Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 16
The Efficacy of GLP-1 Analogues on Appetite Parameters, Gastric Emptying, Food Preference and Taste Among Adults with Obesity: Systematic Review of Randomized Controlled Trials
Authors Aldawsari M , Almadani FA, Almuhammadi N, Algabsani S, Alamro Y, Aldhwayan M
Received 31 August 2022
Accepted for publication 14 January 2023
Published 2 March 2023 Volume 2023:16 Pages 575—595
DOI https://doi.org/10.2147/DMSO.S387116
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Gian Paolo Fadini
Malikah Aldawsari,* Fatima A Almadani,* Nujud Almuhammadi, Sarah Algabsani, Yara Alamro, Madhawi Aldhwayan
Community Health Sciences Department, King Saud University, Riyadh, Saudi Arabia
*These authors contributed equally to this work
Correspondence: Malikah Aldawsari, Tel +966114670000, Fax +966114677580, Email [email protected]
Background: Obesity is an epidemiological issue that negatively affects public health and has led to a high global burden on the healthcare system. Several approaches to control and overcome the obesity crisis have been established. However, Nobel discoverers found that glucagon-like peptide-1 analogues (GLP-1 analogues) positively regulate appetite and food intake, eventually leading to weight loss.
Objective: The present systematic review aims to summarize the currently available evidence of the impact of GLP-1 analogues on appetite, gastric emptying, taste sensitivity, and food preferences among adults with obesity without other chronic diseases.
Methods: A systematic literature search was conducted from October 2021 to December 2021 from three electronic databases (PubMed, Scopus, and ScienceDirect), including only randomized clinical trials (RCTs). Studies were based on the use of GLP-1 analogues, of any dosage and duration among adults with obesity without other medical diseases; studies measured appetite, gastric emptying, food preferences, and taste as a primary or secondary outcome. The risk of publication bias in each study was assessed independently using the updated Cochrane risk-of-bias tool (RoB2).
Results: Twelve studies met the inclusion criteria with a total sample size of 445 participants. All the included studies measured at least one or more of the primary outcomes. The promising effect was evidenced by most studies showing appetite suppression, delayed gastric emptying, and changes in taste and food preferences.
Conclusion: GLP-1 analogues are effective obesity management therapy that could decrease food intake and eventually reduce weight by suppressing appetite, reducing hunger, decreasing gastric emptying, and altering food preferences and taste. However, high-quality, long-term, large sample size studies are crucial to examine the efficacy and effective dose of GLP-1 analogues intervention.
Keywords: GLP-1 analogues, Liraglutide, Semaglutide, appetite, satiety, gastric emptying, palatability
Introduction
Obesity is a growing health crisis that places a significant burden on health-care systems worldwide, with an estimated global prevalence of 2.1 billion people suffering from overweight or obesity in adults.1–3 In Saudi Arabia, the prevalence of obesity was estimated to be 24.7% in 2020.4 Weight loss can be achieved by several strategies, including dietary approaches, physical activity, pharmacotherapies, and surgical approaches (bariatric surgeries).5 There are relatively few medical interventions (including pharmacotherapies) approved for weight management.6 Five drugs were approved by Food and Drug Administration (FDA) for weight management, including; Orlistat, Lorcaserin, Phentermine/topiramate, Naltrexone/bupropion, and GLP-1 analogues (Liraglutide).7 Obesity can alter many biological functions. It is associated with increased fat mass, altered levels of gastrointestinal (GI) hormones, increased appetite, and dysregulated satiety and satiation mechanisms, which are now transformed to be the targets of some weight loss drugs.8
The central nervous system (CNS) regulates body weight and energy balance. It receives signals from peripheral organs, such as the gut, pancreas, and adipose tissue, regulating food consumption, digestion, absorption, and storage.9 Gut-derived hormones such as the anorexigenic hormone Glucagon-like peptide-1 (GLP-1) have been identified as a player in conveying meal-related information of hunger and/or satiety to the brain.9 It has demonstrated that alteration in the gut-derived hormones (GLP-1) post-bariatric surgery is one of the mechanisms leading to sustained weight loss post-surgery.10 GLP-1 is a peptide composed of 30 amino acids. It is produced and secreted in the L cells of the small intestine in response to food intake. It is an incretin and neuroendocrine hormone.11–13 There are different forms of GLP-1 analogues in the pharmacotherapy industry, the most commonly used are Liraglutide, GLP-1 infusion, Semaglutide, and Exendin. GLP-1 analogues induce weight loss through multiple mechanistic properties, including insulin stimulation, inhibition of glucagon secretion, delaying gastric emptying, enhancing satiety, reducing hunger, reducing energy intake, and regulating appetite and food reward.11,13,14 A multiplex brain-gut interaction orchestrates the role of physiologic and pharmacologic GLP-1 in the modulation of appetite.15 The effect of GLP-1 analogues on appetite, food preferences, and GI hormones using different doses, duration, and assessment methods have been investigated in several studies.16–18 Additionally, multiple clinical studies have demonstrated a successful impact of exogenous GLP-1 analogues on humans in promoting weight loss via dose-dependent reduction in appetite scores, and ad libitum caloric consumption, making GLP-1 analogues therapy an attractive option in the management of a patient with obesity.19,20 To the best of our knowledge, there is no previous systematic review investigated the efficacy of GLP-1 analogues versus placebo or no intervention on appetite, gastric emptying, food preference, and taste among adults with obesity.
Therefore, the present systematic review aims to summarize the currently available evidence of the impact of GLP-1 analogues on appetite, gastric emptying, food preferences, and taste among adults with obesity.
Materials and Methods
This systematic review was registered at the PROSPERO international prospective register of systematic reviews (CRD42022297683). It was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. It considers the finding of the clinical trials, and below will clarify the systematic review question and PICOTS, study eligibility, search strategy, data collection and extraction, and validity assessment of risks of bias in included studies. No ethical approval was required for this study.
Systematic Review Question and PICOTS
This systematic review was conducted to investigate the effect of GLP-1 analogues versus placebo or any other intervention on appetite, gastric emptying, food preferences, and taste among adults with obesity without any other medical diseases.
For the review’s PICOTS [Population (P), Intervention (I), Comparison (C), Outcome (O), Time (T), and Study design (S)], criteria were defined before the literature search and are detailed in Table 1. Concisely, our study question was, in adults with obesity without any other medical diseases (P), does GLP-1 analogues (I), compared with placebo or any other intervention (C), have an impact on appetite, gastric emptying, food preferences, and/or taste (O), for any time and any duration (T) in randomized clinical trials (RCTs) (S)?.
 |
Table 1 PICOS Structure Used for Search Strategy |
Primary Outcomes
The primary outcome of the analysis was to evaluate the impact of GLP-1 analogues on appetite, gastric emptying, food preferences, and/or taste in adults with obesity without any other medical diseases.
Data Sources and Search Strategy
This systematic review was conducted according to the recommendations of the Cochrane Collaboration. A systematic search was conducted using three electronic databases (PubMed, Scopus, and ScienceDirect) to include all published trials. Search terminology and keyword searches were used to optimize the investigation with no time restrictions to avoid missing relevant publications. The searches were conducted from October 2021 to December 2021 to identify relevant studies. A search strategy was conducted to systematically assess the literature for providing an updated review of all the available published RCTs investigating the effect of GLP-1 analogues on appetite, gastric emptying, food preferences, and/or taste among adults with obesity without any other medical diseases. Supplementary Materials report search methods and electronic search strategies’ text (Appendix A).
Study Eligibility
Inclusion Criteria
Studies were included if they met the PICOTS criteria described in Table 1. Briefly, studies had only published RCTs that implemented a method for randomization and blinding. Studies used GLP-1 analogues of any dosage and any duration among adults with obesity without diabetes or any other medical diseases, compared with a placebo or any other intervention. No time frame determines; studies measured appetite, gastric emptying, food preferences, and/or taste as a primary or secondary outcome.
Exclusion Criteria
Excluded non-human studies, participants with diabetes or with any other medical diseases, meeting or conference abstracts, unpublished studies, different study designs (case-report study, case-control study, cross-sectional study, reviews, observational study, letter to the editor, or theses), different languages other than English.
Data Collection and Extraction
Five reviewers conducted data extraction. The first screening by reading titles and abstracts of all retrieved articles divided by five reviewers (Alamro, Aldawsari, Algabsani, Almadani, and Almuhammadi), and the second screening by reading the full articles divided by four reviewers (Aldawsari, Algabsani, Almadani, and Almuhammadi). The tabulation table for all studies included in this systematic review was extracted by two researchers (Almadani and Alamro). Data extracted in the tabulation table of the eligible studies included in the systematic review are first author, published year, country, study design, sample size, population characteristic, intervention (type and dose), placebo (type and dose), duration, outcomes, and tools (Table 3). The full extracted data table is shown in Supplementary Materials (Appendix B).
 |
Table 2 Characteristics of the Population in This Systematic Review |
 |
Table 3 Characteristics for All RCTs Studies Included in This Systematic Review |
Validity Assessment
The studies included in this systematic review were carefully evaluated by four researchers (Alamro, Aldawsari, Algabsani, and Almuhammadi). Independently assessed the quality of all the included trials using the Cochrane Collaboration to evaluate the risk of bias tool. Six studies by (Alamro, and Aldawsari) and the other six studies by (Algabsani, and Almuhammadi), and if there were any disagreements between the authors, they resolve the disagreements through a discussion with (Almadani). Risk is assessed as a judgment (low risk, high risk, or unclear) for the six domains of bias selection, performance, detection, attrition, and reporting bias, and one additional domain (other bias). Statistical analysis was conducted using Review Manager 5 software (version 5.4).
Results
Literature Search
The current systematic review follows the Preferred Reporting Items of Systematic Reviews and Meta-Analysis (PRISMA) guidelines for reporting systematic reviews.21 The flowchart of the study selection process is described in Figure 1. A total of 1222 studies were identified through all electronic database searches (Figure 1), and 1195 studies remain after duplication removal (Microsoft Excel 16.55). One thousand one hundred and ninety-five articles were screened based on the title and abstract, 1148 irrelevant studies were excluded, and 47 studies were eligible for full-text review. Finally, excluding 35 studies because they did not meet the inclusion criteria;16 due to different populations, 12 due to different outcomes, 6 due to different study designs, and 1 for different language, the remaining 12 RCTs were included.16–18,20,22–29 The 12 RCTs were screened and included in the tabulation table and the risk of bias assessment tables shown in the Supplementary Materials (Appendix B and C).16–18,20,22–29 The reasons for excluding studies at each stage of the literature screening are reported in the PRISMA Flow Diagram shown in Figure 1.
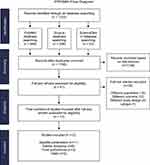 |
Figure 1 PRISMA flow diagram for the study. Notes: PRISMA figure adapted from Page MJ, Moher D, Bossuyt PM, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:n160. Creative Commons.30 |
Characteristics of Included Studies
Population characteristics: twelve randomized clinical trials, either single or double-blind studies, either compared the intervention group with the controlled group (placebo) or with other intervention, were included to examine the efficiency of GLP-1 analogues use on appetite parameters, gastric emptying, food preferences, and/or taste published between 1998 and 2021.16–18,20,22–29 The duration of the interventions varied between 5 days to 52 weeks, and the sample size ranged between 6 and 113, yielding 445 participants in this systematic review. Eight out of twelve studies were crossover designs, with a total of 171 participants.16,18,20,22,26–29 Four out of twelve were parallel-group studies;17,23–25 three out of four divided the participants into two groups (the GLP-1 analogous group and the placebo group); the total number of participants included in the GLP-1 analogous group for three studies was 81, and 80 participants were in the placebo group.17,23,24 A study by Tronieri et al 2020 divided the intervention into three groups: the intensive behavioral therapy (IBT) group (n = 36), IBT with Liraglutide group (n = 37), and the IBT with Liraglutide and meal replacement shake group (n = 40).25 Participants aged 18 −75 years old, with obesity as defined as BMI greater than or equal to 30 kg/m2, without any other medical conditions. We placed no restrictions on gender or ethnicity. However, four studies included only males,16,27–29 and the remaining eight included both genders.17,18,20,22–26 These studies were performed in different countries; three studies were conducted in the United States,17,24,25 one study in the Netherlands,26 three studies in Sweden,20,27,28 two studies were conducted in Denmark16,29 f, two in the United Kingdom,18,22 and one in Germany.23 Characteristics of this systematic review are in Table 2. Eleven out of twelve assessed the effect of GLP-1 analogues on appetite profile.16–18,20,23–29 Eight out of twelve evaluated the effect of GLP-1 analogues on gastric emptying.16,20,22–24,26–28 Three out of twelve assessed the effect of GLP-1 analogues on food preferences,18,23,27 and two out of twelve evaluated the effect of GLP-1 analogues on taste.16,17 The characteristics of each study are presented separately in Table 3.
Quality Assessments
The risk of bias was assessed for each study. All the information for each study is available, with the comments for each domain in Supplementary Materials (Appendix C). In addition, the summary of the risk of bias is shown in Figures 2 and 3 for all included studies. The assessment of the risk of bias within these studies revealed the following results.16–18,20,22–29 Briefly, the generation of random allocation for participants was unclear in eight, and low risk of bias in four studies. Concealment of allocation was described in four studies, unclear in five studies, and low risk in three studies. One study had a high risk of blinding participants and personnel (performance bias), two studies had an unclear risk, and nine had a low risk. Blinding outcome assessments (detection bias) were described in two studies, unclear in six, and low risk in four studies. Incomplete outcome data (attrition bias) was described in one study, unclear in two, and low risk in nine studies. Additionally, three studies had unclear od bias for selection reporting (reporting bias), and nine studies showed a low risk of bias.
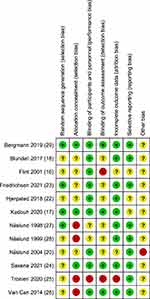 |
Figure 2 Summary of risk of bias for all included studies. |
 |
Figure 3 Risk of bias graph for all included studies. |
Summary of Findings
The effect of GLP-1 analogues was evaluated in eleven out of twelve studies on appetite profile,16–18,20,23–29 eight out of twelve studies on gastric emptying,16,20,22–24,26–28 three out of twelve assessed the effect of GLP-1 analogues on food preferences,18,23,27 and two out of twelve studies on taste.16,17
Effect of GLP-1 Analogues on Appetite
The term satiety, hunger, and fullness are the most assessed markers for appetite.31 Eleven out of twelve studies assessed the effect of GLP-1 analogues on appetite profile.16–18,20,23–29 All the studies used a 100-m VAS tool to assess the appetite. A total of 90 out of 220 participants were recruited in the GLP-1 analogues group in parallel group studies,17,23–25 and 182 participants were recruited in crossover design studies.16,18,20,26–29 So, the total of participants who used GLP-1 analogues were 272 participants. Four studies out of eleven used Liraglutide,17,24–26 five out of eleven used GLP-1 infusion,16,20,27–29 and two out of eleven used Semaglutide.18,23
Liraglutide
Four studies used Liraglutide as GLP-1 analogues to assess the appetite profile.17,24–26 A cross-over study by Van Can et al 2014 used two doses of Liraglutide (1.8 mg and 3.0 mg). All Liraglutide doses showed similar significant effects on overall appetite score (reduced appetite), increased satiety and fullness, and decreased hunger compared to placebo.26
The second study was a parallel design study by Tronieri et al 2020 that divided the intervention groups into three groups. Behavioral therapy alone, behavioral therapy with Liraglutide 3.0 mg/day (dose started at 0.6 mg/day and increased weekly by 0.6 mg), and behavioral therapy with Liraglutide 3.0mg/day (dose started at 0.6 mg/day and increased weekly by 0.6 mg) and meal replacement shake for 52 weeks. Behavioral therapy with the Liraglutide group reported greater improvements in reduced hunger, increased fullness, and reduced food preoccupation than behavioral therapy alone at 24 weeks. IBT-Liraglutide participants reported larger reductions at week 6 in hunger (P = 0.005) and food preoccupation (P = 0.002) and more significant increases in fullness (P = 0.001) compared to IBT alone. However, there were no statistical differences between groups in appetite at week 52.25
The third study by Kadouh et al 2020, was a parallel-group study that included 17 participants that used Liraglutide 3.0 mg/daily (starting with 0.6 mg per week to a maintenance dose of 3.0 mg per day) for 16 weeks. Liraglutide group reported more fullness (P = 0.02) and lower prospective food consumption (P = 0.03) compared to baseline.17
The last study by Saxena et al 2021, was a parallel study design that used Liraglutide 3.0 mg (dose started by 0.6 mg per day and escalated by 3.0 mg per week) for six weeks. The Liraglutide group showed an increase in satiety and fullness compared to the placebo group, but no statistical significance was found between the groups. However, they found significant differences in appetite (decreased appetite) at weeks 3 and 6, in prospective food consumption in 30-min postprandial at weeks 3 and 6, and for hunger at week 3 only.24
GLP-1 Infusion
Five studies used GLP-1 infusion to assess the appetite profile.16,20,27–29 Three studies used the same dose of GLP-1 infusion 0.75 pmol GLP−1/ kg−1 /min−1 for one time.16,27,28 All of them found the same conclusion; GLP-1 infusion decreases hunger (P < 0.05).16,27,28
A study by Näslund et al (1998) concluded that hunger and prospective food consumption were significantly lower (P = 0.03; P = 0.04, respectively) with GLP-1 than with placebo (saline infusion). In contrast, feelings of fullness were not significantly different between GLP-1 infusion and placebo (saline infusion) (P = 0.4).27 The same results were found in the Flint et al 2001 study, which decreased the ratings of hunger and prospective food consumption decreased in the GLP-1 infusion compared with saline (P < 0.05).16 A study by Näslund et al 1999, using GLP-1 infusion during the period between breakfast and lunch and between lunch and dinner decreased the ratings of hunger (P < 0.05; P = 0.01 respectively) and prospective consumption (P < 0.05; P = 0.07, respectively) and fullness (P < 0.05; P = 0.09, respectively).28
A crossover study by Näslund et al (2004) used two methods to administer GLP-1 for five days in a row for each intervention. Subjects received GLP-1 infusion or saline (placebo) as a continuous subcutaneous infusion (CSI) or GLP-1 infusion or saline as prandial subcutaneous injections (PSI) for 5 d in a row, with a wash-out period of 9 d in between. The first method was a PSI dose of 76 nmol 30 min before meals, four times daily, a total of 302·4 nmol/24 hours, and the second was CSI dose of 12·7 nmol/h; a total of 304·8 nmol/24 hours. They found no significant differences between the effects of GLP-1 infusion as PSI or CSI compared with placebo on hunger and satiety ratings 300 min after the meal. Group PSI reported increased satiety ratings compared with the placebo group after day one (P = 0·03), but after day five, they did not find significant differences in satiety. Group CSI has no effects on satiety and hunger. Briefly, when taking the PSI group and CSI group together to evaluate the effect of GLP-1 infusion on satiety and hunger. Satiety increased before the meal and 60min after the meal during the placebo group and GLP-1 infusion groups (P = 0·05). Hunger on day one and day five showed significant suppression (P = 0·01; P = 0·03 respectively) before the meal and 60 min after the meal during the placebo group and GLP-1 infusion groups.20
The last study in the GLP-1 infusion section has been done by Bergmann et al 2019. In the third study, each participant was included in 5 study days for different interventions. GLP-1 infusion (1 pmol kg−1 min−1) with 50 g oral glucose tolerance test (OGTT) has been used in two separate study days, once alone and once with glucose-dependent insulinotropic polypeptide (GIP). No significant differences among the interventions were observed for the appetite profile at the beginning of the study. But at the end of the study, hunger and prospective food consumption were significantly lower (P = 0.031; P = 0.028, respectively) on the days of study that used GLP-1 infusion as part of the intervention than the placebo study day.29
Semaglutide
Two studies used Semaglutide as GLP-1 analogues to assess the appetite profile.18,23 The first study by Blundell et al 2017, was a crossover study that included 30 participants. That used subcutaneous Semaglutide, dose-escalated to 1.0 mg once weekly (starting dose was 0.25 mg (4 weeks), escalating to 0.5 mg (4 weeks) and then 1.0 mg (4 weeks)) for 12 weeks. The overall appetite suppression score indicated decreased appetite (P = 0.0023).18 The second study by Friedrichsen et al 2021, was a parallel‐group design that included 36 participants in the intervention group, used subcutaneous Semaglutide 2.4 mg/weekly for 20 weeks. Results showed higher overall postprandial appetite suppression score with the Semaglutide group (P = 0.001). Also, an increase in fullness and satiety and decrease in hunger and prospective food consumption with the Semaglutide group compared to the placebo (P < 0.02).23
Effect of GLP-1 Analogues on Gastric Emptying
Eight out of twelve studies assessed the effect of GLP-1 analogues on gastric emptying.16,20,22–24,26–28 All clinical trials used the paracetamol absorption test technique as an assessment method. A total of 64 out of 126 participants were recruited in the GLP-1 analogues group in parallel group studies,23,24 and 126 participants were recruited in crossover design studies.16,20,22,26–28 So, the total of participants who used GLP-1 analogues were 190 participants. Two studies out of eight used Liraglutide,24,26 four studies out of eight used GLP-1 infusion,16,20,27,28 and two studies out of eight used Semaglutide.22,23 Among all studies, only Liraglutide and GLP-1 infusion studies showed consistent results in lowering gastric emptying compared to placebo, as was reflected by the declined absorption rate of paracetamol (P < 0.05).19,20,24,26–28
Liraglutide
Studies that used Liraglutide found consistent results in lowering gastric emptying compared to placebo (P < 0.05).24,26 A study by Van Can et al (2014) found a significant difference between the Liraglutide 3.0 mg group and the placebo group after 1 hour. The Liraglutide 3.0 mg group was lower by 23% compared to the placebo group (P = 0.007), but there was no significant difference between the placebo group and the Liraglutide 1.8 mg group in which gastric emptying was reduced by 13% only (P = 0.14).26 Also, Saxena et al (2021) found that Liraglutide caused a significant delay in gastric emptying when administered over six weeks. There was a statistically significant difference between the groups at week 6 (1 hour, P = 0.0348; 5 hours, P = 0.0152).24
GLP-1 Infusion
Studies that used GLP-1 infusion for one time found the same results; there was a significant difference between GLP-1 infusion group and saline infusion group.16,20,27,28 Gastric emptying was significantly slower during GLP-1 infusion (P = 0.001, P = 0.001, and P = 0.0001) in study by Näslund et al 1998, used GLP-1 infusion (0.75 pmol GLP−1/ kg−1/min−1) for 210 min,27 Näslund et al 1999, used GLP-1 infusion (0.75 pmol GLP-1/kg−1/min−1) 5 mL/hour,28 and Flint et al 2001, used GLP-1 infusion (0.75 pmol GLP-1/kg−1/min−1),16 respectively. Näslund et al 2004, is a crossover study that used two methods to administer GLP-1 for five days. They found that the gastric emptying rate was reduced by the two methods, and there was a significant difference in PSI (P < 0.001) and CSI (P < 0.05). However, it was more effective and faster with the PSI of GLP-1.20
Semaglutide
The two studies that used Semaglutide found no statistically significant effect after administering Semaglutide.22,23 A crossover study by Hjerpsted et al (2018) administered Semaglutide gradually, 0.25 mg/4 weeks, escalating to 0.5 mg/4 weeks and then to 1.0 mg/4 weeks, showed that the Semaglutide group was 27% lower gastric emptying during the first hour than the placebo (P = 0.0012). However, there was no significant difference between treatments for overall postprandial gastric emptying (P = 1.01).22 The second study by Friedrichsen et al (2021) used subcutaneous Semaglutide (dose-escalated to 2.4 mg/week) for 20 weeks. No differences were found between Semaglutide and placebo (P = 0.8474).23
Effect of GLP-1 Analogues on Food Preferences
Three studies investigated the effect of GLP-1 on food preference by using different assessment methods (a forced-choice list, The Leeds Food Preference Task (LFPT) and Control of Eating Questionnaire (CoEQ)).18,23,27 One study out of three used GLP-1 infusion27 and two studies out of three used Semaglutide.18,23
GLP-1 Infusion
The first study by Näslund et al 1998, was a crossover study in which they used GLP-1 infusion (0.75 pmol GLP-1/ kg−1/min−1) and measured food preferences by the forced-choice list method, which is designed to reveal a specific preference for proteins or carbohydrates; over two occasions, five days apart. It was found that there was no significant difference between the GLP-1 analogous (GLP-1 infusion) group and the placebo group in the proportion of food selected as carbohydrate, protein, fat, or low energy from the food-choice list (data not shown). Also, no significant difference in the ratio of high-carbohydrate to high-protein items selected between the GLP-1 analogous (GLP-1 infusion) group and the placebo group (P = 0.7). There was a significant difference in the number of items selected from the food-choice list. It was significantly lower in the GLP-1 analogous (GLP-1 infusion) group than in the placebo group immediately after food intake and four hours after intake (P = 0.03).27
Semaglutide
The second study by Blundell et al (2017) used subcutaneous Semaglutide, dose-escalated to 1.0 mg once weekly (starting dose was 0.25 mg (4 weeks), escalating to 0.5 mg (4 weeks) and then 1.0 mg (4 weeks)) for 12 weeks. Researchers measured food preferences by LFPT. The study evaluated the effect of Semaglutide on hedonics (food preferences and cravings), and results showed a lower liking for high-fat and non-sweet foods (P = 0.0016).25 In addition, lower ratings of wanting to high-fat and non-sweet foods (P = 0.0203) compared to placebo.18
Finally, the third study by Friedrichsen et al (2021) used subcutaneous Semaglutide (dose-escalated to 2.4 mg/week), and intervention period was 20 weeks. This study used CoEQ to assess food preferences, which resulted in a significantly less craving for sweet, savory, and dairy products after 20 weeks of Semaglutide intervention compared to placebo (P < 0.05).23
Effect of GLP-1 on Taste
Two studies (one Liraglutide and one GLP-1 infusion) investigated the effect of GLP-1 analogues on taste using different methods.16,17
Liraglutide
A study by Kadouh et al (2020) used Liraglutide 3.0 mg/daily (escalated by 0.6 mg per week to a maintenance dose of 3.0 mg per day). Measured taste by a standardized nutrient drink test; the period was 16 weeks. They found that GLP-1 analogous (Liraglutide) resulted in a significantly less desire for sweet, salty, fatty, and savory foods with (P < 0.05) when compared to placebo.17
GLP-1 Infusion
Flint et al 2001 used GLP-1 infusion (0.75 pmol GLP-1 · kg-1 · min-1) to assess palatability using the ratings of palatability (appearance, smell, taste, after-taste, and overall palatability). They found no differences in the palatability ratings of the breakfast between groups. However, lunch appearance was more attractive during the GLP-1 infusion than the placebo (P < 0.05).16
Discussion
This systematic review was undertaken to summarize the currently available evidence of the effect of GLP-1 analogues on appetite parameters, gastric emptying, food preferences, and taste in adults with obesity. One thousand two hundred and twenty-two journal articles from three databases were screened and assessed for eligibility. Twelve RCTs were included with the enrollment of 445 adults with obesity without other medical diseases.16–18,20,22–29 Administration of GLP-1 analogues varies depending on the type (Liraglutide, GLP-1 infusion, or Semaglutide) and the route of administration to participants (intravenous infusion or subcutaneous injections). The findings suggested that GLP-1 analogues in adults with obesity could alter the appetitive and consummatory reward value. However, the effect depends on the type of GLP-1 analogues used and the dosage.
The effect of GLP-1 analogues was evaluated in eleven out of twelve studies on appetite profile,16–18,20,23–29 eight out of twelve studies on gastric emptying,16,20,22–24,26–28 three out of twelve assessed the effect of GLP-1 analogues on food preferences,18,23,27 and two out of twelve studies on taste.16,17
Appetite
The improvements in the appetite profile (hunger, fullness, and prospective food consumption) upon GLP-1 analogues treatment of all types (Liraglutide, GLP-1 infusion and Semaglutide) were shown in 11 studies that measured appetite in this review.16–18,20,23–29 These findings are consistent with the results of previous study that aimed to investigate the mechanisms underlying the effect of GLP-1 analogues (Liraglutide) on weight loss in patients with type 2 diabetes mellitus and suggest that the mechanisms of the GLP-1 analogues induced weight-loss may involve the improvement of appetite control.14 Van Can et al 2014, used two doses of Liraglutide (1.8 mg and 3.0 mg) and concluded that all Liraglutide doses showed similar significant effects on overall appetite score (indicating reduced appetite), increased satiety and fullness, and decreased hunger in comparison with placebo.26 All the studies used a 100-m VAS tool,16–18,20,23–29 a widely used tool to assess appetite and subsequent food intake in nutrition research.32 VAS questionnaires were used in the behavior research to evaluate the pre- and postprandial hunger, fullness, prospective food consumption, and desire to eat, and in controlled studies.33,34 It has been established that this approach has been validated in the literature and considered as the best strategy to assess appetite and satiety.33–36 The studies have been sectioned into three parts depending on the type of GLP-1 analogues (Liraglutide, GLP-1 Infusion, Semaglutide) used. Most of the studies used GLP-1 infusion.
All five studies that used GLP-1 infusion has similar findings on lowering hunger feelings and prospective food consumption significantly, despite different dosage used.16,20,27–29 The administration of GLP-1 infusion could influence the appetite parameter. For instance, a study by Näslund et al 2004 was conducted using two GLP-1 infusion administration methods, either PSI or CSI of GLP-1 (dose ranging from 45 to 75 pmol/kg). In general, the groups that used GLP-1 infusion despite the administration methods reported significant hunger suppression on day one and day five before the meal but not after the meal. Satiety ratings were higher in PSI group compared with the placebo group after day one GLP-1 infusion. In contrast, group CSI reported no effects on satiety and hunger. They stated that due to this choice of assessment, they found less variations in the hunger ratings, and properly they did not record the trough values. In addition, the mean plasma GLP-1 concentration in the CSI group before the meal was 65 pmol/l, which may not be sufficient to suppress food intake.20
Semaglutide has been used in two studies to assess the appetite profile with different doses.18,23 Blundell et al 201718 used 1.0 mg/weekly and Friedrichsen et al 202123 used 2.4 mg/weekly. Both demonstrated a significantly higher overall appetite suppression score with the Semaglutide group compared to the placebo group. Moreover, Friedrichsen et al 2021 reported increases in fullness and satiety and decreases in hunger and prospective food consumption with the Semaglutide group compared to the placebo. More related appetite outcomes may be related to the higher dose of Semaglutide in the second study.23
As demonstrated by recent animal studies, the GLP-1 receptor can be mediated through multiple sites in the brain, including the brainstem and hypothalamic nuclei that regulate homeostatic feeding appetite.37,38 A randomized, crossover, placebo-controlled trial by van Bloemendaal et al 2014 included 48 patients with type 2 diabetes (T2DM) and patients with normoglycemia and obesity aimed to determine the acute effects of intravenous administration of the GLP-1 analogues exenatide, with or without prior GLP-1 receptor blockade. Using functional magnetic resonance imaging (fMRI), they found that exenatide decreased food intake and food-related brain responses in T2DM patients and participants with obesity compared to placebo. This finding offers a novel insight into the GLP-1 analogues mechanisms in regulating the food intake and could provide understanding in how weight loss achieved after GLP-1 analogues administration.9
Gastric Emptying
All eight studies that assessed the effect of GLP-1 analogues on gastric emptying used the same paracetamol absorption test technique as their assessment method.16,20,22–24,26–28 This technique is accepted as an indirect measure of gastric emptying,39 it measures gastric emptying through its capacity to absorb the medication over 4–6 hours.40 Among all studies, only Liraglutide and GLP-1 infusion (despite using different dosages and administration methods) studies showed consistent results in lowering gastric emptying compared to placebo, as was reflected by the declined absorption rate of paracetamol.19,20,24,26–28 Hjerpsted et al 2018 and Friedrichsen et al 2021 found no significant effects of Semaglutide administration on gastric emptying in the intervention group compared to the placebo group.22,23 Hjerpsted et al 2018 also stated that the effect of Semaglutide on lowering gastric emptying was only observed during the first hour of Semaglutide administration, and there was no significant difference between treatments for overall postprandial gastric emptying.22
The gastric emptying rate is a major determinant of food intake and weight loss41 and has been associated with reduced postprandial blood glucose in patients with type 2 diabetes mellitus.42 Halawi et al 2017 suggested that the retardation of gastric emptying may be attributed to the increased subjective sensation of fullness, satiation, and satiety that was previously strongly associated with decreased calorie intake (in a nutrient drink test and a buffet meal) in about 280 participants.43 The effects of GLP-1 analogues as an obesity treatment were partly mediated by reduced gastric emptying.40
Food Preferences
Three studies investigated the effect of GLP-1 on food preference using different assessment methods.18,23,27 Näslund et al (1998) measured the food preferences after GLP-1 Infusion by the forced-choice list; and they found significant difference between the GLP-1 infusion group and the placebo group in number of items selected from the food-choice list, but not in the proportion of food selected.27 Blundell et al 2017, examine effect of Semaglutide on the food preferences by LFPT. They demonstrated a lower liking and wanting for high-fat and non-sweet foods among intervention group.18 Friedrichsen et al (2021) used Semaglutide to assess the food preferences by CoEQ, reported a significantly less craving for sweet, savory, and dairy products intervention.23
Taste
A study by Kadouh et al (2020) measured the taste by standardized nutrient drink test; found a significant less desire for sweet, salty, fatty, and savory foods with Liraglutide when compared to placebo.17 A study by Flint et al 2001 assessed palatability using the ratings of palatability (appearance, smell, taste, after-taste, and overall palatability). They only found significant effect of GLP-1 infusion on the lunch appearance with differences on the other parameters of palatability in the intervention group compared to the placebo.16 This might be due to the short intervention period of one day infusion and the assessment method used (Ratings of palatability).
The GLP-1 analogues used in all studies included in this systematic review are the Liraglutide dose (3.0 mg), the GLP-1 infusion dose range from (0.75 pmol/kg/min to 1.0 pmol/kg/min), and the Semaglutide dose range from (1.34 to 2.4 mg). This present study suggests that GLP-1 analogues significantly improved the appetite parameter. In regard to gastric emptying, all types and administration routes of GLP-1 analogues showed consistent results in lowering gastric emptying. The present review suggested that the alteration in food preferences and taste sensitivity could be one of the mechanisms of weight loss after GLP-1 analogues intervention. The results of included studies indicated that GLP-1 analogues might have a positive impact on suppressing the desire for sweet, salty, and fatty foods with longer intervention periods ranging from 12 to 20 weeks.18,44 Regardless of the assessment and administration methods differences. In addition, the effects of GLP-1 analogues are similar whether given by once-weekly or one-day infusion injection in patients with obesity.18,44 However, outcomes of food preferences and taste cannot be generalized because the number of studies included, and types of GLP-1 analogues are limited compared with other variables and each study used different assessment tool.
Strengths and Limitations
This study had several strengths. First, this systematic review follows the PRISMA guidelines. Second, the Cochrane Collaboration risk of bias tool was used to assess the risk of bias and the quality of evidence for all studies included. Third, the sole inclusion of RCT. Furthermore, it is the first to investigate the mechanism of the GLP1 analogues on appetite, gastric emptying, food preference, and/or taste. However, some limitations are worth considering. No meta-analysis was performed due to different research methods. Studies on certain variables (food preferences and taste) were limited. Finally, the total number of included studies was small.
More RCTs with considerable sample size and precise, accurate methods during and after the intervention are needed to support the efficacy of GLP1 analogues mechanisms.
Conclusion
GLP-1 analogues proved to be an effective weight management therapy to overcome obesity and its physiologic and metabolic complications. Based on the supported data provided in this systematic review, GLP-1 analogues reduced weight by lowering appetite, gastric emptying, and changing food preferences among adults with obesity. However, more high-quality studies with larger sample sizes and longer durations are required to provide a comprehensive clinical judgment.
Acknowledgments
We would like to express our profound gratitude to King Saud University. We would also like to thank Ms. Lana Mahrous and all the anonymous referees who provided useful and detailed comments on previous research articles.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Garvey WT, Mechanick JI, Brett EM, et al. American Association of Clinical Endocrinologists and American College of Endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity. Endocr Pract. 2016;22:1–203. doi:10.4158/EP161365.GL
2. Yumuk V, Tsigos C, Fried M, et al. European guidelines for obesity management in adults. Obes Facts. 2015;8(6):402–424. doi:10.1159/000442721
3. Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the global burden of disease study 2013. Lancet. 2014;384(9945):766–781. doi:10.1016/S0140-6736(14)60460-8
4. Althumiri NA, Basyouni MH, Almousa N, et al. Obesity in Saudi Arabia in 2020: prevalence, distribution, and its current association with various health conditions. Healthc. 2021;9(3):1–8. doi:10.3390/healthcare9030311
5. Kushner RF. Weight loss strategies for treatment of obesity. Prog Cardiovasc Dis. 2014;56(4):465–472. doi:10.1016/j.pcad.2013.09.005
6. Erratum: Addendum. Obesity management for the treatment of type 2 diabetes: standards of medical care in diabetes-2020. Diabetes Care. 2020;43(Suppl.1):S89–S97. doi:10.2337/dc20-ad08b
7. Daneschvar HL, Aronson MD, Smetana GW. FDA-approved anti-obesity drugs in the United States. Am J Med. 2016;129(8):879.e1–879.e6. doi:10.1016/j.amjmed.2016.02.009
8. Camilleri M. Peripheral mechanisms in appetite regulation. Gastroenterology. 2015;148(6):1219–1233. doi:10.1053/j.gastro.2014.09.016
9. Van Bloemendaal LG, IJzerman RS, Ten Kulve J, Barkhof F. GLP-1 receptor activation modulates appetite- and reward-related brain areas in humans. Diabetes. 2014;2014:1–43.
10. Abdeen GN, Miras AD, Alqahtani AR, le Roux CW. Vertical sleeve gastrectomy in adolescents reduces the appetitive reward value of a sweet and fatty reinforcer in a progressive ratio task. Surg Obes Relat Dis. 2019;15(2):194–199. doi:10.1016/j.soard.2018.10.033
11. Müller TD, Finan B, Bloom SR, et al. Glucagon-like peptide 1 (GLP-1). Mol Metab. 2019;30:72–130. doi:10.1016/j.molmet.2019.09.010
12. Pais R, Gribble FM, Reimann F. Stimulation of incretin secreting cells. Ther Adv Endocrinol Metab. 2016;7(1):24–42. doi:10.1177/2042018815618177
13. Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87(4):1409–1439. doi:10.1152/physrev.00034.2006
14. Horowitz M, Flint A, Jones KL, et al. Effect of the once-daily human GLP-1 analogue liraglutide on appetite, energy intake, energy expenditure and gastric emptying in type 2 diabetes. Diabetes Res Clin Pract. 2012;97(2):258–266. doi:10.1016/j.diabres.2012.02.016
15. Shah M, Vella A. Effects of GLP-1 on appetite and weight. Rev Endocr Metab Disord. 2014;15(3):181–187. doi:10.1007/s11154-014-9289-5
16. Flint A, Raben A, Ersbøll AK, Holst JJ, Astrup A. The effect of physiological levels of glucagon-like peptide-1 on appetite, gastric emptying, energy and substrate metabolism in obesity. Int J Obes. 2001;25(6):781–792. doi:10.1038/sj.ijo.0801627
17. Kadouh H, Chedid V, Halawi H, Burton DD, Clark MM, Khemani D. GLP-1 analog modulates appetite, taste preference, gut hormones and regional body fat stores in adults with obesity hoda. Cad Saude Publica. 2020;12(1):1–30.
18. Blundell J, Finlayson G, Axelsen M, et al. Effects of once-weekly semaglutide on appetite, energy intake, control of eating, food preference and body weight in subjects with obesity. Diabetes Obes Metab. 2017;19(9):1242–1251. doi:10.1111/dom.12932
19. Verdich C, Flint A, Gutzwiller JP, et al. A meta-analysis of the effect of glucagon-like peptide-1 (7-36) amide on Ad Libitum energy intake in humans. J Clin Endocrinol Metab. 2001;86(9):4382–4389. doi:10.1210/jc.86.9.4382
20. Näslund E, King N, Mansten S, et al. Prandial subcutaneous injections of glucagon-like peptide-1 cause weight loss in obese human subjects. Br J Nutr. 2004;91(3):439–446. doi:10.1079/bjn20031064
21. Kamioka H. Preferred reporting items for systematic review and meta-analysis protocols (prisma-p) 2015 statement. Japanese Pharmacol Ther. 2019;47(8):1177–1185.
22. Hjerpsted JB, Flint A, Brooks A, Axelsen MB, Kvist T, Blundell J. Semaglutide improves postprandial glucose and lipid metabolism, and delays first-hour gastric emptying in subjects with obesity. Diabetes Obes Metab. 2018;20(3):610–619. doi:10.1111/dom.13120
23. Friedrichsen M, Breitschaft A, Tadayon S, Wizert A, Skovgaard D. The effect of semaglutide 2.4 mg once weekly on energy intake, appetite, control of eating, and gastric emptying in adults with obesity. Diabetes Obes Metab. 2021;23(3):754–762. doi:10.1111/dom.14280
24. Saxena AR, Banerjee A, Corbin KD, Parsons SA, Smith SR. Energy intake as a short-term biomarker for weight loss in adults with obesity receiving liraglutide: a randomized trial. Obes Sci Pract. 2021;7(3):281–290. doi:10.1002/osp4.486
25. Tronieri JS, Wadden TA, Walsh O, et al. Effects of liraglutide on appetite, food preoccupation, and food liking: results of a randomized controlled trial. Int J Obes. 2020;44(2):353–361. doi:10.1038/s41366-019-0348-6
26. Van Can J, Sloth B, Jensen CB, Flint A, Blaak EE, Saris WHM. Effects of the once-daily GLP-1 analog liraglutide on gastric emptying, glycemic parameters, appetite and energy metabolism in obese, non-diabetic adults. Int J Obes. 2014;38(6):784–793. doi:10.1038/ijo.2013.162
27. Näslund E, Gutniak M, Skogar S, Rössner S, Hellström PM. Glucagon-like peptide 1 increases the period of postprandial satiety and slows gastric emptying in obese men. Am J Clin Nutr. 1998;68(3):525–530. doi:10.1093/ajcn/68.3.525
28. Näslund E, Barkeling B, King N, et al. Energy intake and appetite are suppressed by glucagon-like peptide-1 (GLP-1) in obese men. Int J Obes. 1999;23(3):304–311. doi:10.1038/sj.ijo.0800818
29. Bergmann NC, Lund A, Gasbjerg LS, et al. Effects of combined GIP and GLP-1 infusion on energy intake, appetite and energy expenditure in overweight/obese individuals: a randomised, crossover study. Diabetologia. 2019;62(4):665–675. doi:10.1007/s00125-018-4810-0
30. Page MJ, Moher D, Bossuyt PM, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:n160. doi:10.1136/bmj.n160
31. Rate GE, Response G. Suppression of oral sweet sensations during consumption of sweet food in humans: e ff ects on; 2020.
32. Douglas SM, Leidy HJ. Novel methodological considerations regarding the use of visual analog scale (VAS) appetite questionnaires in tightly controlled feeding trials. Curr Dev Nutr. 2019;3(6):1–4. doi:10.1093/cdn/nzz061
33. Hill AJ, Rogers PJ, Blundell JE. Techniques for the experimental measurement of human eating behaviour and food intake: a practical guide. Int J Obes Relat Metab Disord J Int Assoc Study Obes. 1995;1995:361–375.
34. Blundell J, De Graaf C, Hulshof T, et al. Appetite control: methodological aspects of the evaluation of foods. Obes Rev. 2010;11(3):251–270. doi:10.1111/j.1467-789X.2010.00714.x
35. Stubbs RJ, Hughes DA, Johnstone AM, et al. The use of visual analogue scales to assess motivation to eat in human subjects: a review of their reliability and validity with an evaluation of new hand-held computerized systems for temporal tracking of appetite ratings. Br J Nutr. 2000;84(4):405–415. doi:10.1017/s0007114500001719
36. Flint A, Raben A, Blundell JE, Astrup A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes. 2000;24(1):38–48. doi:10.1038/sj.ijo.0801083
37. Secher A, Jelsing J, Baquero AF, et al. The arcuate nucleus mediates GLP-1 receptor agonist liraglutide-dependent weight loss. J Clin Invest. 2014;124(10):4473–4488. doi:10.1172/JCI75276
38. Dickson SL, Shirazi RH, Hansson C, Bergquist F, Nissbrandt H, Skibicka KP. The glucagon-like peptide 1 (GLP-1) analogue, exendin-4, decreases the rewarding value of food: a new role for mesolimbic GLP-1 receptors. J Neurosci. 2012;32(14):4812–4820. doi:10.1523/JNEUROSCI.6326-11.2012
39. Medhus AW, Lofthus CM, Bredesen J, Husebye E. Gastric emptying: the validity of the paracetamol absorption test adjusted for individual pharmacokinetics. Neurogastroenterol Motil. 2001;13(3):179–185. doi:10.1046/j.1365-2982.2001.00249.x
40. Werner U. Effects of the GLP-1 receptor agonist lixisenatide on postprandial glucose and gastric emptying - Preclinical evidence. J Diabetes Complications. 2014;28(1):110–114. doi:10.1016/j.jdiacomp.2013.06.003
41. Acosta A, Camilleri M, Burton D, et al. Exenatide in obesity with accelerated gastric emptying: a randomized, pharmacodynamics study. Physiol Rep. 2015;3(11):1–10. doi:10.14814/phy2.12610
42. Trahair LG, Horowitz M, Marathe CS, et al. Impact of gastric emptying to the glycemic and insulinemic responses to a 75-G oral glucose load in older subjects with normal and impaired glucose tolerance. Physiol Rep. 2014;2(11):1–10. doi:10.14814/phy2.12204
43. Halawi H, Camilleri M, Acosta A, et al. Relationship of gastric emptying or accommodation with satiation, satiety, and postprandial symptoms in health. Am J Physiol. 2017;313(5):G442–G447. doi:10.1152/ajpgi.00190.2017
44. Halawi H, Khemani D, Eckert D, et al. Effects of liraglutide on weight, satiation, and gastric functions in obesity: a randomised, placebo-controlled pilot trial. Lancet Gastroenterol Hepatol. 2017;2(12):890–899. doi:10.1016/S2468-1253(17)30285-6
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
