pain Catastrophizing Scale for children (PCS-C) and the PCS for parents (PCS-P) assess negative thinking associated with pain for identification of individuals at risk for psychological consequences, which may be needed for further psychosocial assessment. It is comprised of 13 Likert items rated on a 5-point scale with both intensity and frequency information, with the following five levels of response: (0) not at all, (1) to a slight degree, (3) to a moderate degree, (4) to a great agree, and (5) all the time. The total score derivates the sum of items. Higher scores indicated higher levels of catastrophic thinking. Internal reliability for the current sample was 0.90 for the PCS-C and 0.88 for the PCS-P.27
Children’s Depression Inventory (CDI) is an instrument widely used in the USA (constructed by MariaKovács and Beck, United States).28 The CDI is a self-reported inventory prepared for children aged seven to 17 years. It comprises 27 items to measure the presence and severity of symptoms of depression as well as to assess its various relevant clinically dimensions: affectivity (eg, mood lowered, loneliness and irritability); cognitive (eg, negative self-image, self-blame and negative expectations in a decision); motivational (eg, seclusion, avoidance and suicidal ideation); vegetative (eg, appetite and sleep disturbance) and psychomotor.29 Higher scores indicated the higher severity of symptoms. Internal reliability for the current sample was 0.91.
Functional Disability Inventory (FDI)30 is a scale that assesses children’s self-reported difficulty in physical and psychosocial functioning due to their physical health. The scale consists of 15 items concerning perceptions of activity limitations during the past two weeks. Higher scores indicate greater disability, and total scores are obtained by summing the items. The original FDI has good reliability and validity.31 Higher scores indicated higher levels of disability. Internal reliability for the current sample was 0.91.
Strengths and Difficulties Questionnaire (SDQ) can be completed by 11–16 year olds themselves.32,33 The SDQ can be used for screening, as part of a clinical assessment, as a treatment-outcome measure and as a research tool.34,35 The SDQ asks about 25 attributes, some positive and others negative; respondents use a 3-point Likert scale to indicate how far each attribute applies to the target child. The 25 items are divided between five scales of five items each, generating scores for emotional symptoms, conduct problems, hyperactivity-inattention, peer problems and prosocial behavior; are summed to create a total difficulties score. Internal reliability for the current sample was 0.78.
The dosage of BDNF: Participants who received instruction had not consumed any food or drink, or brushed their teeth, for two hours before sample collection. All participants collected approximately 3 ml of saliva unstimulated by passive expectoration into a 5-ml conical tube. Tubes were centrifuged for 10 mins at 4,500 rpm at 4 °C. Upon thawing, the samples were centrifuged once more to ensure complete debris removal.36 Saliva was stored at −80 °C. Saliva-mediator concentrations were determined using BDNF (Chemicon CYT306, lower detection limit 7.8 pg/mL; EMD Millipore, Billerica, MA, USA) enzyme-linked immunosorbent assay kits, according to the manufacturer’s instructions.
Statistical analysis
Descriptive statistics were conducted to examine the underlying assumptions of normality for all concerned variables. We assessed the items on the BrP-FOPQ for adolescents and parents’ measures to check significant skewing or kurtotic response patterns. Internal consistency was assessed using Cronbach’s alpha for the BrP-FOPQ-A and BrP-FOPQ-P measures to compare it with the English version. Item-total correlations were calculated for both tests. Confirmatory factory analysis (CFA) for the FOPQ-C BrP-FOPQ-A as well as the maximum likelihood factor analyses with oblique rotation was conducted. For the BrP-FOPQ-P factor analysis, the principal component analysis was performed using Promax rotation. For both measures, a loading of items of 0.3 was considered relevant, and thus when the loading was less than 0.3, the item was not retained.37 Factors with eigenvalues greater than one were also excluded. Convergent validity was evaluated by the Pearson’s correlation coefficient between BrP-FOPQ-A total scores and subscales and the BrP-FOPQ-P with the following tools that evaluate the aspects related to pain: depressive symptoms, pain catastrophizing, emotion and conducts problems and physical and psychosocial functioning due to their physical health. We expected a moderate positive correlation coefficient between the BrP-FOPQ-A total scores and other scales, not exceeding 0.7, as this criterion is considered satisfactory for establishing construct validity between scales that measure a comparable concept.38 Criterion-group’s validity was assessed by screening the accuracy of the BrP-FOPQ for adolescents and parents in distinguishing between chronic pain subjects and pain-free healthy control subjects. We used the nonparametric receiver operating characteristics (ROC) analysis. The area under the curve (AUCs) with exact binomial of 95% confidence intervals (CI) is presented. Standard errors (SEs) were calculated using Hanley’s method.39 The cutoff values with the highest Youden index, with 90% sensitivity and 100% specificity, are presented for BrP-FOPQ-A and QIF with a ROC AUC of 0.70. A Generalized Mixed Model demonstrated the main effect of the group for the BrP-FOPQ-A scores adjusted by gender and age, which was used to assess the correlation between the BrP-FOPQ-A and the BDNF. A priori sample size was estimated based on the ratio of the number of volunteers to the number of items. In this case, the FOPQ-A has 24 items. Based on this criterion, we needed 240 volunteers. Considering the potential of ending up with insufficient data, we increased the sample size by 15%.24,40 For all statistical analyses, significance was set at P<0.05. Data were analyzed using SPSS version 22.0 (IBM, Armonk, NY, USA).
Results
Phase III: Assessment of psychometric properties and the validity of the final version of the BrP-FOPQ-A and BrP-FOPQ-P
Sample characteristics
Table 1 presents the demographic characteristics, depressive symptoms, pain catastrophizing, emotion and conducts problems and physical and psychosocial functioning due to their physical health. There was a disproportionate number of females (n=174) in our sample. The mean score of the BrP-FOPQ-A for males was 29.13 (17.17), and for females, it was 41.62 (16.36) (t= −6.16, P<0.001). The mean score of the BrP-FOPQ-A for the total subject sample was 36.62 (SD 17.72). The median was 36, and the range interquartile [(IQ25-75) 24; 50].
Psychometric properties of the BrP-FOPQ-A and BrP-FOPQ-P
The BrP-FOPQ-A final 24-item had strong internal consistency (α=0.92). The sample mean for the total scale was 34.13 (SD =15.12). The reliability of subscale of Fear of Pain and Avoidance of Activities were α=0.89 and α=0.86, respectively. The sample mean for the subscale of Fear of Pain was 20.00 (SD =9.96), and the sample mean for the subscale of Avoidance of Activities was 16.25 (SD =8.25).
The BrP-FOPQ-P final 21-item had strong internal consistency (α=0.91). In BrP-FOPQ-P, Factor 1 labeled avoidance (contains five items α=0.73); the sample mean was 5.89 (SD =4.23). Factor 2 labeled fear of pain and movement (contains nine items, α=0.82); the sample mean was 20.44 (SD =7.99). Factor 3 labeled fear of school (contains three items α=0.62); the sample mean was 3.30 (SD =2.68).
A CFA was performed to investigate whether the original English FOPQ-C study dimensionality and factor-loading pattern were like the Brazilian subject sample. The mean (SD) of each item and the standardized factor loadings, including the specific BrP-FOPQ-A question items, contributing to factors with item loading higher than 0.3 are shown in Table 2. No items violated assumptions of normality (skew and/or kurtosis >2.0). Twenty four items were entered into a maximum likelihood factor analysis with oblique rotation. The mean (SD) of each item is presented in Table 2. According to the factor structure suggested by Cattell’s elbow criteria on the screen plot, 2-factor solution best explained the structure of the BrP-FOPQ-A with 46.09% of the variance accounted for. The two factors were intercorrelated (see Table 2). The avoidance factor comprises ten items and fear of pain comprises 14 items. The cross-cultural validity of the BrP-FOPQ-A to Brazilian population was demonstrated by the high factorial load of all items and by the similarity of the best-explained structure with the original scale.
 |
Table 2 Factor loadings for Brazilian Portuguese Fear of Pain Questionnaire for Adolescents (n=286) |
A confirmatory factor analysis was performed to investigate whether the original English FOPQ-P study dimensionality and factor-loading pattern were like the Brazilian subject sample. The mean (SD) of each item and the standardized factor loadings, including the specific BrP-FOPQ-P question items, contributing to factors with item loading higher than 0.3 are shown in Table 3. No items violated assumptions of normality (skew and/or kurtosis >2.0). Twenty one items were entered into a maximum likelihood factor analysis with oblique rotation. According to the factor structure suggested by Cattell’s elbow criteria on the screen plot, 3-factors solution best explained the structure of the FOPQ-P with 50.76% of the variance accounted for across the three subscales. The three factors were intercorrelated (see Table 5).The avoidance factor comprises nine items;the fear of pain comprises nine items; and the fear of school factor comprises three items.
 |
Table 3 Factor loadings for Brazilian Portuguese Fear of Pain Questionnaire for Parents (n=286) |
 |
Table 4 Intercorrelation, mean, and SD values for Fear of Pain Questionnaire for Parents (n=286) |
 |
Table 5 Intercorrelation among adolescents and parent fear of pain questionnaire with depressive, catastrophizing and disability symptoms (N=286) |
In the Brazilian version, parents better understood the “movement” factor as an avoidance response. This possibly justifies the migration of the items that had constituted the movement factor in the original scale to the avoidance factor in the current study. In the same way, in our sample, item 6, “my child’s pain controls my life”, presented higher factorial load in the factor of the fear of pain, whereas in the English version, this item is in the avoidance activities factor. We interpreted migration of this item based on the premise that the fear of parents can do make children’s pain worse. The item 4, “I believe that my child cannot go back to school until his/her pain is treated”, did not fit in the “school” factor in our study, which is possibly because for parent’s interpretation of how the child was out the school environment.
Although two items migrated to another factorin the factorial structure of BrP-FOPQ-P, their content is congruent with that the migration factor. Moreover, all items demonstrated high factorial load with a variance explained by the best structure, likewise the original scale. Thus, these results demonstrate the cross-cultural validity of the BrP-FOPQ-A.
The intercorrelation between the BrP-FOPQ-A and BrP-FOPQ-P total scale and subscale scores is displayed in Table 4. Concerning subscales, both fear and avoidance subscale for the child, was positively associated with the total score to BrP-FOPQ-P (r=0.50 and 0.56), respectively.
Convergence validity for the total score of BrP-FOPQ-A measure is supported with significant relations found for the adolescents’ depressive symptoms, catastrophizing and greater functional disability. Convergence-related validity is also supported by significant associations between higher BrP-FOPQ-A scores of subscales (Fear of Pain and Activity Avoidance), respectively. All variables were positively correlated with higher levels of pain related to fear for the BrP-FOPQ-A in either the total scale or subscale scores. Significant correlations were detected concerning pain catastrophizing and emotional pain with the BrP-FOPQ fear subscale (r=0.79 and r=0.50), respectively. Concerning avoidance subscale for the child, it was also most highly correlated with the pain catastrophizing in the adolescents (r=0.68).The fear of pain subscale was positively correlated to emotional symptoms (r=0.54).
The responsiveness of the BrP-FOPQ-A can be seen by the mean (SD) of the total score, in healthy subjects, and for the ones with chronic pain, it was 36.02 (17.47) vs 42.10(19.33) (P=0.08). While in healthy subjects and those with chronic pain, the score in the subscales of fear was 19.95 (9.94) vs 24.10(9.47) (0.03) and avoidance was 16.06 (8.70) vs 18.00(10.60) (P=0.26), respectively. It is possible to see that the scores of scale and subscales are tending to be higher in adolescents with chronic pain. It means that these tools have properties to capture differences between volunteers free of pain and those who have chronic pain.
Criterion-group validity was assessed by the screening accuracy of the discriminate between chronic pain subjects (n=257) and healthy control subjects (n=29). Nonparametric ROC analysis of BrP-FOPQ-A showed AUCs with exact binomial 95% confidence intervals (CI) on BrP-FOPQ-A. The cutoff point was 11, and the AUC was 0.72 (CI 95%=0.57–0.85). The sensibility was 1, and the specificity was 0.92. The BrP-FOPQ-P for a cutoff point was equal to 12; the AUC was 0.69 (CI 95%=0.58–80); sensibility was equal 1, and the specificity was 0.92. These findings showed that, according to this cutoff point, both BrP-FOPQ were classified correctly (ie, specificity), and more than 90% of them presented chronic pain conditions.
Assessment of saliva BDNF and its correlation with the Br-FOPQ-A scores
The subjects of a subsample (n=146) from the identified sample previously detailed were made the dosage of BDNF on the saliva. Age of healthy adolescents in this subsample was 13.83 (1.83) and 14.00 (1.88) for patients. Years of school for healthy subjects was 8.03 (1.57), and for patients, it was 7.92 (1.32). The number of females in the sample of healthy subjects was 75 (56.8%) and 8 (53.33%) for that of subjects with chronic pain. The saliva BDNF measured in healthy adolescents showed a mean (SD) equal to 2.66 (1.93), and in adolescents with chronic pain, it was 5.03 (4.02).
A Generalized Mixed Model showed the main effect for group for the Br-FOPQ-A scores. Adolescents with chronic pain presented higher scores compared to healthy subjects, Wald χ2= (17.80; Df=1, P<0.0001). The results of multivariate analysis after adjustments by multiple comparisons by Bonferroni Test are presented in Table 6. The model revealed that the BDNF was positively correlated with the score of BrP-FOPQ-A when considering the age. That is, older adolescents presented higher levels of saliva BDNF; similarly, female gender presented higher levels compared to male. Also, the model confirms that subjects with chronic pain even considered the effect of gender and age and showed higher levels of BDNF.
Discussion
The present study displays data about the cross-cultural, adapted English version of the FOPQ for the child and parent to Brazilian Portuguese by the semantic equivalence with the original scale. Also, it demonstrated the psychometric properties assessed by internal consistency, reliability, construct validity, convergence validity, criterion-group validity and the responsiveness for both, BrP-FOPQ for adolescents and parents to identify the severity of fear of pain, according to an adolescent with a pain condition, and pain-free healthy control subjects. The process of translating and back-translating the English FOPQ for child and parents to Brazilian Portuguese version was carried out by stringently following established guidelines.41 A panel of experts, as well as persons with experience in translate translating instruments, assisted us in maintaining the semantic equivalence and the content of items according to cultural variation. The set of items of the BrP-FOPQ for both child and parents presented satisfactory internal reliability with Cronbach’s alpha coefficients higher than 0.9, which is similar to the original English version.4,23 These results indicated an adequate construct validity and internal consistency of these FOPQ translated and adapted to Brazilian Portuguese.42
The content validity is evidenced by the high scores of the questionnaire items for readability, clarity and comprehensiveness as demonstrated by the scores on the VAS in the assessment of the expert’s committee. However, significant and moderate correlations of the total score of BrP-FOPQ-A and its subscales with the child depression symptoms, pain catastrophizing and greater functional disability confirm the construct validity (see Table 5). These results underscore the convergent validity by different measures, which assess the same concept in different ways yields.43 Moreover; they suggest that catastrophizing pain overlaps with the fear of pain phenomenon, which leads to behavioral and emotional changes in order to avoid the suffering related to chronic pain. Another measure that showed the theoretical construct of the BrP-FOPQ-A is the criterion-group validity to differentiate those with chronic pain from those without the diagnosis. Thereby, this result reveal how meaningful fear and avoidance is in the practical use of the instrument.43
Indeed, the fear of pain and the avoidance activities due to pain are reactions that involve emotional facilitates that can encode and help the retrieval of information efficiently. Thus, the positive correlation between BrP-FOPQ-A and pain catastrophizing can be understood in this line, because the catastrophizing pain scale is a cognitive construct characterized by feelings of helplessness, active rumination and excessive magnification toward the painful situation.44 The pain catastrophizing is distinct to fear of pain, which is an emotional construct that comprises negative affect reaction to pain that provokes escape or avoidance. Moreover, we observed that the BrP-FOPQ-A shows a positive correlation with other measures related to depressive symptoms, emotion and conducts problems and physical and psychosocial functioning due to their physical health. All associations were small and moderate, but they point in the same direction that the fear of pain and avoidance behavior identifies subjects prone to a maladaptive response to the consequences of pain-related behavior.44
These results demonstrated that BrP-FOPQ-A and BrP-FOPQ-P are multidimensional constructs with items that contribute to discriminate fear avoidance of pain either in the adolescents or in parents. The factorial analyses of BrP-FOPQ-A showed that the best solution was a factorial structure with two factors, which comprise a set of items to assess fear and avoidance similar to that proposed in the original scale.4 The CFA demonstrated that all items of both factors to assess fear and avoidance in the BrP-FOPQ-A showed a load factorial higher than 0.5. This result indicates that all elements of each factor of the CFA converge to a common point to constitute a construct. Thus, our result confirms how well our analyzed variables represent the original constructs.4 Further, the BrP-FOPQ-A demonstrated that the Brazilian version presents satisfactory properties to discriminate subjects with higher fear pain and avoidance activities, with specificity higher than 90% for a cutoff point equal to 11.
Indeed, this scale showed a satisfactory accuracy to identify children with a higher fear of pain and avoidance of activities due to pain. This is a positive feature of these measures as they permit planning specific interventions, such as educative programs to improve outcomes and optimize the cost-utility for long-term pain management. Given the previous evidence, a decreased fear of pain in patients exposed to feared activities may make them more confident on their ability to perform such movements, which might be the reason for the readjustment of their beliefs. At the same way, the disconfirmation of negative feelings of threat by performing feared activities have probably resulted in improved pain, hypervigilance behavior, a sense of danger, decreased anxiety and catastrophizing.45 These approaches to treating fear of pain and avoidant activities are based on the fear-avoidance model (FA). The FA model finds support in clinical outcomes into musculoskeletal pain conditions, such as knee pain,46 neck pain47 and fibromyalgia.48 Little is known about the neural correlates postulated in the paradigm of fear and avoidance model despite the FA model’s pervasiveness.
The BrP-FOPQ-P CFA found the best solution with a structure of three factors. Cultural differences may explain the distinct distribution of items compared to the original scale. This explanation is plausible as a three factor structure showed the best relationship between the content of elements of each factor (fear of pain, fear of school and avoidance activities). Another finding that indicates an adequate factorial structure of the BrP-FOPQ-P was its properties to discriminate the healthy adolescent’s sample compared to the sample of individuals with chronic pain.23 In the Brazilian version, the migration of items that constituted the “movement” factor was better understood by parents as an avoidant response it can also be interpreted in the sense that parents’ fear and anxiety can make children’s pain worse. Also, it is theoretically plausible that a more catastrophic description of these items induced bad postures of parents, which can have caused a positive reinforcement of their children’s fear and avoidance behavior.49
The close correlation between the BrP-FOPQ-P scale with the total score and subscales scores of BrP-FOPQ-A shown in Table 4 indicates that an effect of vicarious conditioning can influence the child’s behavior. According to conceptual postulates in the vicarious conditioning, the individual child’s behavior can be acquired by the observation of their parent’s model.50 For example, if a child saw that their parents demonstrate an aversion to a stimulus, they may copy their parents’ model. Even though the present result related to fear and avoidance can be explained by in part by this conditioning, we need to have parsimony in the interpretation of this possible association, once a correlation between the scores in the scales of pain catastrophizing of child with the score of the pain catastrophizing of their parents was not observed. Another factor to consider is the influence of age in the cognitive aspects.51
Our results highlight that the score in the BrP-FOPQ-A is positively correlated with saliva BDNF after to adjust for age, and at the same way, we found higher levels of BDNF in female and in the subsample of chronic pain. This is an exciting finding that confirms the validity of BrP-FOPQ-A using an objective biological marker of the neuroplasticity processes, which presents compelling evidence of its association with pain conditions and different measures of neuroplasticity as demonstrated in pre-clinical and clinical studies.52–54 It is important to emphasize that, during adolescence, the fear circuitry is mainly plastic. Also, the BDNF has been established as a significant regulator of adult fear circuitry function as well as expression of fear behavior.55 The neurotrophic hypothesis is one of the comprehensive molecular frameworks thought to underlie mood and anxiety disorder. It postulates that a sustained environmental and physiological stressor leads to altered neural plasticity in key regions implicated in anxiety and fear responses, including the hippocampus, prefrontal cortex and amygdala.56 It is important to point out that chronic stress increases BDNF expression in the amygdala, whereas it has an opposite effect in the hippocampus. Although we cannot evaluate the neurotrophic factor in specific structures in a clinical study, the positive correlation between saliva BDNF and the score of BrP-FOPQ-A permits to identify the relationship between changes in the neurotrophic factor and fear to pain and avoidance activities. Therefore, a higher score on the BrP-FOPQ-A may be useful in helping to identify subjects prone to fear pain and avoidance or those with a higher propensity to develop chronic pain. Future studies should investigate if BrP-FOPQ-A scores can be a useful self-reported screening that might reduce the impact of one of the most critical factors that lead to chronic pain-related disability.57
Main limitations of our study that should be addressed. First, a cross-sectional design does not allow concluding causal relationships between the increase in the BDNF with the higher score of BrP-FOPQ-A. Second, we selected our sample of chronic pain in a specialized university pain clinic, and they presented different pain sites that may have distinct limitations in daily life, treatments, and relevant fears. Accordingly, the sample of chronic pain in our study might be representative of patients with complex pain problems. Third, the sample consisted primarily of healthy subjects and female adolescents. These adolescents were recruited from public schools, where usually the social income family is lower. Although this demographic pattern might limit the generalizability of our results, this is a corresponding pattern of our country and several other countries. However, one could realize that despite the difference of sample, we found similar results than those reported in the study of the author’s scale with subjects recruited at a tertiary pain clinic.4,23 Fourth, mostly mothers completed the parent’s measures in this study, which might be a limitation of the current study, since extensive literature has shown differences between sex in pain perception and the emotional reaction to pain.58 Thereby, we can consider that the response to confrontation of avoided activities due to pain and reengagement with activities of daily living change according to mothers’ or fathers’ perception. Fifth, the study is based on self-reported measures. Thus, the comprehension of the content of the assessment instruments may have implications for the internal validity of the survey as there may be an overlap in the constructs measured in the study. Finally, further longitudinal studies are required with a more significant number of clinical samples and their parents to examine how prior parent fears and avoidance behaviors influence subsequent child avoidant behaviors and outcomes.
This survey provides evidence for the consistent psychometric properties of the BrP-FOPQ-A and BrP-FOPQ-P. It demonstrates good discriminative properties, and the validity of BrP-FOPQ-A was confirmed by its positive correlation with depressive symptoms, emotion and conducts problems, physical and psychosocial functioning due to their physical health and a biological marker of neuroplasticity (ie, BDNF). Therefore, these results suggest that both scales represent valuable instruments for use in scientific studies and in the clinical setting involving early adolescents prone to develop chronic pain or institute therapeutic approaches to improve the negative feelings related to fear of pain.
Acknowledgments
This research was supported by grants and material support from the following Brazilian agencies: Committee for the National Council for Scientific and Technological Development (CNPq; grants to ILST and WC), the Postgraduate Program in Medical Sciences at the School of Medicine of the Federal University of Rio Grande do Sul (material support), the Postgraduate Research Group at the Hospital de Clínicas de Porto Alegre (FIPE-HCPA; material support), and the Brazilian Innovation Agency (FINEP; process number 1245/13; to WC).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Melzack R. Pain and the neuromatrix in the brain. J Dent Educ. 2001;65(12):1378–1382.
2. Rolf-Detlef T, Winfried R, Antonia B, et al. Chronic pain as a symptom or a disease: the IASP classification of chronic pain for the International Classification of Diseases (ICD-11). Pain. 2019;160:9–27.
3. King S, Chambers CT, Huguet A. The epidemiology of chronic pain in children and adolescents revisited: a systematic review. Pain. 2011;152(12):2729–2738. doi:10.1016/j.pain.2011.02.053
4. Simons LE, Sieberg CB, Carpino E, et al. The Fear of Pain Questionnaire (FOPQ): assessment of pain-related fear among children and adolescents with chronic pain. J Pain. 2011;12(6):677–686. doi:10.1016/j.jpain.2010.12.008
5. la Buissonnière-Ariza V, Hart D, Schneider SC. Quality and correlates of peer relationships in youths with chronic pain. Child Psychiatry Hum Dev. 2018;49(6):1–10.
6. Parr JJ, Borsa PA, Fillingim RB. Pain-related fear and catastrophizing predict pain intensity and disability independently using an induced muscle injury model. J Pain. 2012;13(4):370–378. doi:10.1016/j.jpain.2011.12.010
7. van Wijk AJ, Hoogstraten J. Dutch translation of the fear of pain questionnaire: factor structure, reliability and validity. Eur J Pain. 2006;10(6):479–486. doi:10.1016/j.ejpain.2005.06.008
8. McCracken LM, Gross RT, Aikens J, Carnrike CLM. The assessment of anxiety and fear in persons with chronic pain: a comparison of instruments. Behav Res Ther. 1996;34(11–12):927–933. doi:10.1016/S0005-7967(96)00057-5
9. Turk DC, Wilson HD. Fear of pain as a prognostic factor in chronic pain: conceptual models, assessment, and treatment implications. Curr Pain Headache Rep. 2010;14(2):88–95. doi:10.1007/s11916-010-0094-x
10. Varni JW, Waldron SA, Gragg RA. Development of the Waldron/Varni pediatric pain coping inventory. Pain. 1996;67(1):141–150. doi:10.1016/0304-3959(96)03077-1
11. Lynch-Jordan AM, Kashikar-Zuck S, Szabova A, et al. The interplay of parent and adolescent catastrophizing and its impact on adolescents’ pain, functioning, and pain behavior. Clin J Pain. 2013;29(8):681–688. doi:10.1097/AJP.0b013e3182757720
12. Tupper SM, Rosenberg AM, Pahwa P, Stinson JN. Pain intensity variability and its relationship with quality of life in youths with juvenile idiopathic arthritis. Arthritis Care Res. 2013;65(4):563–570. doi:10.1002/acr.21850
13. Eller-Smith O, Nicol A, Christianson J. Potential mechanisms underlying centralized pain and emerging therapeutic interventions front. Cell Neurosci. 2018;12:35. doi:10.3389/fncel.2018.00035
14. Quach TT, Lerch JK, Honnorat J, et al. Neuronal networks in mental diseases and neuropathic pain: beyond brain derived neurotrophic factor and collapsin response mediator proteins. World J Psychiatry. 2016;6(1):18. doi:10.5498/wjp.v6.i1.18
15. Stefani LC, IL DST, De Souza ICC, et al. BDNF as an effect modifier for gender effects on pain thresholds in healthy subjects. Neurosci Lett. 2012;514(1):62–66. doi:10.1016/j.neulet.2012.02.057
16. Allen AL, McCarson KE. Estrogen increases nociception-evoked brain-derived neurotrophic factor gene expression in the female rat. Neuroendocrinology. 2005;81:193–199. doi:10.1159/000087002
17. Harris AP, Lennen RJ, Brydges NM, et al. The role of brain‐derived neurotrophic factor in learned fear processing: an awake rat fMRI study. Genes Brain Behav. 2016;15(2):221–230. doi:10.1111/gbb.12277
18. Ledoux J. Emotion circuits in the brain. The science of mental health: fear and anxiety. Annu Rev Neurosci. 2000;23:155–18. doi:10.1146/annurev.neuro.23.1.155
19. Korte M, Carroll P, Wolf E, Brem G, Thoenen H, Bonhoeffer T. Hippocampal long-term potentiation is impaired in mice lacking brain-derived neurotrophic factor. Proc Natl Acad Sci USA. 1995;92:8856–8860. doi:10.1073/pnas.92.19.8856
20. Minichiello L, Korte M, Wolfer D. Essential role for TrkB receptors in hippocampus-mediated learning University of Heidelberg. Neuron. 1999;24:401–414.
21. Tsukinoki K, Saruta J, Sasaguri K, et al. Immobilization stress induces BDNF in rat submandibular glands. J Dent Res. 2006;85:844–848. doi:10.1177/154405910608500913
22. Meiera ML, Stampflib P, Humphreysa BK, et al. The impact of pain-related fear on neural pathways of pain modulation in chronic low backpain. Pain Rep. 2017;9–2:e601. doi:10.1097/PR9.0000000000000601
23. Simons LE, Smith A, Kaczynski K, et al. Living in fear of your child’s pain : the parent fear of pain questionnaire. Pain. 2015;156:694–702.
24. Prinsen CAC, Mokkink LB, Bouter LM. COSMIN guideline for systematic reviews of patient-reported outcome measures. Qual Life Res. 2018;27(5)1147–1157.
25. Beaton DE, Bombardier C, Guillemin F, Ferraz MB. Guidelines for the process of cross-cultural adaptation of self-report measures. Spine. 2000;25:3186–3191. doi:10.1097/00007632-200012150-00014
26. Deyo RA. Pitfalls in measuring the health status of Mexican Americans: comparative validity of the English and Spanish sickness impact profile. Am J Public Health. 1984;74(6):569–573. doi:10.2105/AJPH.74.6.569
27. Crombez G, Bijttebier P, Eccleston C. The child version of the pain catastrophizing scale (PCS-C): a preliminary validation. Pain. 2003;104(3):639–646. doi:10.1016/S0304-3959(03)00121-0
28. Smucker MR, Craighead WE, Craighead LW, Green BJ. Normative and reliability data for the children ’s depression inventory. J Abnorm Child Psychol. 1986;14(1):25–39. doi:10.1007/BF00917219
29. Siegel LJ. Factor analysis of the children’s. Psychol Rep. 1983;53(3Pt 1):759–763. doi:10.2466/pr0.1983.53.3.759
30. Walker LS, Greene JW. The functional disability inventory : measuring a neglected dimension of child health status. J Pediatr Psychol. 1991;16(1):39–58. doi:10.1093/jpepsy/16.1.39
31. Claar RL, Walker LS. Functional assessment of pediatric pain patients : psychometric properties of the functional disability inventory. Ból. 2006;121:77–84.
32. The Strengths GR. Difficulties questionnaire: a research note. J Child Psychol Psychiatry. 1997;38(5):581–586. doi:10.1111/j.1469-7610.1997.tb01545.x
33. Meltzer H. The strengths and difficulties questionnaire: a pilot study on the validity of the self-report version. Intern Rev Psych. 1998;130:125–130.
34. Garr ME, Yates P, Higginson I. Child and adolescent mental health service use HoNOSCA as an outcome measure. Br J Psych. 2000;177(1):52–59. doi:10.1192/bjp.177.1.52
35. Goodman R, Renfrew D. Predicting type of psychiatric disorder from strengths and Dif ® culties Questionnaire (SDQ) scores in child mental health clinics in London and Dhaka. Eur Child Adole Psych. 2000;134:129–134. doi:10.1007/s007870050008
36. Mandel AL, Ozdener H, Utermohlen V. Brain-derived neurotrophic factor in human saliva: ELISA optimization and biological correlates. J Immunoassay Immunochem. 2011;32:18–30. doi:10.1080/15321819.2011.538625
37. DeVellis RF. Scale Development: Theory and Applications.
38. Terwee CB, Van Der Slikke RM, Van Lummel RC, et al. Self-reported physical functioning was more influenced by pain than performance-based physical functioning in knee-osteoarthritis patients. J Clin Epidemiol. 2006;59(7):724–731. doi:10.1016/j.jclinepi.2005.11.019
39. Ruopp MD, Perkins NJ, Whitcomb BW, et al. Youden index and optimal cut-point estimated from observations affected by a lower limit of detection. Biom J. 2008;50:419–430.
40. Rouquette A, Falissard B, Rouquette A, et al. Sample size requirements for the internal validation of psychiatric scales. Int J Methods Psychiatr Res. 2011;20:235–249.
41. Guillemin F, Bombardier C. Cross-cultural adaptation of health-related quality of life measures: literature review and proposed guidelines. J Clin Epidemiol. 1993;46(12):1417–1432.
42. Taber KS. The use of Cronbach’ s alpha when developing and reporting research instruments in science education. Res In Scien Edu. 2018;48(6):1273–1293. doi:10.1007/s11165-016-9602-2
43. Bolarinwa OA. Principles and methods of validity and reliability testing of questionnaires used in social and health science researches. Niger Postgrad Med J. 2015;22(4):195–201. doi:10.4103/1117-1936.173959
44. Drahovzal DN, Stewart SH, Sullivan MJL. Tendency to catastrophize somatic sensations: pain catastrophizing and anxiety sensitivity in predicting headache. Cogn Behav Ther. 2006;35(4):226–235. doi:10.1080/16506070600898397
45. Barke A, Preis MA, Schmidt-Samoa C, et al. Neural correlates differ in high and low fear-avoidant chronic low back pain patients when imagining back-straining movements. J Pain. 2016;17(8):930–943. doi:10.1016/j.jpain.2015.12.006
46. Sanchis-alfonso V. Changes in catastrophizing and kinesiophobia are predictive of changes in disability and pain after treatment in patients with anterior knee pain. Knee Surg Sports Traumat Arth. 2014;22(10):2295–2300. doi:10.1007/s00167-014-2968-7
47. Landers MR, R V C, C V B, et al. The use of fear-avoidance beliefs and nonorganic signs in predicting prolonged disability in patients with neck pain. Man Ther. 2008;13:239–248. doi:10.1016/j.math.2007.01.010
48. Nijs J, Roussel N, Van OJ. Fear of movement and avoidance behaviour toward physical activity in chronic-fatigue syndrome and fibromyalgia: state of the art and implications for clinical practice. Clin Rheumatol. 2013;1121–1129. doi:10.1007/s10067-013-2277-4
49. Asmundson GJG, Noel M, Petter M, et al. Pediatric fear-avoidance model of chronic pain: foundation, application and future directions. Pain Res Manag. 2012;17(6):397–405. doi:10.1155/2012/541751
50. Bandura A. Self-efficacy. In: Ramachaudran VS, editor. Encyclopedia of human behavior. New York: Academic Press. Reprinted in H. Friedman [Ed.]. Encyclopedia of mental health. San Diego: Academic Press, 1998;4:71-81.
51. Salthouse TA. Relations Between Cognitive Abilities and Measures of Executive Functioning. Neuropsychology. 2005;19:532-545.doi:10.1037/0894-4105.19.4.532
52. Botelho LM, Morales-Quezada L, Rozisky JR, et al. A framework for understanding the relationship between descending pain modulation, motor corticospinal, and neuroplasticity regulation systems in chronic myofascial pain. Front Hum Neurosci. 2016;10:1–12. doi:10.3389/fnhum.2016.00308
53. Caumo W, Deitos A, Carvalho S, et al. Motor cortex excitability and BDNF levels in chronic musculoskeletal pain according to structural pathology. Front Hum Neurosci. 2016;10:1–15. doi:10.3389/fnhum.2016.00357
54. Zanette SA, Dussan-sarria JA, Souza A, et al. Higher serum S100B and BDNF levels are correlated with a lower pressure-pain threshold in fibromyalgia. Mol Pain. 2014;10:1–9. doi:10.1186/1744-8069-10-46
55. Andero R, Choi DC, Ressler KJ. BDNF-TrkB receptor regulation of distributed adult neural plasticity, memory formation, and psychiatric disorders. Prog Mol Biol Transl Sci. 2014;122:169-192. doi: 10.1016/B978-0-12-420170-5.00006-4
56. Dincheva I, Lynch NB, Lee FS. The role of BDNF in the development of fear learning. Depress Anxiety. 2016;33(10):907–916. doi:10.1002/da.2016.33.issue-10
57. Vlaeyen JW, Linton SJ. Fear-avoidance model of chronic musculoskeletal pain: 12 years on. Pain. 2012;153(6):1144–1147. doi:10.1016/j.pain.2011.12.009
58. Bartley EJ, Fillingim RB. Sex differences in pain: a brief review of clinical and experimental findings. Br J Anaesthesia. 2013;111(1):52–58. doi:10.1093/bja/aet127
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.