Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 17
The Expression of miR-377-3p in Patients with DKD and the Regulatory Mechanism of miR-377-3p on the Inflammatory Response of HK-2 Cells Through TGF-β
Authors Xing C , Lu Y, Liu G, Chen F, Hou Z, Zhang Y
Received 14 November 2023
Accepted for publication 20 February 2024
Published 23 February 2024 Volume 2024:17 Pages 903—911
DOI https://doi.org/10.2147/DMSO.S449791
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Konstantinos Tziomalos
Chenhao Xing,1 Yamin Lu,2 Guangxia Liu,2 Fang Chen,2 Zhan Hou,2 Yiwen Zhang2
1Graduate School of Hebei North University, Hebei North University, Zhangjiakou, Hebei, People’s Republic of China; 2Department of Nuclear Medicine, Hebei General Hospital, Shijiazhuang, Hebei, People’s Republic of China
Correspondence: Yamin Lu, Department of Nuclear Medicine, Hebei General Hospital, Shijiazhuang, 050051, People’s Republic of China, Tel +86 15833956939, Email [email protected]
Objective: The purpose of the study was to investigate the expression levels and correlation of inflammatory factors such as miR-377-3p and TGF-β in patients with diabetic kidney disease (DKD), and to investigate the regulatory mechanism of transfection of miR-377-3p on the inflammatory response of HK-2 cell induced by high glucose.
Methods: According to UACR, patients were divided into normal albuminuria group (Con, n = 29), microalbuminuria group (Micro, n = 31) and macroalbuminuria group (Macro, n = 30), analyzed the correlation and influencing factors between DKD and inflammatory factor. HK-2 cells were randomly divided into four groups: normal control group (NC), high glucose group (HG), miR-377-3p overexpression group (MIN), and miR-377-3p inhibition group (IN). After transfection of miR-377-3p mimics and inhibitors, the contents of TGF-β, IL-6 and IL-18 were detected by RT-PCR and Western blot.
Results: The levels of miR-377-3p, TGF-β, IL-6 and IL-18 in both Micro group and Macro group were significantly higher than those in Con group (P < 0.05); Pearson correlation analysis showed that miR-377-3p was positively correlated with UACR, TG, TGF-β, IL-6 and IL-18, and negatively correlated with GFR (P < 0.05). Cell experiment: RT-PCR and Western blot results showed that miR-377-3p, TGF-β, IL-6 and IL-18 in HG group were significantly higher than those in NC group (P < 0.05). After transfection with miR-377-3p inhibitor, the levels of miR-377-3p, TGF-β, IL-6 and IL-18 in IN group were significantly decreased compared with HG group and MIN group.
Conclusion: miR-377-3p expression was elevated both in serum of DKD patients and in HK-2 cells with high glucose induced injury, overexpression of miR-377-3p exacerbates the damage to HK-2 cells and promotes the progression of DKD. Silencing miR-377-3p can potentially regulate the levels of inflammatory factors in HK-2 cells by targeting downregulation of TGF-β expression, thereby mitigating the damage to HK-2 cells and delaying the development of diabetic kidney disease.
Keywords: miR-377-3p, transforming growth factor beta, interleukin 6, interleukin 18, renal tubular epithelial cells
In recent years, with the improvement of residents’ living standards and the change of lifestyle, the incidence of diabetes has increased year by year. As a common complication of diabetes, the incidence of diabetic kidney disease (DKD) also increases with the increase of diabetic patients.1 Renal tubule injury is considered to be the main cause of the pathogenesis of DKD.2 Sustained stimulation of high glucose causes damage to renal tubular epithelial cells, leading to the secretion of several inflammatory cytokines, such as interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), transforming growth factor-β (TGF-β) and vascular cell adhesion molecule-1 (VCAM-1), which promote the inflammatory response. Activate TGF-β and other molecular pathways,3,4 thereby triggering interstitial inflammation and fibrosis, and mediating the occurrence and development of diabetic kidney disease.5
MicroRNAs (miRNAs) are important regulators of gene expression in many diseases and can affect many cellular processes, including cell proliferation, differentiation, and apoptosis.2 It has been reported that miRNA-615-3p is involved in the inflammatory response related to osteoarthritis by increasing the expression of IL-1β, IL-6, interleukin-α and other inflammatory cytokines.6 miR-10b-5p is one of the earliest miRNAs found to be abnormal in human cancer, and it is involved in inflammation control and inflammation-related diseases by regulating T cells.7 miR-377 is a new pro-inflammatory factor that can regulate cellular inflammatory response and insulin resistance. Studies have reported that miR-377-3p is closely related to the pathogenesis of DKD.8 Wang et al believed that miR-377 could increase the expression of matrix proteins and fibronectin, leading to renal tubulointerstitial inflammation and renal fibrosis.9 TGF-β signaling pathway is the key to the occurrence of diabetic kidney disease and renal tubulointerstitial fibrosis. There may be a regulatory correlation between miR-377-3p and TGF-β, but the exact mechanism of this relationship is still unclear. In this study, the expression levels of inflammatory factors such as miR-377-3p and TGF-β in patients with diabetic kidney disease were analyzed to explore their correlation. The purpose of the study was to investigate the mechanism of transfection of miR-377-3p on the inflammation of renal tubular epithelial cells induced by high glucose.
Data and Methods
The Ethics Committee of the Hebei General Hospital in China gave its approval for conducting this study, which adhered to the Declaration of Helsinki. The patients in this study were admitted to the Hebei General Hospital in Shijiazhuang, China. A total of 90 patients with type 2 diabetes diagnosed in out-patient and in-patient hospitals from October 2022 to June 2023 were collected, 33 healthy subjects were selected as control group (NC). All participants signed an informed consent form to participate in this study. Patients were screened and assessed based on the inclusion and exclusion criteria.
The following were the inclusion criteria: According to the Chinese Guidelines for the Prevention and Treatment of T2DM (2020 Edition)10: Diabetes mellitus diagnoses based on the results of fasting glucose of ≥7 mmol/l, abnormal 2 h oral glucose tolerance test, and symptoms (polyuria, polydipsia, and unexplained weight loss) of hyperglycaemia with a random blood glucose of >11.1 mmol/l. The participants included in the study were diagnosed with T2DM.
According to urinary albumin/creatinine ratio (UACR), T2DM patients were divided into the following 3 groups: normal albuminuria group (Con, n = 29, UACR < 30mg/g), microalbuminuria group (Micro, n = 31, 30 mg/g ≤ UACR ≤ 300 mg/g) and macroalbuminuria group (Macro, n = 30, UACR > 300 mg/g).
Inclusion criteria of normal subjects: Non-diabetic patients with normal blood glucose, liver function and renal function who came to our hospital for physical examination during the same period.
The following were the exclusion requirements: Patients had neurological disease, severe respiratory disease, radiation therapy and chemotherapy for tumors, pregnancy or lactation, serious mental illness and infectious disease.
Cells: HK-2 human renal proximal convoluted tubule cells were derived from China Center for Type Culture Collection (CCTCC). Main reagents: Fetal bovine serum was purchased from Sciencell Company in the United States, RNA extraction and separation kit, cDNA first chain synthesis kit and fluorescence quantitative detection kit were purchased from TIANGEN Company. TGF-β mouse monoclonal antibodies (TA506583S), IL-6 mouse monoclonal antibodies (TA500067S), and IL-18 rabbit polyclonal antibodies (TA324190S) were purchased from ORIGENE, and horseradish peroxidase labeled goat anti-Rabbit IgG and goat anti-mouse IgG were sourced from Servicebio. miR-377-3p mimics and miR-377-3p inhibitors were purchased from Guangzhou Ruibo Biotechnology Co, LTD, and the transfection kit Lipofectamine 2000 was purchased from Thermo Fisher Technologies. Human interleukin 18 ELISA kit (MM-0139H2), human interleukin 6 ELISA kit (MM-0050H2) and human TGF-β ELISA kit (MM-1774H2) was purchased from Jiangsu Meimian Industrial Co., Ltd.
Cell Culture and Group
Human renal tubular epithelial cells HK-2 were cultured with DMEM medium containing 10% fetal bovine serum, 1% penicillin (100 U/mL) and streptomycin (100μg/mL) in a 5% CO2 incubator at 37°C. HK-2 cells were cultured with DMEM medium containing 5.5 mmol/L glucose as normal control group (NC). The high glucose (HG) group was cultured with DMEM medium containing 30 mmol/L glucose. miR-377-3p mimics and miR-377-3p inhibitor were transfected into renal tubular epithelial cells and cultured with DMEM medium containing 30 mmol/L glucose, which were denoted as miR-377-3p overexpression group (MIN) and miR-377-3p inhibition group (IN).
CCK-8 Cell Proliferation Experiment
After digestion, the concentration of HK-2 cells was adjusted to 5.0 × 104 cells/mL and inoculated into 96-well plates at 100 μL/well. Forty-eight hours later, CCK-8 solution was added to each well for 10 μL and incubated at 37°C for 2 h. The value of absorbance (A450 nm) at 450 nm was measured by enzymoleter to evaluate cell proliferation activity. Cell survival rate = (experimental hole – blank hole)/(control hole – blank hole).
Transfection
The miR-377-3p mimics and miR-377-3p inhibitors were transfected into HK-2 cells using the Lipofectamine 2000 kit. The cells were cultured with incomplete medium without serum for 6 hours, and then replaced with complete medium for further culture. After 24 hours, the transfection effect was detected by RT-PCR.
Methods
The general information of each group was recorded, including gender, age, blood sugar, blood lipid and other indicators. Cholesterol (TC) and triglyceride (TG) levels were determined by GPO-POD method, blood glucose was determined by hexokinase method, creatinine was detected by sarcosine oxidase, urea was determined by urease-glutamate dehydrogenase method, high-density lipoprotein and low-density lipoprotein were determined by direct method. The levels of TGF-β, IL-6 and IL-18 in serum were measured by ELISA. Real-time quantitative PCR was used to detect the mRNA content of miR-377-3p in serum. The expression levels of TGF-β, IL-6 and IL-18 were detected by Western blot and real-time quantitative PCR in cells.
RT-PCR was used to detect the level of miR-377-3p, U6 as the internal reference, TGF-β, IL-6, IL-18 mRNA levels, β-ACTIN as the internal reference. The primers are shown in Table 1. Total RNA was extracted by TANGEN kit, purified RNA concentration was detected, and RNA integrity was detected by gel electrophoresis. cDNA was reversed using reverse transcription kit, and the amplification procedure was as follows: predenaturation: 95°C, 15 min, 1 cycle; The relative mRNA expression of the target gene was calculated by 2−ΔΔCT after 40 cycles at 94°C, 60°C and 34s.
 |
Table 1 Primer Sequence |
Western Blotting Experiment Method
The cells were washed with PBS 2–3 times; Appropriate volume of RIPA lysate was added (each protease inhibitor was added within a few minutes before application) into the six-well plate for 3–5 min; The protein concentration was measured with the BCA protein concentration detection kit, SDS-PAGE electrophoresis, membrane transfer and closure were performed, the primary antibody was diluted according to the antibody instructions (1:1000), and the configured primary antibody was added, and incubated at 4°C in a shaker overnight (shaking slowly in a shaker). Recover the primary antibody, use TBST to quickly wash the film for three times, place it on the decolorizing shaker for rapid elution, 5 min each time, wash three times; The secondary antibody was diluted with TBST at a ratio of 1:5000, and then added to the incubator, which was slowly shaken on a shaking table and incubated at room temperature for 1h; Mix liquid ECL A and B in a 1:1 ratio and set aside. Take out the eluted PVDF film and put it on absorbent paper, slightly absorb the liquid above the film, put the film on the chemiluminescence meter shelf, add the mixed ECL luminescent liquid, let the liquid completely soak the film, after the reaction for 1 min, use absorbent paper to absorb the excess liquid above, put it into the chemiluminescence meter, start chemiluminescence according to the preset procedure, after the exposure is completed. The original image was saved in TIFF format, and the relative expression level of target protein was expressed by the gray value of target protein band/gray value of internal reference protein band.
Statistical Analysis
Statistical methods SPSS 25.0 software was used for statistical analysis. The measurement data were represented by mean ± standard deviation (x ± s), and the normal distribution of variables in each group was tested. One-way analysis of variance was used for comparison between groups. The non-conforming distribution was expressed as the median and quartile distance [M(Q1, Q3)], and the Kruskal–Wallis H-test was used for the non-parametric comparison of multiple independent samples. Pearson correlation analysis was used for correlation analysis among variables, and Spearman correlation analysis was used for non-parameters. Multiple linear regression model was used to analyze the influencing factors. P < 0.05 was considered statistically significant.
Results
Comparison of General Clinical Data in Each Group
Table 2 shows the clinical features of the study group. All groups were matched for age and sex. UACR, glomerular filtration rate, fasting blood glucose, urea, creatinine, serum TC, TG, HDL, and LDL, compared with Con group, the levels of UACR, glomerular filtration rate, creatinine, serum TG and LDL-C in Micro group and Macro group were significantly different (P < 0.05), Table 2.
 |
Table 2 Comparison of Basic Biochemical Indexes in Each Group (X±s) |
Comparison of Levels of miR-377-3p and Inflammatory Factors Among Study Subjects in Each Group
Serum levels of TGF-β, IL-18, miR-377-3p and IL-6 were detected in each group. As shown in Figure 1A–D), serum levels of TGF-β, IL-18, miR-377-3p and IL-6 in both Micro group and Macro group were significantly higher than those in Con group and NC, P < 0.05;
Correlation Analysis
Pearson correlation analysis showed that miR-377 was correlated with UACR (r = 0.512) in diabetic proteinuria group. P = 0.000), TG (r = 0.350, P= 0.000), TGF-β (r = 0.688, P = 0.000), IL-6 (r = 0.352, P = 0.000), IL-18 (r = 0.290, P = 0.001) were positively correlated. It was negatively correlated with the glomerular filtration rate (r = −0.592, P = 0.000). (Figure 2A–F).
Multivariate Linear Regression Analysis of Influencing Factors in Patients with Diabetic Kidney Disease
Micro group and Macro group as diabetic renal disease group, Con as control group, TGF-β, IL-18, miR-377-3p and IL-6 as variables, multiple linear regression analysis was performed. The results showed that TGF-β, IL-18, miR-377-3p and IL-6 were influential factors in the occurrence of diabetic kidney disease (P < 0.05), accounting for 50.3% of the total variation in the regression equation. Table 3.
 |
Table 3 Multiple Linear Regression Analysis of Influencing Factors in Patients with Diabetic Kidney Disease |
Comparison of miR-377-3p and Inflammatory Factors in Each Group in HK-2 Cells by PCR
The expression levels of miR-377-3p, TGF-β, IL-6 and IL-18 in HG group were significantly increased, P < 0.05; After transfection with miR-377-3p mimics, the inflammatory level was further increased, while after transfection with miR-377-3p inhibitor, the expression levels of TGF-β, IL-6 and IL-18 were significantly decreased compared with those in HG and MIN groups (P < 0.05). Table 4
 |
Table 4 Expression Levels of TGF-β, IL-6 and IL-18 in HK-2 Cells Induced by High Glucose After Transfection of miR-377-3p Mimics and Inhibitor |
After Transfection of miR-377-3p Mimics and Inhibitors, the Survival Rate of HK-2 Cells in Each Experimental Group Was Detected by CCK-8 Method
Each group was set with 6 multiple holes and repeated three times, as shown in Figure 3. The cell survival rate of HG and MIN groups was significantly lower than that of NC, and the cell survival rate of HG and MIN groups was significantly higher than that of Hg and min groups after transfection with miR-377-3p inhibitor, P<0.05
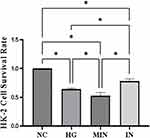 |
Figure 3 HK-2 cell survival per experimental group (NC, HG, MIN and IN) *P<0.05. |
Western Blot Assay Was Used to Detect the Expression of TGF-β, IL-6 and IL-8 in HK-2 Cells of Each Group After Transfection with miR-377-3p Mimics and Inhibitors
The expression levels of TGF-β, IL-6 and IL-18 in HG group were significantly increased, P < 0.05; After transfection with miR-377-3p mimics, the inflammatory level was further increased, while after transfection with miR-377-3p inhibitor, the expression levels of TGF-β, IL-6 and IL-18 were significantly decreased (P < 0.05) Figure 4.
Discussion
Diabetic kidney disease is a multi-factorial progressive disease with extremely complex pathogenesis. Tubular injury is an important pathological feature of diabetic kidney disease, and inflammation is an important mechanism for the occurrence and development of DKD.11
High glucose treatment can induce an inflammatory response in HK-2 cells, resulting in renal tubule injury accompanied by increased levels of pro-inflammatory cytokines such as IL-6.12 Inflammatory factors destroy the structure of the kidney, leading to the overproduction of ECM, which leads to renal fibrosis. Renal fibrosis is the common pathway of most progressive nephropathy.13 TGF-β, JAK/STAT, Notch and other signaling pathways play a role in renal fibrosis.14 IL-6 is a pro-inflammatory cytokine whose main biological function is to regulate immune response and inflammatory response. IL-6 can not only directly stimulate the proliferation of mesangial cells, damage islet cells and destroy islet function, but also damage renal vascular endothelial cells and promote renal interstitial fibrosis.15 IL-18, a pro-inflammatory marker, is positively correlated with the level of proteinuria in DKD patients16 and is a biomarker for renal tubule injury and repair.17 NOD-like receptors (NLRPs and NLRCs) are activated in the diabetic micropig model to promote the expression of downstream inflammatory factors such as IL-6 and IL-18 through TGF-β pathway, causing metabolic inflammation of kidney tissue, and ultimately leading to the occurrence and development of diabetic kidney disease.18 In this experiment, we found that the serum levels of TGF-β, IL-6 and IL-18 in diabetic kidney disease patients were significantly higher than those in normal diabetic albuminuria group and healthy control group, and were significantly higher in diabetic microalbuminuria. Person analysis found that inflammatory factors IL-6, IL-18 and TGF-β were significantly positive with UACR, and significantly negatively correlated with glomerular filtration rate, indicating that inflammatory response had already occurred in the early stage of diabetic kidney disease and the degree of inflammation was positively correlated with the disease. In the cell experiment, HK-2 cells treated with HG significantly decreased cell viability, and the levels of TGF-β, IL-6 and IL-18 inflammatory cytokines significantly increased, which was consistent with clinical results.
Sustained high glucose stimulation may cause damage to renal tubular epithelial cells and lead to inflammatory response, thereby destroying renal tissue.19 TGF-β is a key regulator of DKD, and TGF-β signaling pathway is a key pathway for inducing renal fibrosis, which can cause inflammation, renal tubular epithelial fibrosis and abnormal deposition of extracellular matrix.20 Studies have shown that abnormal expression of Egr1 in HK-2 cells treated with high glucose may activate TGF-β1/Smad pathway through up-regulation of PAR1 transcription, thus promoting the synthesis of fibrosis gene protein.21 In this study, sustained high glucose stimulation may activate TGF-β signaling pathway by up-regulating the content of inflammatory factors such as IL-6 and IL-18. Resulting in increased levels of inflammation.22
The function of miRNAs has become a hot topic in the occurrence and development of various diseases, and miRNAs is becoming a key regulator of autoimmune response and inflammation.23 Inflammation is the key to renal tubular epithelial cell injury, miRNAs and inflammation are closely related, miRNAs may be a new target for DKD treatment. Studies have shown that miR-377 is upregulated in DKD mice and HG-treated mesangial cells, and promotes the production of fifientin to a large extent, thus promoting mesangial cell fibrosis.24 In this study, miR-377-3p was significantly elevated in patients with diabetic kidney disease. miR-377-3p was positively correlated with UACR, TGF-β, IL-6 and IL-18, and negatively correlated with glomerular filtration rate. Studies have shown that miR-377 regulates many basic biological processes and plays a key role in tumor cell proliferation, migration and inflammation.25 These results indicate that miR-377-3p and inflammatory factors play a role in diabetic kidney disease. In this study, multiple linear regression also confirmed that miR-377-3p, TGF-β, IL-6 and IL-18 are important factors affecting the occurrence of diabetic kidney disease. miR-377-3p may have a regulatory relationship with TGF-β signaling pathway and inflammatory factors, and TGF-β may be the downstream target gene of miR-377-3p, but the specific mechanism is still unclear. We conducted cell experiments and found that the contents of TGF-β, IL-6 and IL-18 in the HG group were significantly higher than those in the NC group. After transfection of miR-377-3p mimics, the contents of TGF-β were further increased, and the degree of inflammation was further aggravated, while the expression of miR-377-3p was inhibited, and TGF-β was significantly decreased. The activity of HK-2 cells was significantly enhanced, suggesting that miR-377-3p has a targeted regulatory relationship with TGF-β signaling pathway and inflammatory factors, and miR-377-3p may be an upstream regulator of TGF-β. miR-377-3p may promote the increase of expression of inflammatory factors IL-6 and IL-18 by regulating TGF-β signaling pathway, leading to renal tubular cell injury, and participating in the occurrence and development of diabetic kidney disease. However, the specific regulatory mechanisms of miR-377-3p and TGF-β need to be further explored. In summary, persistent high glucose leads to tubular epithelial cell damage and induces the production of inflammatory factors, and TGF-β, IL-6 and IL-18 are significantly positively correlated with the occurrence of diabetic kidney disease. miR-377-3p may be the upstream regulator of TGF-β. By regulating the expression of miR-377-3p, the expression level of TGF-β can be effectively regulated, the release of inflammatory factors can be reduced, and the damage of renal tubular epithelial cells can be reduced, which has a positive effect on the prognosis of diabetic kidney disease.
Data Sharing Statement
The primary data for this study is available from the Yamin Lu on direct request.
Ethics Approval and Consent to Participate
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of Hebei General Hospital (Scientific research ethics No. 218 of 2022), All participants signed the informed consent form
Consent for Publication
The research article is original, has not already been published in any other journal (medical, or otherwise) or is not currently under consideration for publication by another journal, and does not infringe any existing copyright or any other rights prescribed by law
Acknowledgments
Thanks to Hebei General Hospital China for their support.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by 2023 Medical Science Research Program of Hebei Province (NO: 20230388), Hebei Provincial government funded the training project of clinical medical talents (NO: 2020, 124).
Disclosure
The authors declare that there are no conflicts of interest regarding the publication of this article.
References
1. Dong HY, Zheng JB, Zhng YJ, et al. The mechanism of lncRNA CASC9 targeting miR-513a-5p in regulating renal tubular epithelial cell injury induced by high glucose. Chin J Immunol. 2019;38(22):2706–2711.
2. Zhao N, Luo Q, Lin R, et al. MiR-142-3p ameliorates high glucose-induced renal tubular epithelial cell injury by targeting BOD1. CLIN EXP NEPHROL. 2021;25(11):1182–1192. doi:10.1007/s10157-021-02102-y
3. Li Y, Deng X, Zhuang W, et al. Tanshinone IIA down-regulates -transforming growth factor beta 1 to relieve renal tubular epithelial cell inflammation and pyroptosis caused by high glucose. Bioengineered. 2022;23:13.
4. Zhang L, Liu X, Liang J, et al. Lefty-1 inhibits renal epithelial-mesenchymal transition by antagonizing the TGF-β/Smad signaling pathway. J Mol Histol. 2020;24:51.
5. Hu W, Dai Q, Ding G, et al. Effect of overexpression of SNHG5 on apoptosis and oxidative stress of HK-2 cells induced by high glucose [J]. Chin J Cell Bio. 2019;44(06):1056–1068.
6. Wang J, Tao Y, Zhao F, Liu T, Shen X, Zhou L. Expression of urinary exosomal miRNA-615-3p and miRNA-3147 in diabetic kidney disease and their association with inflammation and fibrosis. Ren Fail. 2023;45(1):2121929. doi:10.1080/0886022X.2022.2121929
7. Lauria F, Iacomino G, Russo P, et al. Circulating miRNAs are associated with inflammation biomarkers in children with overweight and obesity: results of the I. Family study. Genes (Basel). 2022;13(4):632. doi:10.3390/genes13040632
8. Abdelghaffar S, Shora H, Abdelatty S, et al. MicroRNAs and risk factors for diabetic nephropathy in Egyptian children and adolescents with type 1 diabetes. Diabetes Metab Syndr Obes. 2020;13:2485–2494. doi:10.2147/DMSO.S247062
9. Xie Y, Lan F, Zhao J, et al. Hirudin improves renal interstitial fibrosis by reducing renal tubule injury and inflammation in unilateral ureteral obstruction (UUO) mice. INT IMMUNOPHARMACOL. 2020;81:106249. doi:10.1016/j.intimp.2020.106249
10. Jia W, Weng J, Zhu D. Diabetes society of Chinese medical association. Chin J Diabet. 2021;13(4):315–409.
11. Zhang J, Zhao X, Zhu H, Wang J, Ma J, Gu M. Crocin protects the renal tubular epithelial cells against high glucose-induced injury and oxidative stress via regulation of the SIRT1/Nrf2 pathway. Iran J Basic Med Sci. 2022;25(2):193–197. doi:10.22038/IJBMS.2022.51597.11708
12. Zhou J, Zhang S, Sun X, Lou Y, Yu J. Hyperoside Protects HK-2 Cells Against High Glucose-Induced Apoptosis and Inflammation via the miR-499a-5p/NRIP1 Pathway. Pathol Oncol Res. 2021;27:629829. doi:10.3389/pore.2021.629829
13. Rende U, Guller A, Goldys EM, et al. Diagnostic and prognostic biomarkers for tubulointerstitial fibrosis. J PHYSIOL-LONDON. 2023;601:1.
14. Tserga A, Pouloudi D, Saulnier-Blache JS, et al. Proteomic analysis of mouse kidney tissue associates peroxisomal dysfunction with early diabetic kidney disease. Biomedicines. 2022;10(2):216. Doi:10.3390/biomedicines10020216
15. Liu GW, Zeng JE, Li LF. Correlation analysis of serum IGF-1 and IL-6 and urinary albumin/creatinine ratio in patients with type 2 diabetic kidney disease. Front Endocrinol. 2022;13. doi:10.3389/fendo.2022.1082492
16. Petrica L, Vlad A, Gadalean F, et al. Mitochondrial DNA changes in blood and urine display a specific signature in relation to inflammation in normoalbuminuric diabetic kidney disease in type 2 diabetes mellitus patients. Int J Mol Sci. 2023;24(12):9803. doi:10.3390/ijms24129803
17. Shi C, He A, Wu X, et al. Urinary IL-18 is associated with arterial stiffness in patients with type 2 diabetes. Front Endocrinol. 2022;13:956186. doi:10.3389/fendo.2022.956186
18. Ren Y, Cui S, Hong Q, et al. Role of NOD-like receptors in a miniature pig model of diabetic renal injuries. Mediators Inflamm. 2022;2022:5515305. doi:10.1155/2022/5515305
19. Wang L, Wang H, Lan H. TGF-β signaling in diabetic nephropathy: an update diabetic nephropathy. Diabetic Nephr. 2023;23:1.
20. Tang J, Liu F, Cooper ME, et al. Renal fibrosis as a hallmark of diabetic kidney disease: potential role of targeting transforming growth factor-beta (TGF-β) and related molecules. EXPERT OPIN THER TAR. 2022;26(8):721–738. doi:10.1080/14728222.2022.2133698
21. Xu P, Zhan H, Zhang R, et al. Early growth response factor 1 upregulates pro-fibrotic genes through activation of TGF-β1/Smad pathway via transcriptional regulation of PAR1 in high-glucose treated HK-2 cells. MOL CELL ENDOCRINOL. MOL CELL ENDOCRINOL. 2023;572:111953. doi:10.1016/j.mce.2023.111953
22. Sueud T, Hadi NR, Abdulameer R, et al. Assessing urinary levels of IL-18, NGAL and albumin creatinine ratio in patients with diabetic nephropathy. DIABETES METAB SYND. 2019;13:1.
23. Wen L, Wang X, Ji F, et al. Renal megalin mRNA downregulation is associated with CKD progression in IgA nephropathy. AM J NEPHROL. 2022;53(6):481–489. doi:10.1159/000524929
24. Wu R, Niu Z, Ren G, Ruan L, Sun L. CircSMAD4 alleviates high glucose-induced inflammation, extracellular matrix deposition and apoptosis in mouse glomerulus mesangial cells by relieving miR-377-3p-mediated BMP7 inhibition. Diabetol Metab Syndr. 2021;13(1):137. doi:10.1186/s13098-021-00753-1
25. Akhlaghipour I, Taghehchian N, Zangouei AS, et al. MicroRNA-377: a therapeutic and diagnostic tumor marker. INT J BIOL MACROMOL. 2023;226:1.
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



