Back to Journals » Journal of Hepatocellular Carcinoma » Volume 10
The Extended Surgical Concepts for Hepatocellular Carcinoma in the Era of Immune Checkpoint Inhibitors
Authors Hsu HM, Tsai HI, Lee WC , Wang CC, Yu MC , Lin SM, Lin CY , Wu CH, Lee CW
Received 1 September 2023
Accepted for publication 18 October 2023
Published 24 October 2023 Volume 2023:10 Pages 1873—1880
DOI https://doi.org/10.2147/JHC.S433598
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Mohamed Shaker
Hsiao-Mei Hsu,1 Hsin-I Tsai,2– 4 Wei-Chen Lee,4,5 Chih-Chi Wang,6,7 Ming-Chin Yu,3,4,8 Shi-Ming Lin,4,9 Chun-Yen Lin,4,9 Chi-Huan Wu,4,9 Chao-Wei Lee3– 5
1Department of Surgery, Linkou Chang Gung Memorial Hospital, Taoyuan, Taiwan; 2Department of Anesthesiology, Linkou Chang Gung Memorial Hospital, Taoyuan, Taiwan; 3Graduate Institute of Clinical Medical Sciences, Chang Gung University, Taoyuan, Taiwan; 4College of Medicine, Chang Gung University, Taoyuan, Taiwan; 5Division of General Surgery, Department of Surgery, Linkou Chang Gung Memorial Hospital, Taoyuan, Taiwan; 6Division of General Surgery, Department of Surgery, Kaohsiung Chang Gung Memorial Hospital, Kaohsiung, Taiwan; 7Division of General Surgery, Department of Surgery, Chiayi Chang Gung Memorial Hospital, Chiayi, Taiwan; 8Department of Surgery, New Taipei Municipal Tu-Cheng Hospital (Built and Operated by Chang Gung Medical Foundation), New Taipei City, Taiwan; 9Department of Gastroenterology and Hepatology, Linkou Chang Gung Memorial Hospital, Taoyuan, Taiwan
Correspondence: Chao-Wei Lee, Division of General Surgery, Department of Surgery, Chang Gung Memorial Hospital, Linkou Medical Center, Guishan, No. 5, Fuxing St., Guishan District, Taoyuan City, 33305, Taiwan, Tel +886-975366192, Email [email protected]
Abstract: Surgical resection remains one of the most effective curative therapies for HCC. However, the majority of patients have advanced unresectable diseases upon presentation. It is of paramount importance to raise the resectability of patients with HCC. The remarkable objective response rate reported by Phase III IMbrave150 trial has led to the concept of “Atezo/Bev followed by curative conversion (ABC conversion)” for initially unresectable HCC. With this revolutionary treatment strategy, the concept of surgical resection for HCC should be amended. The current opinion illustrated three extended surgical concepts, which could be integrated into clinical practice in the era of immune checkpoint inhibitors (ICI).
Keywords: hepatocellular carcinoma, liver resection, surgery, immune checkpoint inhibitors, immunotherapy
Introduction
As the most common primary malignancy of the liver, hepatocellular carcinoma or HCC is associated with morbidity and mortality. It ranks the 6th most common cancer worldwide, and in 2020, HCC has an estimated mortality of approximately 830,000 globally.1–4 In Taiwan, HCC is the second most common cause of death secondary to cancer leading to over 7300 deaths annually.5,6 Surgical resection, one of the most effective curative therapies for HCC, can achieve 5-year survival rate of around 70% in selected patients.3,7,8 However, the majority of patients have advanced unresectable diseases upon presentation. A previous study showed that only 5% to 40% of HCC patients in the absence of cirrhosis could undergo liver resection, and this number was even much lower if the patients are complicated with chronic liver disease or overt cirrhosis.9 In order to improve the prognosis of this dismal malignancy, it is therefore of urgent need to raise the resectability of patients with HCC.
Together with poor liver reserve, clinically relevant portal hypertension, and poor performance status, tumor factors also contribute significantly to this low resectable rate. The presence of major vascular invasion or multifocal bilobar disease is considered unresectable by most major clinical guidelines.10,11 The long-term survivals of these subgroups of patients are unsatisfactory after upfront liver resections. However, these patients in the meanwhile usually are not candidates for liver transplantations, and their treatment response to other locoregional therapies such as ablations or transarterial chemoembolizations (TACE) is generally poor. Fortunately, the advent of novel systemic therapies has revolutionized the treatment paradigm of unresectable HCC. The phase III IMbrave150 trial demonstrated that the use of atezolizumab in addition to bevacizumab (Atezo/Bev combination) could substantially prolong the disease-free survival (DFS) and overall survival (OS) of patients with unresectable HCC when compared with sorafenib.12–14 The objective response rate (ORR) reached 30% overall and 44% in patients with intermediate-stage HCC.13,14 The remarkable “tumor shrinkage” effect achieved by Atezo/Bev has led to the concept of “Atezo/Bev followed by curative conversion (ABC conversion)” coined by Professor Masatoshi Kudo.15,16 This new concept has been verified by a recent study that 35% of unresectable and TACE-unsuitable intermediate-stage HCC achieved complete response (CR) after ABC conversion, and 23% of patients overall were drug-free and recurrence-free.17 With this revolutionary treatment strategy, the concept of surgical resection for HCC should be amended. We believe that in the era of immune checkpoint inhibitors (ICI), three extended surgical concepts should be integrated into our daily clinical practice. The following paragraphs would describe and exemplify these promising surgical strategies.
Case Presentation
Neoadjuvant Therapy
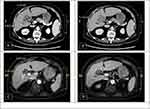
A 64-year-old female patient with underlying diseases of hypertension and diabetes mellitus suffered from body weight loss of around 3 kg within 1 month. Contrast-enhanced computed tomography (CT) revealed a 4.83 × 3.73 cm infiltrative and hypervascular mass at liver S5. The mass had invaded her gallbladder and encased the right hepatic artery (Figure 1A and B). Her serum alpha-fetoprotein (AFP) was 65,197.5 ng/mL. Locally advanced HCC was diagnosed. Surgery was not absolutely contraindicated but adequate safety margin may be more difficult to achieve. Systemic therapy with immune checkpoint inhibitors (ICI) was suggested. She received intravenous nivolumab 100mg as her first induction therapy. Unfortunately, intolerable adverse events of diffuse skin rash, fever, diarrhea, and general weakness developed after the first dose of ICI treatment, and further administrations were cancelled. Magnetic resonance imaging (MRI) found decreased tumor size (from 4.83 cm to 3.21 cm, >30% reduction) and AFP (1604.7 ng/mL). The major vessels including right hepatic artery were spared from tumor invasion (Figure 1C and D). Partial response per RECIST 1.1 was impressed. Due to reduced tumor size and resolved vessel invasion, conversion surgery with en bloc right lobectomy, partial S4b resection, and cholecystectomy was performed 22 days after nivolumab administration. Pathological examination revealed a moderately differentiated HCC with 90% tumor necrosis, ypT1bN0M0, stage I. The section margin was 0.5cm wide. The postoperative recovery was uneventful, and she was discharged on the 9th postoperative day (POD). AFP returned to normal after the operation and there was no evidence of tumor recurrence till 23 months after surgery.
Negative Selection
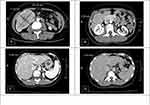
A 47-year-old female had been relatively healthy until she was found to have one 10.32 × 7.15 cm hypervascular tumor at S6 of liver (Figure 2A). She received laparoscopic hepatectomy to remove the tumor. The final pathology was moderately differentiated HCC, pT1bN0M0, stage I. Her serum PIVKA-II dropped from 1615 mAU/mL to 9 mAU/mL after the operation. She was enrolled to IMbrave 050 clinical trial and was randomized to the active surveillance group. One year after the operation, CT found recurrent HCCs at S1 (2.82 cm) and S6 (1.29 cm) of liver (Figure 2B and C). The tumor at S6 was ablated by radiofrequency, and she underwent serial Atezo/Bev combination therapy per trial protocol. The tumor at S1 demonstrated partial response after 8 cycles (from 2.82 cm to 1.94 cm). However, the tumor was found to enlarge at cycle 13 (from 1.94 cm to 2.43 cm) (Figure 2D). Concerning risk of disease progression, conversion therapy was suggested. Since there were no new lesions, liver resection was recommended and she received laparoscopic caudate lobectomy to remove the recurrent tumor. The patient was discharged 4 days after surgery, and she remained disease free for at least 10 months after surgery.
Downstaging
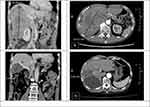
A 64-year-old male HBV carrier was found to have a huge liver tumor when being examined for his upper abdominal pain. CT furthered disclosed a huge conglomerate tumor (18 × 14 × 10 cm) at the right inferior lobe of liver with obliteration of right portal vein (Figure 3A and B). His serum AFP level was 3418 ng/mL. Advanced HCC, cT4N0M0, stage IIIb, was impressed. His Child-Pugh class was A (5 points), and Eastern Cooperative Oncology Group Performance Status was 0. Atezo/Bev combination therapy was administered every 3 weeks to control the disease. The tumor reduced from 18 cm to 12 cm after 7 cycles (30% reduction in size), and it further decreased to 9.44 × 5.66 cm after 14 cycles (nearly 50% reduction). No serious immune-related adverse events occurred during treatment. The tumor remained stationary in size after 17 cycles of Atezo/Bev, and the right PVTT also regressed (Figure 3C and D). Serum AFP level was <2 ng/mL. The disease was successfully down-staged, and conversion surgery with laparoscopic right hepatectomy was conducted. The pathological examination showed extensive tumor necrosis without identifiable residual carcinoma. The patient was discharged 9 days after surgery, and there was no evidence of disease recurrence for at least 8 months after surgery.
Discussion
Neoadjuvant Therapy
Although large tumors (>5cm) or tumors in close proximity to major vessels are not absolutely contraindicated for surgical resection, surgery under these scenarios may still pose a stress for surgeons. In addition, in order not to injure major vessels, a wider safety margin may not be always obtained. With the aid of immune check inhibitors (ICI) such as Atezo/Bev combination therapy, significant “tumor shrinkage” becomes possible. Liver resection can be carried out after serial Atezo/Bev sessions when tumor size reduced or distance from major vessels furthered (Figure 4). A wider safety margin can be obtained, and surgeons can conduct the operations less stressful. Furthermore, it may potentially reduce the risk of early recurrence following liver resections. This “neoadjuvant therapy” was rarely described for HCC since no effective regimen has been discovered before the advent of Atezo/Bev. As for standardized neoadjuvant protocols, strong evidence is still lacking and further prospective studies are warranted. For the time being, we would suggest one to three cycles of neoadjuvant immunotherapy and liver resections can then be conducted by experienced liver surgeons.
 |
Figure 4 Neoadjuvant therapy with ICI or Atezo/Bev combination. |
Negative Selection
Based on the updated BCLC guideline, patients with multinodular disease (>3) were not appropriate for liver resection.10 Similarly, upfront surgery was generally not recommended for those with bilobar multicentric HCC. The rationale supporting nonsurgical treatment is that either multinodular or bilobar disease may indicate intrahepatic metastasis. The risk of early recurrence is high even if the primary tumors are completely resected.18 The latest guidelines have therefore preferred systemic therapy with Atezo/Bev for this subgroup of unresectable and TACE-unsuitable intermediate-stage HCC.10,11 Given satisfactory ORR and disease control rate (44% ORR in intermediate-stage HCC), a substantial proportion of patients would respond to or maintain stable after serial cycles of Atezo/Bev therapies. Nevertheless, these patients would eventually develop progressive disease (PD) if Atezo/Bev is continued to be administered.15,17 Conversion therapy should therefore be considered. Since “no” new lesions have occurred during treatment period, intrahepatic metastasis becomes less likely and the disease may be treated as a localized disease. Under this circumstance, conversion surgery would be a reasonable treatment modality. Atezo/Bev combination therapy thus serves as a gatekeeper to “select” those who do “not” have new lesions after therapy. We name this process as “negative selection” (Figure 5). Since complete tumor response occurred between 5.5 and 7 months after initiation of Atezo/Bev therapy and most PD was observed after 7–9 months of treatment, we would suggest comprehensive tumor reassessment at 6–9 months after first Atezo/Bev to select candidate responders for conversion surgery.19,20 As for the tools for tumor assessment, we would recommend the combination of imaging (CT or MRI) and serum biomarker (AFP and PIVKA-II) studies to determine the response. Conversion surgery can be considered once the response steadies.
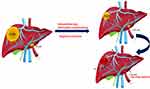 |
Figure 5 Negative selection by ICI or Atezo/Bev combination. |
Downstaging
Liver resection for HCC with Vp3 portal vein tumor thrombus (PVTT) carries higher risks of recurrence and is not suggested by major guidelines.21 Due to superior survival outcome over sorafenib, Atezo/Bev is the recommended first-line therapy for this subgroup of advanced-stage HCC.10,11,13,14 The ORR could reach 27%, and the adverse events were mostly tolerable. Only 12–16% of patients had elevated liver enzymes and only 2% of patients experienced abnormal hepatic functions.13 Given well-preserved liver functions after serial treatments, it is therefore possible to switch to locoregional therapies including surgery once maximal tumor response has been achieved. If the tumor shrinks and PVTT disappears, the disease is successfully down-staged and surgery can subsequently be performed (Figure 6). The “downstaging” strategy, although rather straightforward for cancer surgery, is hardly practicable for HCC until the advent of Atezo/Bev combination therapy. The optimal surgical timing varies and should be individualized. In the meanwhile, based on time to response and disease progression, we would suggest tumor reassessment and surgical consideration for responders at 6–9 months of Atezo/Bev therapy. Continued Atezo/Bev therapy can be considered for those with deep response and tolerable adverse events. Surgery can be performed when the tumor is successfully downstaged and treatment response steadies.
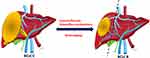 |
Figure 6 Downstaging by ICI or Atezo/Bev combination. |
Conclusion
The combination of Atezo/Bev showed promising results for unresectable HCC. The notable tumor response may convert unresectable disease into resectable disease. Conversion surgery under the concepts of “neoadjuvant therapy”, “negative selection”, and “downstaging” should therefore be considered for selected patients responsive to Atezo/Bev therapy. The treatment goal is to achieve “cancer free” and “drug free” status, as promoted by Professor Masatoshi Kudo. In the meanwhile, the surgical concepts and treatment strategy proposed herein are preliminary. The appropriate treatment duration and timing of conversion surgery are still undetermined. Moreover, although statistically superior to sorafenib, the 30% ORR obtained by Atezo/Bev is still unsatisfactory. The precise selection of potential responders for the administration Atezo/Bev therapy thus warrants further investigations. The novel genetic signatures or panels predictive of treatment response, although promising, are yet not universally validated.22,23 The management for those unresponsive to Atezo/Bev combination therapy, for the time being, is unaddressed, either. Further large scale well-designed prospective studies are therefore necessary to validate and consolidate our proposals.
Data Sharing Statement
All data generated or analyzed during the study are included in this published article. Raw data may be requested from the corresponding author Chao-Wei Lee with the permission of the institution.
Ethics Approval and Consent to Participate
This study was approved by the Institutional Review Boards (CGMH IRB No: 202100628B0) of CGMH.
Consent to Publish
All patients included in the current study have signed the informed consent and agreed the application of their deidentified data.
Acknowledgment
We are grateful to all our colleagues in the Division of General Surgery, Department of Surgery, Linkou, Keelung, Chiayi, and Kaohsiung Chang Gung Memorial Hospitals for patient care and data maintenance. We also appreciate the support from Department of Gastroenterology and Hepatology and Department of Cancer Center, Linkou Chang Gung Memorial Hospital for their technical assistance.
Funding
This study was supported by Chang Gung Memorial Hospital (CMRPG3M1701) and National Science and Technology Council, Taiwan, R.O.C. (MOST 111-2314-B-182A-122 - / NMRPG3M0201).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi:10.3322/caac.21262
2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
3. Llovet JM, Kelley RK, Villanueva A, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7(1):6. doi:10.1038/s41572-020-00240-3
4. Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
5. Department of Health ROC: statistics of cause of death in Taiwan 2020. Executive Yuan, Republic of China; 2021.
6. Department of Health ROC: statistics of cause of death in Taiwan 2021. Executive Yuan, Republic of China; 2022.
7. Zhou XD, Tang ZY, Yang BH, et al. Experience of 1000 patients who underwent hepatectomy for small hepatocellular carcinoma. Cancer. 2001;91(8):1479–1486. doi:10.1002/1097-0142(20010415)91:8<1479::AID-CNCR1155>3.0.CO;2-0
8. Lee CW, Tsai HI, Yu MC, et al. A proposal for T1 subclassification in hepatocellular carcinoma: reappraisal of the AJCC 8th edition. Hepatol Int. 2022;16(6):1353–1367. doi:10.1007/s12072-022-10422-8
9. Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379(9822):1245–1255. doi:10.1016/S0140-6736(11)61347-0
10. Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022;76(3):681–693. doi:10.1016/j.jhep.2021.11.018
11. Singal AG, Llovet JM, Yarchoan M, et al. AASLD practice guidance on prevention, diagnosis, and treatment of hepatocellular carcinoma. Hepatology. 2023:4:1.
12. Kudo M. A New Era in systemic therapy for hepatocellular carcinoma: atezolizumab plus bevacizumab combination therapy. Liver Cancer. 2020;9(2):119–137. doi:10.1159/000505189
13. Cheng AL, Qin S, Ikeda M, et al. Updated efficacy and safety data from IMbrave150: atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol. 2022;76(4):862–873. doi:10.1016/j.jhep.2021.11.030
14. Kudo M, Finn RS, Galle PR, et al. IMbrave150: efficacy and safety of atezolizumab plus bevacizumab versus sorafenib in patients with Barcelona Clinic Liver Cancer stage b unresectable hepatocellular carcinoma: an exploratory analysis of the phase III study. Liver Cancer. 2022;11(1):1–13. doi:10.1159/000521374
15. Kudo M. Atezolizumab plus bevacizumab followed by curative conversion (ABC Conversion) in patients with unresectable, TACE-unsuitable intermediate-stage hepatocellular carcinoma. Liver Cancer. 2022;11(5):399–406. doi:10.1159/000526163
16. Kudo M. A novel treatment strategy for patients with intermediate-stage HCC who are not suitable for TACE: upfront systemic therapy followed by curative conversion. Liver Cancer. 2021;10(6):539–544. doi:10.1159/000519749
17. Kudo M, Aoki T, Ueshima K, et al. Achievement of complete response and drug-free status by atezolizumab plus bevacizumab combined with or without curative conversion in patients with transarterial chemoembolization-unsuitable, intermediate-stage hepatocellular carcinoma: a multicenter proof-of-concept study. Liver Cancer. 2023;12(1):1–18. doi:10.1159/000528979
18. Wu JC, Huang YH, Chau GY, et al. Risk factors for early and late recurrence in hepatitis B-related hepatocellular carcinoma. J Hepatol. 2009;51(5):890–897. doi:10.1016/j.jhep.2009.07.009
19. Lee MS, Ryoo BY, Hsu CH, et al. Atezolizumab with or without bevacizumab in unresectable hepatocellular carcinoma (GO30140): an open-label, multicentre, phase 1b study. Lancet Oncol. 2020;21(6):808–820. doi:10.1016/S1470-2045(20)30156-X
20. Kudo M, Zolota V, Tzelepi V. Changing the treatment paradigm for hepatocellular carcinoma using atezolizumab plus bevacizumab combination therapy. Cancers. 2021;14(1):13. doi:10.3390/cancers14010013
21. Kokudo T, Hasegawa K, Matsuyama Y, et al. Survival benefit of liver resection for hepatocellular carcinoma associated with portal vein invasion. J Hepatol. 2016;65(5):938–943. doi:10.1016/j.jhep.2016.05.044
22. Zhu AX, Abbas AR, de Galarreta MR, et al. Molecular correlates of clinical response and resistance to atezolizumab in combination with bevacizumab in advanced hepatocellular carcinoma. Nat Med. 2022;28(8):1599–1611. doi:10.1038/s41591-022-01868-2
23. Haber PK, Castet F, Torres-Martin M, et al. Molecular markers of response to anti-PD1 therapy in advanced hepatocellular carcinoma. Gastroenterology. 2023;164(1):72–88.e18. doi:10.1053/j.gastro.2022.09.005
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.