Back to Journals » Clinical Epidemiology » Volume 14
The Epidemiology, Treatment Patterns and Economic Burden of Different Phenotypes of Multiple Sclerosis in Italy: Relapsing-Remitting Multiple Sclerosis and Secondary Progressive Multiple Sclerosis
Authors Perrone V, Veronesi C, Giacomini E, Citraro R, Dell'Orco S, Lena F , Paciello A, Resta AM, Nica M, Ritrovato D , Degli Esposti L
Received 24 May 2022
Accepted for publication 17 October 2022
Published 6 November 2022 Volume 2022:14 Pages 1327—1337
DOI https://doi.org/10.2147/CLEP.S376005
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Vera Ehrenstein
Valentina Perrone,1 Chiara Veronesi,1 Elisa Giacomini,1 Rita Citraro,2 Stefania Dell’Orco,3 Fabio Lena,4 Arrigo Paciello,5 Anna Maria Resta,6 Mihaela Nica,7 Daniela Ritrovato,7 Luca Degli Esposti1
1Clicon S.r.l. Società Benefit, Health Economics & Outcomes Research, Bologna, Italy; 2Dipartimento di Scienze della Salute, Università Magna Grecia di Catanzaro, Unita’ Operativa di Farmaco-logia Clinica e Farmacovigilanza, Azienda Ospedaliero-Universitaria “Mater Domini”, Catanzaro, Italy; 3ASL Roma 6, Rome, Italy; 4USL Toscana Sud Est, Grosseto, Italy; 5ATS Bergamo, Bergamo, Italy; 6Struttura Complessa di Farmacia Territoriale Area Vasta 1, Fano, Italy; 7Novartis Farma S.p.A, Origgio, Varese, Italy
Correspondence: Valentina Perrone, CliCon S.r.l. Società Benefit, Health, Economics & Outcomes Research, Via Murri, 9, Bologna, 40137, Italy, Tel +39 3450316494, Email [email protected]
Purpose: A retrospective analysis of real-world data was performed to assess the epidemiology and economic burden of multiple sclerosis (MS), relapsing-remitting MS (RRMS), and secondary-progressive MS (SPMS) in Italy.
Patients and Methods: An observational study on administrative databases from a sample of Italian entities was carried-out. Between 01/2010– 12/2017, patients with ≥ 1 MS diagnosis code (ICD-9-CM:340 and/or exemption code:046) and/or ≥ 1 disease-modifying therapies (DMTs) prescription, were included. Among MS-cohort, SPMS patients were identified by ≥ 2 hospitalizations or by ≥ 2 drug prescriptions related to MS progression. MS patients not fulfilling SPMS criteria were included as RRMS. Mean annual healthcare costs were reported during follow-up and stratified by DMT treatment/untreatment.
Results: Overall, 9543 MS patients were included; 8397 with RRMS and 1146 with SPMS. Estimated prevalence of MS was 141.6/100,000 inhabitants (RRMS 124.4/100,000 and SPMS 17.2/100,000). Mean annual cost for untreated and treated patient was respectively: € 3638 and € 11796 (MS-cohort), € 3183 and € 11486 (RRMS-cohort), € 6317 and € 15511 (SPMS-cohort). The first-line DMT treatment duration averaged 27.4 ± 22.8 months; the mean cost was 19004€ for the whole period. The second-line DMT treatment lasted on average 31.1 ± 24.5 months; the mean cost was 47293€ for the whole period.
Conclusion: This study provided insights into the MS epidemiology in Italy and its economic burden. Healthcare costs associated with MS management were mainly driven by DMTs expenditure. A trend of higher healthcare-resource consumption was observed among SPMS-cohort.
Keywords: real-world evidence, clinical practice, multiple sclerosis, epidemiology, healthcare resource consumption
Introduction
Multiple sclerosis (MS) is a chronic immune-mediated, inflammatory and demyelinating disease of the central nervous system (CNS) that leads to irreversible disability.1 MS is classified in different phenotypes depending on the patterns of cognitive or physical impairment progression: relapsing-remitting MS (RRMS), primary-progressive MS (PPMS), secondary-progressive MS (SPMS), or progressive-relapsing MS (PRMS).2–4 The most common one is RRMS, which is characterized by the alternation of relapses and remission phases. Approximately 85% of patients with MS are initially diagnosed with RRMS, which over time may evolve to a progressive phase (SPMS).3,4 In SPMS, although patients might still have relapses, neurologic deficits accumulate independently of relapses, leading to the continuous progression of disability.3,4 Currently is estimated that MS affects more than 2 million people globally.5 A recent report has shown that the MS prevalence in Europe averages 108 cases per 100,000 inhabitants, and in Italy, the prevalence is estimated to 113/100,000, with a ratio of women to men of 1.75:1.6 Due to its immune-mediated pathogenesis, immunomodulatory agents have been indicated for MS treatment. These targeted drugs, called “disease-modifying therapies” (DMTs), prevent relapses of the disease, and consequently, progression of disability by shifting immune responses from a pro-inflammatory toward an anti-inflammatory status; thus, they were considered first-line options in MS treatment to modify the disease course.7 Several DMTs have been approved for MS treatment: four forms of interferon (IFN) beta, glatiramer acetate, natalizumab, fingolimod, alemtuzumab, teriflunomide, dimethyl fumarate, ocrelizumab, and very recently Siponimod. In addition, mitoxantrone and cladribine (that are immunosuppressive agents) have been approved for the specific treatment of active forms of RRMS and SPMS.8 Although remarkable advancements have occurred in the therapy of MS, the rate of progressive disability is still worrisome. The substantial disability of MS, (SPMS, or RRMS) makes this disease of great importance for the Italian national health system (ie, Sistema Sanitario Nazionale, SSN) in terms of reimbursed costs.
Few studies have been focused on MS annual management costs in Italy.9–11 Generally, most studies involving MS patients estimated direct and indirect costs based on data collected using a questionnaire completed by patients; thus, they were subject to selection and/or recall bias.11,12 In this setting, administrative databases can provide more reliable information on healthcare costs13 related to hospitalizations, specialist visits, and diagnostic exams, to have a clear picture of the direct medical expenditure reimbursed by the SSN for these patients.
Thus, we conducted a retrospective analysis of administrative datasets of MS patients to i) estimate the disease prevalence of MS (specifically in SPMS and RRMS) among the Italian population, ii) describe demographics and clinical characteristics of patients, iii) investigate their treatment patterns and pharmaco-utilization variables and iv) estimate the MS, RRMS, and SPMS patient direct annual cost for the Italian SSN.
Materials and Methods
Data Source
This is an observational study based on the secondary use data extracted from the administrative databases from 3 Italian Local Health Units and 2 Regions, distributed across the Italian territory, covering approximately 6 million inhabitants. Within the administrative flows, the anonymous univocal numeric code assigned to each patient allowed the electronic linkage of all subjects’ records across the databases. Specifically, data-linkage was performed among the following databases: demographic database (collecting data on patients’ demographic), pharmaceutical database [collecting data on prescription of drugs reimbursed by the SSN, in terms of related Anatomical Therapeutic Chemical (ATC) code, and prescription date], hospitalization database [giving information on discharge diagnoses at any level classified according to the International Classification of Diseases, 9-th Revision, Clinical Modification (ICD-9-CM) and date of diagnose], and outpatient diagnostic tests and specialist visits database (containing the date of prescription, type, description of diagnostic tests and procedure for patients in analysis). The anonymous univocal numeric code ensured total compliance with the European General Data Protection Regulation (GDPR) (2016/679). No identifiers related to patients were provided to the authors. All the results of the analyses were produced as aggregated summaries, and thus data cannot be assigned, either directly or indirectly, to a single institution, department, doctor, individual, or individual prescribing behaviors. Based on the Privacy Guarantor Authority general authorization for personal data treatment for scientific research purposes – n.9/2014, informed consent was not required, as its’ collection would be impossible for organizational reasons. According to the Italian law on conducting observational studies, the ethics committee of each participating entity was notified and approved the analysis (the details are reported in the Supplementary Material).
Study Cohorts
All patients with a diagnosis of MS, SPMS, RRMS from January 1st, 2010 to December 31st, 2017 were included in the study. The cohorts’ definition criteria has been reported below, in accordance with those previously reported.2,14,15
MS Cohort
We included patients with:
- at least one hospitalization with primary or secondary discharge diagnosis for MS (ICD-9-CM code 340),
- and/or an exemption code for MS (code: 046),
- and/or at least one prescription of drugs indicated for MS [as reported in details in Supplementary Table 1].
In this cohort, the date of the first match of inclusion criteria was defined as the index date.
SPMS Cohort
SPMS patients were identified among the MS cohort as those presenting at least two consecutive events associated with the progression of MS disease, as previously described:2
- at least two consecutive hospitalizations with a main or secondary discharge diagnosis for spasticity (ICD-9-CM code 781.0), or impaired ambulation (ICD-9-CM code 719.7), or bladder and sexual dysfunction (ICD-9-CM codes 596.59, 302.70), or muscle weakness (ICD-9-CM code 728.87), or paraplegia (ICD-9-CM code 344.1), or hemiplegia (ICD-9-CM code 342.x), or ataxia (ICD-9-CM code 334.3), or gait disturbance (ICD-9-CM code 781.2), or
- at least two subsequent prescriptions for a drug indicated for the management of symptoms related to SM progression: baclofen (ATC code M03BX01) for spasticity; fampridine (ATC code N07XX07) or cannabinoids (ATC code N02BG10) for gait disturbance.
Among the two hospitalizations and two drug prescriptions, the first hospitalization/prescription must have occurred at least six months after MS diagnosis, while the second hospitalization/prescription must have occurred at least after three months respect to the first hospitalization/prescription date. For this cohort, the date when the second progression symptom occurred was defined as the index date.
RRMS Cohort
MS patients without SPMS symptoms/criteria within 12 months after MS diagnosis were defined as RRMS. For these patients, the index date was the date after 12 months of the MS diagnosis date.
All patients had a characterizing period of one year before the index date and were followed up for at least one year after the index date; during the follow-up period the index-date was included in the analyses.
Analysis of Baseline Characteristics
For all patients included in the study, baseline characteristics in terms of age, gender were identified at the index date; the occurrence of previous hospitalizations and treatments were evaluated during the characterization period. The comorbidity profile was also assessed during the characterization period using the Charlson Comorbidity Index (CCI), which assigns a single score (minimum 0, maximum 6) to patients by weighting each concomitant disease identified in the 12 months before the index date.16 The comorbidities were identified from the discharge diagnosis at primary and secondary level. When a diagnosis was not available, the prescriptions of specific drugs were used as a proxy to determine the specific comorbidity.
Analysis of Treatment Patterns and Pharmaco-Utilization
The treatment patterns were evaluated in all included patients at index date and during the follow-up (all available periods post inclusion, including the index date). Patients were stratified accordingly to an intention-to-treat analysis17 based on the initial DMT treatment assignment. Specifically, MS patients were defined as “treated” if they presented at least one prescription of DMT drugs indicated for MS (Supplementary Table 1) during the first three months after the index date. In MS-treated patients, treatment discontinuation was evaluated and defined as the absence of MS medication prescriptions during the last three months of the follow-up (a one year observation period started from index date prescription). In addition, treatment switch was evaluated and defined as any change of the index DMTin particular, as the presence of at least one different DMT, from the index medication, during 12 months of follow-up.
Analysis of Direct Cost Covered by the SSN
In treated and untreated patients (defined on the basis of their initial DMT treatment during the first three months of follow-up), the direct healthcare costs were evaluated over the follow-up period and were related to the following resource consumption: hospitalizations (determined by using the DRGs tariffs), drug costs (evaluated for those drugs reimbursed by the Italian SSN and using the SSN purchase price), and the outpatient specialist service costs (including all specialistic visits, diagnostic tests) accordingly to Regional tariffs. Data were reported as the mean (95% Confidence Interval, CI) and median annual healthcare cost per patient. In addition, a focused analysis on the mean cost of first- and second-line DMTs during all available period post-inclusion was performed. Specifically, first and second-line treatments DMTs were defined based on the first or second DMT drugs prescribed among the Agenzia Italiana del Farmaco (AIFA) reimbursement criteria (explained in the Note 65): i) first-line DMT drugs [Interferon β-1a (ATC code L03AB07), Interferon β-1a (ATC code L03AB08), Peginterferon β-1a (ATC code L03AB13), Glatiramer acetate (ATC L03AX13), Teriflunomide (ATC code L04AA31), Dimethyl fumarate (ATC code N07XX09); ii) second-line DMT drugs [Natalizumab (ATC code L04AA23), Fingolimod (ATC code L04AA27), Alemtuzumab (ATC code L04AA34), and Ocrelizumab (ATC code L04AA36)]. The costs related to first and second-line DMTs were estimated.
Statistical Analysis
This is a descriptive analysis. Continuous variables were reported as mean and 95% Confidence Interval (CI); median costs were also reported in Supplementary Table 2. Categorical variables were expressed as numbers and percentages. Considering all available period on the dataset, the prevalence was defined as the number of patients already/newly diagnosed with MS/RRMS/SPMS among 100,000 inhabitants. All analyses have been performed using STATA SE version 17.0 (StataCorp LLC, College Station, TX, USA).
Results
Overall, during the inclusion period, 9543 MS patients were included in the study: mean age was 47.8 (47.5–48.1, 95% CI), 34.6% of which were male. Among them, 8397 were RRMS patients [mean age 47.1 (46.7–47.4), 34.1% male] and 1146 were identified as SPMS [mean age 53.1 (52.5–53.8), 38.4% male] (Table 1). The estimated prevalence of MS was 141.6 cases per 100,000 inhabitants, precisely 124.4/100,000 for RRMS and 17.2/100,000 for SPMS (Figure 1A), in line with available published data in the Italian population.6 As expected, higher MS prevalence was retrieved among female (185.4/100,000) versus male (95.6/100,000) population (Figure 1B).6 In the enrolled patients, the comorbidity index (CCI) averaged 0.9 (0.8–0.9) for MS, 0.9 (0.8–0.9) for RRMS, and 0.8 (0.7–0.8) for SPMS patients (Table 1). A tendency lower extent of MS (27%) and RRMS (25.1%) patients had previous MS-related hospitalizations as opposed to 41.4% of the SPMS patients. On the contrary, a tendency higher extent of MS and RRMS patients (58% and 60.2% respectively) had a previous MS specific treatment prescription, as opposed to 42.1% of the SPMS patients (Table 1).
 |
Table 1 Demographic and Clinical Characteristics of MS Patients |
 |
Figure 1 Prevalence of MS, RRMS and SPMS among the Italian population (A) and prevalence of MS estimated among male or female population (B). |
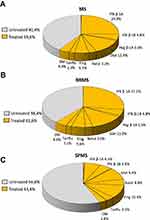
At index date, 51.2% of MS and 53.1% of RRMS patients were treated with DMTs, while among the SPMS cohort only 35.7% of individuals were under DMTs at index date (Figure 2). The most prescribed drugs at index date in MS and RRMS cohorts were interferon beta-1A (22.5% and 24.5%, respectively), glatiramer acetate (10.9% and 11.3%, respectively), and fingolimod (4.7% and 4.2%, respectively) (Figure 2A and B). Among SPMS patients, 9.3% were treated with fingolimod, 7.7% with glatiramer acetate, and 5.8% with interferon beta-1A (Figure 2C).
During the follow-up period 59.6% of MS, 61.6% of RRMS, and 43.4% of SPMS patients were treated with DMTs. As for the index treatment, in MS and RRMS patients, the first drug prescription during the follow-up was interferon-beta-1A (24.9% and 27.2%, respectively), glatiramer acetate (12.6% and 13%, respectively), and fingolimod (6.1% and 5.6%, respectively) (Figure 3A and B). SPMS patients were mainly treated with fingolimod (10.5%), glatiramer acetate (9.4%), and interferon beta-1A (6.3%) (Figure 3C).
The pharmaco-utilization analysis among alive patients during the follow-up has shown that 7.7% of MS, 7.9% of RRMS, and 5% of SPMS patients switched the index treatment to a different DMT (Figure 4A). Overall, 11% of 3666 MS patients discontinued the index treatment: specifically, 10.4% of 3386 RRMS patients and 18.6% of 280 of SPMS patients discontinued the index treatment (Figure 4B).
Overall, in the MS cohort, patients’ management was associated with a mean annual cost/patient of € 11796 (for treated patients) and € 3638 (for untreated patients) (Figure 5A). In the treated patients’ group, the cost related to drug prescriptions was 10520 € (Figure 5A). In MS treated and untreated patients 97.1% and 30.4%, respectively, of the total drug expenditure was accountable for DMT medications. A similar scenario was found in the RRMS cohort: the total annual cost/patient averaged € 11486 (for treated patients) and € 3183 (for untreated patients) (Figure 5B), with the drug prescriptions’ expenditure reaching 10366 € in the treated patients’ group (Figure 5B). In RRMS treated and untreated patients, 97.7% and 34.4%, respectively, of the total drug expenditure was accountable for DMT medications. The management of SPMS patients was associated with cost tendentially higher for treated (15512 €) and for untreated patients (6317 €) (Figure 5C); in the treated SPMS patients’ group, the biggest expense was related to drug prescriptions (12366 €), whereas in untreated patients the drugs (2544 €), hospitalizations (2677 €), and outpatient services (1097 €) were comparable (Figure 5A). In SPMS treated and untreated patients, 90.9% and 14.1%, respectively, of the total drug expenditure was accountable for DMT medications.
All drugs, all hospital stays and all outpatient specialized services during follow up period were considered.
During the follow-up of the overall MS cohort we performed a detailed cost analysis of the patients who had a first-line DMT course followed by a second-line DMT course, with regard to the treatment duration and cost. As shown in Table 2, the mean duration of the first-line DMT treatment averaged 27.4 ± 22.8 months and the mean cost was 19004 € for the whole period. The second-line DMT treatment lasted on average 31.1 ± 24.5 months and the mean cost reached was 47293€ for the whole period (Table 2). The mean annual cost estimated was 8322.93 € for the first- and 18248.04 € for the second-line treatment.
 |
Table 2 Duration and Mean Cost of First-Line DMT Followed by a Second-Line DMT Treatment During All Available Period of Follow-Up in Overall Alive MS Patients |
Discussion
This administrative databases-based observational study provide insights of the epidemiology of MS in Italy, the treatment patterns, and the economic burden associated with the MS disease management. We analyzed three cohorts of MS patients: the overall MS, the relapsing-remitting (RRMS), and the secondary-progressive (SPMS), identified by following previously published reports.2,14,15 Based on the datasets of almost 6 million inhabitants, across 2010–2017, 9543 patients affected by MS were enrolled. Among them, 12% of patients satisfied the SPMS inclusion criteria, while approximately 88% were identified as RRMS. These results are in line with the available published literature, stating that almost 85% of MS patients are diagnosed with a relapsing-remitting phenotype and 15% with a secondary progressive phenotype.3,4 Based on the present data, the estimate prevalence of MS was 141.6 cases/100,000, and specifically 124.4 RRMS cases/100,000 and 17.2 SPMS cases/100,000. These data are in line with previous reports stating that Italy is considered a high prevalence country for MS with about 70,000 people affected and an estimated prevalence of approximately 110 per 100,000 population.18,19 A recent data review by Koch-Henriksen and Magyari20 showed that there is an increasing trend in the incidence of MS plausibly accountable for improved public awareness, better health care, and changing in MS diagnostic criteria. But together with the increase in incidence, the disease course of MS has also changed, with an improvement of survival, resulting from the introduction of DMT and lifestyle changes.20
The analysis of demographic and baseline clinical characteristics of the enrolled patient population has shown that MS patients were quite young, averaging 47 years, and more than 65% of them were female. These data reflect the characteristics of the Italian MS population already reported in a cross-sectional retrospective study conducted in 16 European countries.21 In addition, almost one-third of the MS patients (and in particular RRMS ones) were hospitalized, and two-third of them were under MS treatment with DMTs before the enrollment in the study. Differently, SPMS patients, were highly hospitalized (41% had MS-related hospitalization and 47% any-disease-related hospitalization), and more than half of them did not receive MS specific DMTs during the characterization period or during the follow-up. In fact, although the last decade had seen the approval of various DMTs for relapsing forms of MS, there still exist limited options to treat progressive forms of MS.22 The pharmaco-utilization analysis revealed that the switch of the DMTs accounted only for a small percentage (less than 10%) of MS patients (both RR and SP). Almost 10% of MS and RRMS patients discontinued the DMT treatment during the follow-up period while this percentage was almost doubled among SPMS patients (18%). The scarce literature data available for SPMS patients have tried to enlighten the reason for the treatment withdrawal: i) physician’s decision, if the disease is considered stable or for non-efficacy issues; or ii) other reasons, such as patient’s wish, intolerance, adverse events, desire for pregnancy, or comorbidities.23 Moreover, SPMS still represents a medical challenge as it is difficult for diagnosis and there is still lack of well-established therapy. This scenario may change when new drugs will be available, however, to gain real world perspective takes time as it was for RRMS data patients.
Our study depicted the economic burden of MS in Italy. In line with a previous retrospective study carried out in Italy24,25 the healthcare cost associated with the management of MS patients averaged almost 11800 € in treated and 3600 € in untreated cohort, with an increasing trend in patients with more severe disease features, such as SPMS phenotype. Our data showed that the economic burden of MS is mainly due to medication costs (both in RR and SP phenotypes). In fact, disability at diagnosis and its progression over time can be considered a marker of a more aggressive disease, deserving more active and expensive treatments25,26 and have already been described as one of the main determinants of the economic burden of MS.27 In SPMS patients (especially those untreated with DMTs), we found that in addition to drugs, hospitalizations and outpatient services (including all specialistic visits and diagnostic tests) were accountable for the total healthcare expenditure. It has been well accepted that in advanced stages of SPMS, costs are mainly driven by hospital admissions, palliative treatments, and informal care.10,21 In a recent analysis among Danish population, a cost estimation in almost one million of subjects with brain disorder has been performed.27 The total attributable direct costs in 2015 averaged €5.2 billion and total attributable indirect costs were €11.2 billion. Traumatic brain injury, stress-related disorders, depression and stroke were the most common brain disorders; direct costs related to MS were below €0.25 billion, with inpatient and home care-related costs the most impacting items.27
The limitations of the present study were related to its retrospective observational nature and the use of anonymized data derived from administrative databases. Region/LHUs administrative databases have progressively improved the quality of the collected data. Nevertheless, some information may be missing; if the necessary information was missing for a given patient that patient was excluded from the analysis. In addition, there was a lack or limited clinical information on comorbidities, the disease severity, and other potential confounders that could have influenced the present results. Since the comorbidities and symptoms of MS progression were addressed based proxy of diagnosis, there might be incomplete capture of these variables among patients. Data on pharmacological treatments were captured from medical prescription and dispensing information; thus, the reason for interruption and switch was not retrievable from the dataset. Primary care data were not collected for this study, therefore it was not possible to assess the primary care-based management of the patients in the analysis. Moreover, in the cost analysis, only direct costs have been evaluated.
Conclusion
In conclusion, this retrospective observational database study among the Italian population allowed the identification of MS patients and of two different disease phenotypes: RRMS and SPMS. Medical direct costs associated with the management of MS patients derived mainly for medication supply. A bigger healthcare resource consumption was retrieved for SPMS patients, and despite these patients represented a small percentage of all MS patients, it should be taken into account that some RRMS patients may evolve in the SPMS phenotype.
Disclosure
This study was sponsored by Novartis Pharma S.p.A, under an agreement between Novartis Pharma S.p.A and Clicon S.r.l. Società Benefit. Clicon S.r.l. Società Benefit is an independent company. The agreement sign by Clicon S.r.l. Società Benefit and Novartis Pharma AG does not create any entityship, joint venture or any similar relationship between parties. Neither CliCon S.r.l. Società Benefit nor any of their representatives are employees of Novartis Pharma for any purpose. MN and DR are employees of Novartis Pharma. All other authors report no other conflicts of interest in this work.
References
1. Goldschmidt C, McGinley MP. Advances in the treatment of multiple sclerosis. Neurol Clin. 2021;39:21–33. doi:10.1016/j.ncl.2020.09.002
2. Gross HJ, Watson C. Characteristics, burden of illness, and physical functioning of patients with relapsing-remitting and secondary progressive multiple sclerosis: a cross-sectional US survey. NDT. 2017;13:1349–1357. doi:10.2147/NDT.S132079
3. Lublin FD, Reingold SC. Defining the clinical course of multiple sclerosis: results of an international survey. National Multiple Sclerosis Society (USA) Advisory Committee on clinical trials of new agents in multiple sclerosis. Neurology. 1996;46:907–911. doi:10.1212/WNL.46.4.907
4. Lublin FD, Reingold SC, Cohen JA, et al. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology. 2014;83:278–286. doi:10.1212/WNL.0000000000000560
5. Wallin MT, Culpepper WJ, Nichols E; GBD 2016 Multiple Sclerosis Collaborators. Global, regional, and national burden of multiple sclerosis 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:269–285. doi:10.1016/S1474-4422(18)30443-5
6. EMSP, Multiple sclerosis in Europe. Available from: https://emsp.org/wp-content/uploads/2021/06/MS-in-EU-access.
7. English C, Aloi JJ. New FDA-approved disease-modifying therapies for multiple sclerosis. Clin Ther. 2015;37:691–715. doi:10.1016/j.clinthera.2015.03.001
8. Finkelsztejn A. Multiple sclerosis: overview of disease-modifying agents. Perspect Medicin Chem. 2014;6:65–72. doi:10.4137/PMC.S13213
9. Bergamasco R, Agnello M, Della Giovanna M, et al. Utilità dei database amministrativi per l’analisi del percorso effettivo del paziente con sclerosi multipla: identificazione dei marcatori di ricaduta clinica [Usefulness of administrative databases for the analysis of therapeutic patterns of patients witl multiple sclerosis: identification of markers of clinical relapse]. Supplemento A Politiche Sanitarie. 2012;13:1.
10. Patti F, Amato MP, Trojano M, et al. Multiple sclerosis in Italy: cost-of-illness study. Neurol Sci. 2011;32:787–794. doi:10.1007/s10072-011-0499-2
11. Russo P, Capone A, Paolillo A, et al. Cost-analysis of relapsing-remitting multiple sclerosis in Italy after the introduction of new disease-modifying agents. Clin Drug Investig. 2004;24:409–420. doi:10.2165/00044011-200424070-00004
12. Karampampa K, Gustavsson A, Miltenburger C, et al. Treatment experience, burden, and unmet needs (TRIBUNE) in MS study: results from Italy. Mult Scler. 2012;18:29–34. doi:10.1177/1352458512441566c
13. Riley GF. Administrative and claims records as sources of health care cost data. Med Care. 2009;47:S51–5. doi:10.1097/MLR.0b013e31819c95aa
14. Van Le H, Le Truong CT, Kamauu AWC, et al. Identifying patients with relapsing-remitting multiple sclerosis using algorithms applied to US integrated delivery network healthcare data. Value Health. 2019;22:77–84. doi:10.1016/j.jval.2018.06.014
15. Katz Sand I, Krieger S, Farrell C, Miller AE. Diagnostic uncertainty during the transition to secondary progressive multiple sclerosis. Mult Scler. 2014;20:1654–1657. doi:10.1177/1352458514521517
16. Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi:10.1016/0021-9681(87)90171-8
17. Gupta SK. Intention-to-treat concept: a review. Perspect Clin Res. 2011;2:109–112. doi:10.4103/2229-3485.83221
18. Wade BJ. Spatial analysis of global prevalence of multiple sclerosis suggests need for an updated prevalence scale. Mult Scler Int. 2014;2014:1–7. doi:10.1155/2014/124578
19. Browne P, Chandraratna D, Angood C, et al. Atlas of multiple sclerosis 2013: a growing global problem with widespread inequity. Neurology. 2014;83:1022–1024. doi:10.1212/WNL.0000000000000768
20. Koch-Henriksen N, Magyari M. Apparent changes in the epidemiology and severity of multiple sclerosis. Nat Rev Neurol. 2021;17:676–688. doi:10.1038/s41582-021-00556-y
21. Battaglia M, Kobelt G, Ponzio M, et al. European multiple sclerosis platform. New insights into the burden and costs of multiple sclerosis in Europe: results for Italy. Mult Scler. 2017;23:104–116. doi:10.1177/1352458517708176
22. Bhatia R, Singh N. Can we treat secondary progressive multiple sclerosis now? Ann Indian Acad Neurol. 2019;22:131–136. doi:10.4103/aian.AIAN_345_18
23. D’Amico E, Ziemssen T, Cottone S. To stop or not to stop disease modifying therapies in secondary progressive multiple sclerosis, that is the question. Expert Rev Neurother. 2017;17:847–849. doi:10.1080/14737175.2017.1340831
24. Cortesi GA, Cozzolino P, Capra R, et al. The economic burden of different multiple sclerosis courses: analysis from Italian administrative and clinical databases. Farmeconomia. 2020;21:49–58.
25. Moccia M, Tajani A, Acampora R, et al. Healthcare resource utilization and costs for multiple sclerosis management in the Campania region of Italy: comparison between centre-based and local service healthcare delivery. PLoS One. 2019;14:e0222012. doi:10.1371/journal.pone.0222012
26. Fogarty E, Walsh C, McGuigan C, et al. Direct and indirect economic consequences of multiple sclerosis in Ireland. Appl Health Econ Health Policy. 2014;12:635–645. doi:10.1007/s40258-014-0128-3
27. Vestergaard SV, Rasmussen TB, Stallknecht S, et al. Occurrence, mortality and cost of brain disorders in Denmark: a population-based cohort study. BMJ Open. 2020;10:e037564. doi:10.1136/bmjopen-2020-037564
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.