Back to Journals » Clinical Epidemiology » Volume 15
The Epidemiology of Bile Acid Diarrhea in Denmark
Authors Kårhus ML , Ellegaard AM , Winther-Jensen M , Hansen S, Knop FK , Kårhus LL
Received 15 October 2023
Accepted for publication 17 November 2023
Published 7 December 2023 Volume 2023:15 Pages 1173—1181
DOI https://doi.org/10.2147/CLEP.S442054
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Vera Ehrenstein
Martin L Kårhus,1,* Anne-Marie Ellegaard,1,* Matilde Winther-Jensen,2 Susanne Hansen,2 Filip K Knop,1,3,4,* Line L Kårhus2,*
1Center for Clinical Metabolic Research, Copenhagen University Hospital – Herlev and Gentofte, Hellerup, Denmark; 2Center for Clinical Research and Prevention, Copenhagen University Hospital – Bispebjerg and Frederiksberg, Frederiksberg, Denmark; 3Steno Diabetes Center Copenhagen, Herlev, Denmark; 4Department of Clinical Medicine, Faculty of Health and Medical Science, University of Copenhagen, Copenhagen, Denmark
*These authors contributed equally to this work
Correspondence: Anne-Marie Ellegaard; Line L Kårhus, Email [email protected]; [email protected]
Objective: Bile acid diarrhea (BAD) is a socially debilitating disease with frequent bowel movements, urgency, and fecal incontinence as the main symptoms. It is caused by excessive bile acid levels in the colon and is most commonly treated with bile acid sequestrants. It is estimated that 1– 2% of the population suffers from the disease, but only a fraction of these are properly diagnosed with the gold standard ⁷⁵selenium-homotaurocholic acid (SeHCAT) test. Here, we use nationwide registries to describe the demographic characteristics of individuals suffering from BAD in Denmark.
Methods: Since the International Classification of Diseases diagnosis code for BAD was not used until 2021, we identified the BAD population by referral to SeHCAT testing followed by a prescription of a bile acid sequestrant (colestyramine, colestipol or colesevelam) within 365 days. The study period was from 2003 to 2021.
Results: During the study period, a total of 5264 individuals with BAD were identified with large differences between the five regions in Denmark. The number of prescriptions of colestyramine and colesevelam, the number of SeHCAT tests, and the number of individuals diagnosed with BAD increased during the study period. The BAD population had more co-morbidities and more health care contacts as well as lower levels of education and income compared with age- and sex-matched controls from the general population.
Conclusion: Using the Danish registries, we identified a BAD population, which seems to be inferior in health care and socio-economic parameters compared with the Danish general population.
Keywords: Bile acid diarrhea, epidemiology, nationwide registries
Two Letters to the Editor have been received and published for this article
A Response to Letter by Dr Nurmayanti has been published for this article.
A Response to Letter by Dr Fikri has been published for this article.
Introduction
Bile acid diarrhea (BAD) is a gastrointestinal disease with an estimated prevalence of 1–2% in the general population.1 The main symptoms are loose stools, frequent bowel movements, and urgency, making it a socially debilitating disease.2 The symptoms are caused by a larger spillover of bile acids to the colon than in healthy individuals.3,4 BAD can occur without known underlying pathophysiology or be secondary to other conditions such as inflammatory bowel diseases, cholecystectomy, celiac disease, intestinal resection, or damage after radiotherapy.1,5 In Denmark, the disease is diagnosed with the gold standard ⁷⁵selenium-homotaurocholic acid (SeHCAT) test, where the 7-day retention of orally administered SeHCAT is measured. Even though the SeHCAT test is a well-known and accepted diagnostic tool,6–8 BAD was not registered as a diagnosis in WHO’s International Classification of Diseases Version 10 (ICD10) before 2021. Thus, before this, BAD was registered under, eg, irritable bowel syndrome with diarrhea or unspecified intestinal malabsorption, and other unspecified gastrointestinal diseases. Due to the missing diagnosis code, individuals suffering from BAD are difficult to follow in registries, and epidemiological characterization of this rather large but not well-known patient group is presently lacking. Individuals with BAD may, nevertheless, be identified based on referral to SeHCAT testing and the prescription of bile acid sequestrants, which are widely accepted as the first-line treatment of BAD.9,10 Bile acid sequestrants bind the bile acids in the gut lumen leading to alleviation of the symptoms.
In this nationwide registry-based study, we identify individuals suffering from BAD in Denmark to describe their demographics.
Methods
Data Sources
We used the national Danish registries to collect information on a cohort of individuals with presumed bile acid diarrhea (BAD) in Denmark between 2003 and 2021. All Danish residents are assigned a unique personal ID number at birth or immigration via the Civil Registration System.11 This number is used in all contacts with the health care system and other authorities. We used this ID to link information across all registers. Thus, age, birthdate, and date of death, if deceased, were identified using the Civil Registration System.11
Information on diagnoses, procedures, and treatments was retrieved from the Danish National Patient Register,12 which contains diagnoses and dates of all hospital contacts since 1977. Treatments and surgical procedures have been registered since 1995. Since 1995, diagnoses have been coded using the ICD10 system.13
Using the Populations Education Register, we could assess educational status,14 while the Income Statistics Register made it possible to divide the population into income groups.15
The Register of Medicinal Products Statistics contains all redeemed prescriptions on an individual level since 1995 and was used to identify BAD-relevant prescriptions.16 Prescriptions are coded using Anatomical Therapeutic Chemical Classification (ATC) codes.17 All prescriptions in Denmark are handled by community pharmacies, ensuring that the register contains all redeemed prescriptions.
Contacts with primary health care (general practitioners, practicing specialists) have been registered in the Health Service Register since 1990.18 The reason for contact is not registered, but it is possible to assess the number of contacts based on different providers, eg, general practitioners, physiotherapists, dentists, etc.
Study Population
Since 2021, the ICD10 diagnosis code K90.8B Bile acid diarrhea has been used in Denmark for confirmed BAD. However, before 2021, the ICD10 code used to register BAD was not used. Hence, to identify individuals suffering from BAD we used a combination of procedures and prescriptions. In Denmark, BAD is diagnosed using the SeHCAT test; however, the result of the test is not registered in an easily retrievable way. Thus, we chose to define an individual as diagnosed with BAD if this individual had a SeHCAT test registered (Danish procedural code: WGLGSSEXX) in the National Patient Register followed by redemption of a prescription of bile acid sequestrant (colestipol [ATC code C10AC02], colestyramine [ATC code C10AC01] or colesevelam [ATC code C10AC04]) within 365 days after SeHCAT test. The window of 365 days after the SeHCAT test for the first prescription of bile acid sequestrant was chosen based on clinical experience. The date of BAD diagnosis was defined as the date of redemption of the first prescription. We also investigated redeemed prescriptions within one month, three months, six months, and without a time limit after the SeHCAT test (Table S1), but these cut-offs were not used in the definition of the BAD population.
To be able to compare the BAD population with a non-BAD population, individuals suffering from BAD were matched on sex and date of birth (±1 day) 1:10 with individuals from the general population. Matches were required to be alive on the day of matching. It was possible to be a match to more than one individual suffering from BAD with different match dates. The date of study entry was the date of BAD diagnosis for individuals suffering from BAD and the date of matching for matches.
Covariates
Household income five years before entry was extracted and the average was calculated. Income was then divided into tertiles for comparison.
Educational level was divided into groups based on the highest level of completed education at the time of study entry, corresponding to the International Standard Classification of Education (ISCED). ISCED 0–2 contains pre-primary, primary, and lower-secondary education. ISCED 3+5 entails high school/vocational education and short-cycle tertiary education (no education is consistent with ISCED 4 in Denmark). ISCED 6–8 consists of median-length tertiary education, bachelor, master, and PhD degrees.
Co-morbidities were defined according to the Nordic Multimorbidity Index (NMI), which has been found to predict mortality more accurately than the previously used Charlson comorbidity index.19 The NMI uses hospital diagnoses and redeemed prescriptions to assess co-morbidity status and assigns weights based on this status. We used diagnoses and prescriptions 10 years before study entry.
Health care use outside hospitals (general practitioners and practicing specialists) was counted during the 10 years before the study entry. More than 100 visits were pooled into one category as 100 or more visits. Contacts related to physiotherapy, chiropractic, dentistry, rehabilitation, foot care, psychology, care for eyes or ears, dieticians, and help classified as “others” were not included.
Statistical Analysis
Data are presented as mean + SD or median (25th–75th percentile for continuous data and n (%) for categorical data. No formal statistical tests were performed.
The percentage of BAD patients in each region was calculated as the number of BAD patients diagnosed in each region divided by the total number of inhabitants in the region during the study period. Inhabitants in each region were identified using the Civil Register.
Results
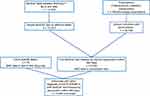
During the study period, a total of 5264 individuals suffering from BAD, defined by a diagnosis of ICD10 K90.8B or a SeHCAT test followed by a prescription of bile acid sequestrant within 365 days, were identified. Figure 1 shows the selection process of the BAD population according to the abovementioned definition and the number of SeHCAT tests and prescriptions during the study period. Only 70 individuals received the ICD10 code K90.8B during the study period. When identifying individuals suffering from BAD by a SeHCAT test followed by a prescription of colestyramine, colestipol, or colesevelam within 365 days, 5230 individuals were identified. Only 36 individuals were identified with both definitions of BAD (Figure 2). Additionally, we investigated redeemed prescriptions within one month, three months, six months, and without a time limit after the SeHCAT test. The number increased from 2694 after one month to 6379 individuals when no time constraint was used (Table S1).
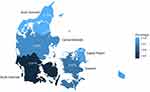
For comparison of the baseline characteristics, 52,640 age- and sex-matched individuals from the general population were identified (Table 1). We found regional differences in Denmark for diagnosis of BAD (Table 1 and Figure 3) with markedly lower numbers in South Denmark (0.01% of the total population in the region) and highest numbers in North Denmark (0.10%) and Capital Region (0.09%). Furthermore, individuals suffering from BAD had more co-morbidities and more health care contacts than the matches from the general population, while individuals suffering from BAD had a lower level of education and a lower income (Table 1).
The number of prescriptions of colestyramine and colesevelam as well as SeHCAT tests increased over time while prescriptions of colestipol decreased (Figure 4). The first SeHCAT test was registered in 2003, and the number of individuals undergoing this test increased to 1319 and 1301 per year in 2020 and 2021, respectively. The number of annual, unique BAD diagnoses—defined by diagnosis with ICD10 code K90.8B or SeHCAT test followed by a prescription of bile acid sequestrant within 365 days—increased from 26 in 2003 to 523 in 2021 (Figure 5).
We have recently shown that the glucagon-like peptide 1 (GLP-1) receptor agonist liraglutide is superior in reducing stool frequency compared with the bile acid sequestrant colesevelam.20 Consequently, some gastroenterologists have started treating individuals suffering from BAD with liraglutide. Since the use of liraglutide in BAD treatment has received increasing attention in recent years, we investigated the number of liraglutide prescriptions (ATC code: A10BJ02) after SeHCAT test among individuals in the BAD population (Table S2). In total, 248 individuals suffering from BAD received a first-time prescription of liraglutide after the SeHCAT test, and 172 individuals received 226 prescriptions with liraglutide after both the SeHCAT test and bile acid sequestrants prescription. However, when including all registered SeHCAT tests in the total population including non-BAD individuals, 262 individuals received a first-time prescription of liraglutide after the SeHCAT test (data not shown). The number of liraglutide prescriptions over time reveals a peak in 2018 (n = 180,439) followed by a rapid decrease (Figure S1).
Discussion
Here, we used the Danish national registries to identify individuals suffering from BAD, defined by a diagnosis with ICD10 code K90.8B or a SeHCAT test followed by a prescription of bile acid sequestrant within 365 days, in Denmark. With this definition, 5264 individuals were identified. We found large regional differences in diagnosis frequency within Denmark and that individuals suffering from BAD had lower income and attained lower education levels than age- and sex-matched controls. To our knowledge, this is the first epidemiological characterization of individuals with BAD.
Based on the fact that the SeHCAT test is specifically used for the diagnosis of BAD and that the bile acid sequestrants are widely accepted as first-line therapy for BAD, we are confident that the individuals identified with the abovementioned definition actually have BAD. We acknowledge the reasonable probability that a potentially substantial number of individuals suffering from BAD are not included in this definition. This includes individuals who have not undergone a SeHCAT test but are treated with bile acid sequestrants prescribed in a trial-and-error approach and individuals who are diagnosed with a SeHCAT test but refrain from medical treatment. Furthermore, an unknown number of individuals suffering from BAD are never diagnosed. As such, our definition does not reflect the true prevalence of BAD in the Danish population; rather it reflects a subset of the population that suffers from the disease with a very high probability. Ideally, we would base our definition on the SeHCAT test results. However, the way these results are registered requires manual extraction of the data, which is not feasible.
The lower level of education and income among individuals suffering from BAD compared with matched controls is unfortunately not surprising. It is known that individuals suffering from the related disease irritable bowel syndrome have more health care contacts, lower work productivity, and lower quality of life21–25. Assuming that the productivity and quality of life of individuals suffering from BAD are similarly affected, we find it a plausible explanation of their reduced educational level and household income reported here. Whether the more frequent health care contacts in the BAD population are due to their BAD symptoms or are related to other diseases and conditions is hard to discern.
Since 2003, when the first SeHCAT test was performed in Denmark, the number of BAD diagnoses has increased. In this study, we found large differences in the diagnosis frequency between the regions of Denmark. We speculate that these differences do not reflect true region-specific differences in prevalence but rather regional differences in clinical practice and diagnostic traditions since the five regions of Denmark are comparable regarding demographics and health-related characteristics.26
Interestingly, individuals suffering from BAD have more co-morbidities. Whether this directly relates to BAD or rather a confounding factor, such as obesity or the higher number of health care contacts, is presently unknown. Further studies are warranted to investigate if certain types of co-morbidities are overrepresented in the BAD population and whether there is a pathophysiological connection between these disorders and BAD.
Even though liraglutide is not approved for the treatment of BAD, we saw 262 individuals redeeming a prescription of liraglutide after the SeHCAT test. This is most likely the result of our published case report and recent clinical proof-of-concept study showing the superiority of liraglutide in reducing stool frequency compared with the bile acid sequestrant colesevelam.20,27
The completeness of the Danish registries and the possibility to cross-reference across registries due to the individual ID numbers are strengths of the study. Furthermore, the specific use of the gold standard SeHCAT test as a diagnostic test with its unique procedure code ensures that individuals referred to the test are suspected to suffer from BAD. As in all register studies, we rely on the registration of diagnoses and procedural tests being registered consistently. As the diagnosis code for BAD is new, no validity studies exist. With regards to SeHCAT tests, these are likely to be registered due to financial incentives. This, in combination with the validity of the Register of Medicinal Products, should ensure that the combination of SeHCAT and redeeming a bile acid sequestrant prescription reflects a conservative, but valid way of detecting BAD in Danish registers.
Conclusions
In conclusion, by using nationwide registries, we provide the first epidemiological characterization of individuals suffering from BAD identified based on ICD10 code K90.8B or a SeHCAT test followed by a prescription of bile acid sequestrant within 365 days showing lower education levels and more health care contacts compared with age- and sex-matched controls.
Abbreviations
BAD, bile acid diarrhea; ICD10, the international classification of diseases version 10; SeHCAT, ⁷⁵selenium-homotaurocholic acid; ATC, the anatomical therapeutic chemical classification; ISCED, the international standard classification of education; NMI, the Nordic multimorbidity index; GLP-1, glucagon-like peptide 1.
Data Sharing Statement
The dataset supporting the conclusions of this article is based on data from Danish national health registers and restrictions apply to the availability of these data, which were accessed through Statistics Denmark’s server under license for the current study. According to Danish law, this information cannot be publicly available. A request for access to the data needs approval from appropriate Danish authorities and are subject to Danish regulations on personal data protection.
Ethics Approval Statement
The study is approved by the Danish Data Protection Agency and registered in the Capital Region’s inventory (P-2022-253). Danish law does not require informed consent for registry studies using administrative data.
Acknowledgments
We thank Janne Petersen for contributing to the conceptualization and planning of the study.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Martin L Kårhus and Anne-Marie Ellegaard share the first-authorship, Filip K. Knop and Line L Kårhus share the last-authorship.
Disclosure
The authors declare no conflicts of interest.
References
1. Wedlake L, A’Hern R, Russell D, Thomas K, Walters JRF, Andreyev HJN. Systematic review: the prevalence of idiopathic bile acid malabsorption as diagnosed by SeHCAT scanning in patients with diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2009;30(7):707–717. doi:10.1111/j.1365-2036.2009.04081.x
2. Vijayvargiya P, Camilleri M. Update on bile acid malabsorption: finally Ready for prime time? Curr Gastroenterol Rep. 2018;20(3):10. doi:10.1007/s11894-018-0615-z
3. Kårhus ML, Sonne DP, Thomasen M, et al. Enterohepatic, Gluco-metabolic, and Gut microbial characterization of individuals with bile acid malabsorption. Gastro Hep Advances. 2022;1(3):299–312. doi:10.1016/j.gastha.2021.12.007
4. Wilcox C, Turner J, Green J. Systematic review: the management of chronic diarrhoea due to bile acid malabsorption. Aliment Pharmacol Ther. 2014;39(9):923–939. doi:10.1111/apt.12684
5. Farrugia A, Attard JA, Khan S, Williams N, Arasaradnam R. Postcholecystectomy diarrhoea rate and predictive factors: a systematic review of the literature. BMJ Open. 2022;12(2):e046172. doi:10.1136/bmjopen-2020-046172
6. Baena García A, Partida Palma F, García Martínez S, de Bonilla Candau M, Pajares Vinardell M. 75Se-Homocholic acid taurine scintigraphy (75SeHCAT®), a standard benchmark test in bile acid malabsorption? Rev Esp Med Nucl Imagen Mol (Engl Ed). 2019;38(5):305–311. doi:10.1016/j.remn.2018.12.005
7. Notghi A, O’Brien J, Low CS, Thomson W. Measuring SeHCAT retention: a technical note. Nucl Med Commun. 2011;32(10):960–966. doi:10.1097/MNM.0b013e32834a36af
8. Sciarretta G, Fagioli G, Furno A, et al. 75Se HCAT test in the detection of bile acid malabsorption in functional diarrhoea and its correlation with small bowel transit. Gut. 1987;28(8):970–975. doi:10.1136/gut.28.8.970
9. National Institute for Health and Care Excellence (NICE) Guidelines: Bile Acid. Malabsorption: Colesevelam; 2013. Available from: https://www.nice.org.uk/advice/esuom22/chapter/Key-points-from-the-evidence.
10. Orekoya O, McLaughlin J, Leitao E, Johns W, Lal S, Paine P. Quantifying bile acid malabsorption helps predict response and tailor sequestrant therapy. Clin Med. 2015;15(3):252–257. doi:10.7861/clinmedicine.15-3-252
11. Pedersen CB. The Danish civil registration system. Scand J Public Health. 2011;39(7_suppl):22–25. doi:10.1177/1403494810387965
12. Schmidt M, Schmidt SAJ, Sandegaard JL, Ehrenstein V, Pedersen L, Sørensen HT. The Danish national patient registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449–490. doi:10.2147/CLEP.S91125
13. World Health Organization, International classification of diseases, 10th version; January, 2023. Available from: https://icd.who.int/browse10/2016/en.
14. Jensen VM, Rasmussen AW. Danish education registers. Scand J Public Health. 2011;39(7_suppl):91–94. doi:10.1177/1403494810394715
15. Baadsgaard M, Quitzau J. Danish registers on personal income and transfer payments. Scand J Public Health. 2011;39(7_suppl):103–105. doi:10.1177/1403494811405098
16. Wallach Kildemoes H, Toft Sørensen H, Hallas J. The Danish national prescription registry. Scand J Public Health. 2011;39(7_suppl):38–41. doi:10.1177/1403494810394717
17. World Health Organization. Anatomical Therapeutic Chemical (ATC) classification. Available from: https://www.who.int/tools/atc-ddd-toolkit.
18. Sahl Andersen J, De Fine Olivarius N, Krasnik A. The Danish national health service register. Scand J Public Health. 2011;39(7_suppl):34–37. doi:10.1177/1403494810394718
19. Kristensen KB, Lund LC, Jensen PB, et al. Development and validation of a Nordic multimorbidity index based on hospital diagnoses and filled prescriptions. Clin Epidemiol. 2022;14:567–579. doi:10.2147/CLEP.S353398
20. Kårhus ML, Brønden A, Forman JL, et al. Safety and efficacy of liraglutide versus colesevelam for the treatment of bile acid diarrhoea: a randomised, double-blind, active-comparator, non-inferiority clinical trial. Lancet Gastroenterol Hepatol. 2022;7(10):922–931. doi:10.1016/S2468-1253(22)00198-4
21. Paré P, Gray J, Lam S, et al. Health-related quality of life, work productivity, and health care resource utilization of subjects with irritable bowel syndrome: baseline results from logic (longitudinal outcomes study of gastrointestinal symptoms in Canada), a naturalistic study. Clin Ther. 2006;28(10):1726–1735. doi:10.1016/j.clinthera.2006.10.010
22. Andrae DA, Patrick DL, Drossman DA, Covington PS. Evaluation of the Irritable Bowel Syndrome Quality of Life (IBS-QOL) questionnaire in diarrheal-predominant irritable bowel syndrome patients. Health Qual Life Outcomes. 2013;11(1):208. doi:10.1186/1477-7525-11-208
23. Frändemark Å, Jakobsson Ung E, Törnblom H, Simrén M, Jakobsson S. Fatigue: a distressing symptom for patients with irritable bowel syndrome. Neurogastroenterol Motil. 2017;29(1):e12898. doi:10.1111/nmo.12898
24. Frändemark Å, Törnblom H, Jakobsson S, Simrén M. Work productivity and activity impairment in Irritable Bowel Syndrome (IBS): a multifaceted problem. J Am Coll Gastroenterol. 2018;113(10):1540. doi:10.1038/s41395-018-0262-x
25. Håkanson C, Sahlberg-Blom E, Nyhlin H, Ternestedt BM. Struggling with an unfamiliar and unreliable body: the experience of irritable bowel syndrome. J Nurs Healthcare Chronic Illness. 2009;1(1):29–38. doi:10.1111/j.1365-2702.2008.01001.x
26. Henriksen DP, Rasmussen L, Hansen MR, Hallas J, Pottegård A. Comparison of the five Danish regions regarding demographic characteristics, healthcare utilization, and medication use—a descriptive cross-sectional study. PLoS One. 2015;10(10):e0140197. doi:10.1371/journal.pone.0140197
27. Kårhus ML, Brønden A, Røder ME, Leotta S, Sonne DP, Knop FK. Remission of bile acid malabsorption symptoms following treatment with the glucagon-like peptide 1 receptor agonist liraglutide. Gastroenterology. 2019;157(2):569–571. doi:10.1053/j.gastro.2019.04.002
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.