Back to Journals » Journal of Pain Research » Volume 16
The Effects of Intravenous Dexamethasone on Rebound Pain After Nerve Block in Patients with Ankle Fracture: A Randomized Controlled Trial
Authors Gao M, Li Y, Yu J, Li W, Qin S, Zhang Y, Zhu L, Hou Z, Wang Q
Received 30 November 2022
Accepted for publication 20 March 2023
Published 31 March 2023 Volume 2023:16 Pages 1127—1136
DOI https://doi.org/10.2147/JPR.S399660
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Ellen M Soffin
Mingyang Gao,1,* Yanan Li,1,* Jiaxu Yu,1 Wei Li,1 Shiji Qin,2 Yahui Zhang,3 Lian Zhu,4 Zhiyong Hou,4 Qiujun Wang1
1Department of Anesthesiology, Third Hospital of Hebei Medical University, Shijiazhuang, Hebei Province, People’s Republic of China; 2Department of Foot and Ankle Surgery, Third Hospital of Hebei Medical University, Shijiazhuang, Hebei, People’s Republic of China; 3Department of Nursing, Third Hospital of Hebei Medical University, Shijiazhuang, Hebei, People’s Republic of China; 4Department of Orthopaedics, Third Hospital of Hebei Medical University, Shijiazhuang, Hebei, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Qiujun Wang, Department of Anesthesiology, Third Hospital of Hebei Medical University, No. 139, Ziqiang Road, Shijiazhuang City, Hebei, People’s Republic of China, Tel/Fax +86-311-8860-2072, Email [email protected]
Purpose: A single-injection nerve block provides excellent analgesia in a short time, but rebound pain after the nerve block disappears has attracted researchers’ attention. The aim of this study is to evaluate the effect of intravenous dexamethasone on rebound pain after adductor canal block (ACB) and popliteal sciatic nerve block in patients with ankle fracture.
Methods: We recruited 130 patients with ankle fractures scheduled for open reduction and internal fixation (ORIF), each of whom received ACB and popliteal sciatic nerve block. Patients were divided into two groups: C (ropivacaine only) and IV (ropivacaine with intravenous dexamethasone). The primary outcome was the incidence of rebound pain. Secondary outcomes included the following: pain scores at 6 h (T1), 12 h (T2), 18 h (T3), 24 h (T4), and 48 h (T5) after operation; duration of the nerve block; number of presses of the analgesia pump and rescue analgesic consumption in the three-day postoperative period; quality of recovery scale (QoR-15 score); postoperative sleep quality; satisfaction of patients; and levels of serum inflammatory markers (IL-1β, IL-6, and TNF-α) six hours after surgery.
Results: Compared with group C, the incidence of rebound pain in group IV was significantly reduced, and the duration of nerve block was extended by approximately nine hours (P< 0.05). Moreover, patients in group IV had significantly lower pain scores at T2-T4, lower levels of serum inflammatory markers (IL-1β, IL-6, and TNF-α), higher QoR-15 score two days after the operation, and satisfactory sleep quality the night after surgery (P< 0.05).
Conclusion: Intravenous dexamethasone can reduce the rebound pain after adductor block and sciatic popliteal nerve block in patients with ankle fracture surgery, prolong the duration of nerve block, and improve the quality of early postoperative recovery.
Keywords: dexamethasone, nerve block, postoperative analgesia, ankle fracture, rebound pain
Introduction
Patients with ankle fractures often have acute pain after surgery. Peripheral nerve blocks are widely used in patients with ankle fractures because of their precise analgesic effect, which can reduce postoperative opioid use and accelerate postoperative recovery.1 However, in recent years, rebound pain after nerve block has become a reason for concern.2,3 Patients who receive nerve blocks can get adequate analgesic effect shortly after surgery, but once the block effect disappears, some patients experience severe pain; the intensity and frequency of rebound pain seem to be higher after upper limb rather than lower limb surgery.4,5 In a qualitative study of patients’ experiences, Henningsen et al6 found that, although nonsteroidal drugs were given preemptively, some patients still suffered severe pain after ankle fracture surgery. Therefore, it is necessary to further explore the rebound pain in lower limb orthopedic surgery. Nowadays, many such operations are performed on an outpatient basis, and the occurrence of rebound pain may lead to excessive prescription of opioid medications and more demand of medical resources.4,7 Therefore, it is urgent to find an effective method to relieve rebound pain after surgery and ensure that pain is adequately managed to improve patient prognosis and rehabilitation.
Continuous peripheral nerve block and dexamethasone as an adjuvant mixed with local anesthetic perineural administration are currently effective measures to prevent rebound pain.5,8 However, continuous peripheral nerve block has shortcomings, such as catheter displacement and fluid leakage, and the need of follow-up care limits its application.9 Dexamethasone, as a long-acting glucocorticoid commonly used in the perioperative period, has anti-inflammatory, analgesic and postoperative nausea and vomiting prevention effects when applied intravenously;10,11 as an adjuvant to nerve block, it can prolong its duration.12 Fang et al8 reported that perineural administration of 8 mg dexamethasone is an effective preventive measure for rebound pain after nerve block. However, perineural dexamethasone is off-label, and although there are no reports of nerve damage caused by dexamethasone used in nerve blocks, its safety still needs to be evaluated in large-scale clinical trials.13
At present, there are few reports on intravenous dexamethasone for the prevention of rebound pain after nerve block in patients undergoing ankle fracture surgery. Therefore, the purpose of this study was to observe the effect of intravenous dexamethasone on rebound pain after adductor canal block (ACB) combined with popliteal sciatic nerve block in patients with ankle fractures, and explore the mechanism of its action to provide a reference for postoperative pain reduction and improvement of analgesic programs.
Methods
This randomized, double-blind clinical study, conducted in the Third Hospital of Hebei Medical University, was approved by our Institutional Ethical Board (NO.2021-020-1) and registered in the Chinese Clinical Trial Registry (ChiCTR2100049075). This study was conducted in accordance with the Declaration of Helsinki (October 2013) and clinical practice guidelines. Informed consent was obtained from all subjects involved in the study.
Study Design and Participants
In our study we selected patients age 18 or older with American Society of Anesthesiology (ASA) status I-II, which underwent open reduction and internal fixation (ORIF) of an ankle fracture between July 2021 and March 2022. Eligible fracture patterns were bimalleolar or trimalleolar fracture. The following exclusion criteria were applied: body mass index ≥35kg/m2; medical contraindication to nerve block (eg coagulopathy, allergy to local anesthetics, or localized infectious and neurologic disease) or dexamethasone; multiple injuries or fractures; time from fracture to operation over five days; cognitive or psychiatric dysfunction causing inability to use intravenous analgesia pump and/or inability to verbally communicate pain scores; pregnancy; receiving chronic pain treatment; having drug abuse history; having respiratory or cardiac diseases; and nerve block failure expressed as pain of at least three points in the numeric rating scale (NRS) three hours after surgery.
Randomization
Patients were equally distributed in two groups (C and IV) using random numbers. Before the operation, patients in group C received 2 mL of intravenous normal saline and those in group IV received 10 mg of intravenous dexamethasone (5 mg/mL, batch number: 2201201, Tianjin Jinyao Pharmaceutical Co., Ltd, China). All patients, anesthesiologists, outcome assessors and the person who analyzed the data were unaware of the patients’ group assignments.
Anesthesia
After the patients entered the operation room, peripheral vein were opened and oxygen facemasks were installed. Routine intraoperative monitoring included oxygen saturation (SpO2), electrocardiography (ECG), non-invasive blood pressure (NBP), and sedation with intravenous midazolam (2 mg) and sufentanil (3 μg).
Patients were placed in a supine position, and the injured limb was slightly abducted. After skin disinfection, the ultrasound probe (line probe, frequency 5–10 MHz, Sonosite, USA) was placed on the medial side of the mid-thigh. The sartorius, adductor longus, vastus medialis muscles, and the femoral artery were identified. Using an in-plane technique, the nerve block needle reached the adductor canal, and 20 mL of 0.375% ropivacaine (Batch number: LCCU, AstraZeneca AB, Sweden) was injected into the lateral triangular region of the femoral artery. Subsequently, patients were placed in the lateral decubitus position, and the probe was placed in the popliteal fossa with a short-axis; to identify separate tibial and common peroneal nerves and the movement of the probe, tibial and common peroneal nerves were brought together above the popliteal crease. After skin disinfection, using an in-plane technique, the nerve block needle was passed through the biceps femoris muscle to the vicinity of the nerve and 20 mL of 0.375% ropivacaine was injected.
General anesthesia consisted of sequential intravenous injections of midazolam 0.02–0.08 mg/kg, propofol 1.5–2 mg/kg, sufentanil 0.2–0.4 μg/kg, and cisatracurium 0.1–0.2 mg/kg. After patient’s loss of consciousness, a laryngeal mask was placed to conduct mechanical ventilation. Anesthesia was maintained using propofol 3–5 mg·kg−1·h−1 and remifentanil 0.1–0.3 μg·kg−1·h−1. Intermittent intravenous injection of cisatracurium provided muscle relaxation. No patients received additional narcotics, local anesthetics or other corticosteroids. All operations were performed by the same team of foot and ankle surgeons, and all patients had inflatable tourniquets. Palonosetron 0.25 mg was given intravenously 30 minutes before the end of surgery to prevent postoperative nausea and vomiting.
Postoperative Management
All patients were connected with patient-controlled intravenous analgesia (PCIA) pump (1.5 μg/kg sufentanil, saline diluted to 100mL; flow rate:2 mL/h; bolus: 0.5 mL; lockout time: 15 mins). When they felt the nerve block was ineffective, the patients were instructed to open PCIA independently, and they could press the button for the PCIA bolus when experiencing severe pain. If the patient needed more analgesics in the ward, ketorolac tromethamine 30 mg was administered intravenously.
Blood Samples
A venous blood sample (4 mL) was extracted from each patient before anesthesia (T0), and 6 hours after surgery (T1). After 1000 g centrifugation during 15 min the serum was sub-packed and stored at −80°C.
Outcome Assessment
The primary outcome were the pain rebound, including the incidence, onset and duration of rebound pain. Referring to the definition of rebound pain by Barry et al:2 an increase from well-controlled pain (NRS pain score ≤3) to severe pain (NRS pain score ≥7) typically within 12 to 24 hours of resolution of the nerve block.
The secondary outcomes were: (a) postoperative pain level of patients at 6h (T1), 12h (T2), 18h (T3), 24h (T4) and 48h (T5) after operation; (b) the duration of the nerve block, which was defined as the time from completion of the block to the first sensation of pain at the surgical site; (c) the time of the first analgesic request, total number of presses of the analgesia pump and rescue analgesic in the postoperative period of three days; (d) quality of recovery scale (QoR-15 score) used to evaluate the early postoperative recovery at postoperative day two; (e) sleep quality on the night of surgery and postoperative day 1; (f) patient satisfaction was collected at postoperative day two; (g) postoperative nausea and vomiting (PONV), and other postoperative adverse events within three day. The sleep quality before the injury was used as a criterion; sleep quality was assessed using a numeric rating scale (0–10 points). Patients’ satisfaction was evaluated by questioning whether they would like to receive the same analgesic method in the future (0–10 points). Postoperative follow-up was done by a research assistant.
Serum Index Detection
Serum levels of interleukin-1β (IL-1β), interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) were determined by commercial IL-1β, IL-6 and TNF-α (4A Biotech Co., Ltd, China) ELISA kits, respectively.
Sample Size Estimation and Statistical Analyses
The sample size for the study was calculated using SAS version 9.1. According to the published literature,14 the incidence of rebound pain is about 50%. We aimed to reduce rebound pain by 25% in group IV compared to group C. We calculated that 58 patients per group were needed (power = 80%, α = 0.05), but to allow for the incidence of patient withdrawals, we planned for 65 patients per group.
Kolmogorov–Smirnov test was used to assess distribution of the variables. The measurement data of normal distribution was expressed by mean±SD, the comparison between groups was performed by two independent samples t-test, the measurement data of skewness distribution was expressed by median and IQR, and the comparison between groups was expressed by Mann–Whitney test. The duration of nerve block, the time of first analgesia and QoR-15 were estimated by Hodges-Lehmann Estimation at 95% CI. Duration of nerve block was estimated using the Kaplan-Meier method and compared using the Log rank test. Categorical data were expressed as frequency (percentage), using Pearson’s chi-squared test or Fisher’s exact probabilities test.
All statistical analyses were performed with IBM SPSS software version 25.0 (SPSS Inc, Chicago, IL). The significance level for each hypothesis was a two-sided P < 0.05.
Results
One hundred forty patients were initially included in this study; ten of them were excluded because they did not meet the inclusion criteria. Finally, one hundred and thirty patients were enrolled. Figure 1 presents the group allocation process according to the Consolidated Standards of Reporting Trials statement.
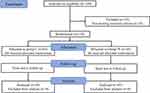 |
Figure 1 The consolidated standards of Reporting Trials statement. |
There were no statistically significant differences between the two groups regarding the baseline demographic, anesthetic, or surgery characteristics (Table 1).
 |
Table 1 Clinical Baseline Characteristics and Perioperative Data in Two Groups (n=65) |
The incidence of rebound pain was significantly lower in Group IV (15%) than in group C (44%, P<0.001). Rebound pain onset was after 12 (5, 30) min in group C and 30 (12, 67) min in group IV (P>0.05). There was no significant difference in the duration of rebound pain between the two groups, which was about 3 hours (P>0.05). The duration of nerve block for groups C and IV were 16 (14–18) hours and 24 (22.5–27.5) hours, respectively (Figure 2). Compared with group C, the duration of nerve block of group IV was 9 hours longer (P<0.001). Compared with group C, patients in group IV had significantly lower NRS scores from T2 to T4 (P<0.05). There were no significant differences in NRS scores at T1 and T5 (P>0.05). The time to first analgesic request was 17 (15–19) hours in the group C and 26 (23–30) hours in the group IV (P<0.001). Patients pressed the analgesia pump and rescue analgesia more frequently in group C than in group IV (P<0.05), as illustrated in Table 2.
 |
Table 2 Rebound Pain and Postoperative Data in Two Group (n=65) |
 |
Figure 2 Kaplan–Meier plot describing nerve block duration in the study groups. The median (95% CI) difference in nerve block duration for Group IV compared with Group C was 9 (8–10) hours. |
Before surgery, there were no significant differences between two groups for TNF-α, IL-1β, or IL-6. In group C, IL-1β, IL-6, and TNF-α levels were increased at T1 compared with before surgery (P<0.05). The level of TNF-α, IL-1β, and IL-6 were lower in the group IV compared with that of the group C at T1 (P<0.05), as shown in Figure 3.
 |
Figure 3 (A) TNF-α, (B) IL-1β, and (C) IL-6 before the surgery and at 6 hours after surgery. *P<0.05. |
Compared with group C, patients in group IV had significantly higher QoR-15 scores on the second day after surgery, and significantly higher sleep score on the night of surgery (P<0.05). There were no significant differences on the night of the first postoperative day and patient satisfaction (P>0.05). There were no significant differences between the groups in the incidence of postoperative adverse effects, including postoperative nausea and vomiting, pruritus, infection, and nerve damage. (Table 3)
 |
Table 3 QoR-15, Sleep Score, Patient Satisfaction and Postoperative Adverse Events in Two Group (n=65) |
Discussion
The present study shows that intravenous dexamethasone as an adjuvant effectively reduces the occurrence of rebound pain after nerve block, prolongs the duration of ACB and popliteal sciatic nerve block in patients with ankle fracture, reduces the consumption of postoperative analgesic drugs, and improves the quality of the postoperative recovery of patients.
In this study, patients with a bimalleolar or trimalleolar fracture with resection and internal fixation were selected, and the medial and lateral ankle joints were independently innervated by the saphenous nerve branch of the femoral nerve and the sciatic nerve. Therefore, we chose here to use ACB combined with popliteal sciatic nerve block. All nerve blocks were performed under ultrasound guidance by the same physician, skilled in nerve block techniques, which could ensure the effectiveness of the nerve block, and patients were excluded from this study if they had significant pain (NRS≥3) three hours after surgery. At present, there is no optimal dose of intravenous dexamethasone for nerve block, and this study is based on the results of Kim et al.15
The mechanism by which dexamethasone reduces rebound pain may be related to its prolonged duration of nerve block and anti-inflammatory effect. The results of this study showed that the duration of nerve block in the IV group was nine hours longer than that in the C group, and the incidence of rebound pain was reduced by 29%. A recent randomized controlled trial of perineural dexamethasone showed that it decreases the incidence of rebound pain in patients undergoing arthroscopic shoulder surgery under single interscalene block combined general anesthesia and that perineural dexamethasone in the dexamethasone group prolonged the duration of the rebound pain by 8.1 h and reduced the incidence of the rebound pain by 45.8%,4 This is basically consistent with our results. In a study by Williams et al5 they found that the rebound pain score decreased with the increase of the duration of peripheral nerve block. Approximately 33 hours of additional nerve block time is required to reduce rebound pain scores by one unit. Yun et al16 found that the continuous interscalene brachial plexus block reduced the patient’s average pain score, and it remained below 3 points for three days after surgery. In addition, studies have shown that the incidence of rebound pain in upper limb surgery is much higher than in lower limb surgery,2 which may be related to the longer duration of nerve block in the lower extremities.
The reason for prolonged nerve block duration with intravenous dexamethasone is still speculative, and several mechanisms may explain this phenomenon: systemic anti-inflammatory and analgesic effects of dexamethasone, pain reduction may cause patients to experience a subjective sensation of longer block duration, but some findings show evidence of prolonged motor block also with intravenous dexamethasone.17 Alternatively, the systemic effect of intravenous dexamethasone on the nervous system may be one of the potential mechanisms.
Tissue and peripheral nerve injury from surgery lead to a local inflammatory response resulting in elevated levels of pro-inflammatory cytokines, including IL-1β, IL- 6, and TNF-α; these cytokines induce sensitization of the peripheral and central nervous systems, resulting in pain hypersensitivity.18,19 Because we cannot accurately estimate the time of rebound pain after the nerve block subsides, we measured serum cytokines while the effects of the nerve block were still present. Our results showed that serum IL-1β, IL-6, and TNF-α levels were elevated in control patients six hours after surgery compared to before surgery. At this point, although the nerve block was still effective, the patients were still exposed to higher levels of pain-causing cytokines. Dexamethasone is a long-acting glucocorticoid with anti-inflammatory effects. Studies showed that preoperative intravenous dexamethasone can reduce postoperative cytokine levels and improve postoperative outcomes.20,21 Our results also showed that serum IL-1β, IL-6, and TNF-α levels were reduced six hours after surgery in group IV compared with group C. Tissue injury caused an inflammatory response, and the release of inflammatory and nociceptive cytokines peaked within 24 h.22 Ropivacaine temporarily blocks sensory nerve transmission by blocking nerve fibre Na+ channels.23 And when the effect of nerve block wears off, patients may still be exposed to higher levels of inflammatory and nociceptive cytokines, leading to the development of rebound pain; the prolonged duration of nerve block by dexamethasone, as well as its well-known anti-inflammatory effect, may be responsible for the prevention of rebound pain.
Dexamethasone administered perineurally as an adjuvant has been shown to be effective in preventing rebound pain, but the mechanism of action of perineural dexamethasone remains controversial, with some studies suggesting that intravenous dexamethasone also prolongs the duration of nerve blocks.24,25 Marhofer et al found, in a randomized triple-blind crossover study of healthy volunteers, that neither intravenous nor perineural dexamethasone could prolong block time.26 And in a pharmacokinetic study of dexamethasone as an adjunct to brachial plexus nerve block, the maximum serum concentration (Cmax) for systemic absorption, the mean time to Cmax, and the area under the concentration curve were found to be similar for both routes of administration (similar blood concentrations were achieved).27 In addition, a retrospective study of ambulatory surgery showed that absence of perioperative intravenous dexamethasone was associated with a higher incidence of rebound pain.2 Therefore, the mechanism of perineural dexamethasone in preventing rebound pain may also be a consequence of its systemic effect after local absorption.
The QoR-15 is a valid, reliable, responsive, and easy-to-use method to measure the quality of a patient’s postoperative recovery.28 Pain is an essential indicator of this. This study showed a statistically significant difference in QOR-15 scores between the C and IV groups second day after surgery, close to the minimal clinically important difference (MCID > 8),29 suggesting that intravenous dexamethasone improves the quality of postoperative recovery in patients. Postoperative sleep quality is closely related to pain, and the results of most studies report that rebound pain occurs mainly at night, which is related to the fact that the surgery was performed during the day and the nerve block effect starts to fade at night, which we also observed during our follow-up. We used the quality of sleep before the patient’s injury as a criterion, and the results showed that the group IV had a higher quality of sleep at night after surgery than group C. A meta-analysis reported that a single dose of dexamethasone in the perioperative period does not pose a risk of postoperative infection and causes only a mild increase in blood glucose in non-diabetic patients.30 Diabetic patients have been excluded from this study, and there were no postoperative infections, suggesting that a single-dose dexamethasone can be safely used in patients with ankle fractures. Our results showed no statistically significant differences in the incidence of postoperative nausea, vomiting, and patient satisfaction between the two groups. The sample size of this study was calculated based on the incidence of rebound pain, which may be related to the small sample size.
This study has some limitations. First, only patients with ankle fractures were included, and further clinical trials are needed regarding the effect of dexamethasone on other types of nerve blocks and rebound pain after nerve blocks. Second, thigh tourniquets were used during surgery in all cases, which may have aggravated postoperative pain levels and affect measurement accuracy. Third, postoperative pain and sleep quality are subjective, and considering that patients need to rest after surgery, we arranged the follow-up time every morning, and helped the patient recall the pain level in the previous day, therefore, the data collected may be inaccurate. Finally, to reduce opioid consumption after surgery, we did not apply PCIA after surgery; instead, patients were instructed to open PCIA on their own after the effect of nerve block began to subside. However, lack of multimodal analgesia is a known risk factor for increasing the incidence and severity of rebound pain.
Conclusion
Compared with using ropivacaine alone, preoperative intravenous administration of dexamethasone is effective in reducing the occurrence of rebound pain, prolonging the duration of nerve block, reducing the consumption of postoperative analgesic drugs, and helping patients recover faster.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Acknowledgments
We thank the patients and staff who participated in this study.
Funding
This work was supported by Hebei Province technology Innovation guide Project Science and Technology Winter Olympics special project (19977790D); Hebei Provincial government funded the specialty capacity building and specialty leader training program.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sort R, Brorson S, Gogenur I, et al. Peripheral nerve block anaesthesia and postoperative pain in acute ankle fracture surgery: the AnAnkle randomised trial. Br J Anaesth. 2021;126(4):881–888. doi:10.1016/j.bja.2020.12.037
2. Barry GS, Bailey JG, Sardinha J, Brousseau P, Uppal V. Factors associated with rebound pain after peripheral nerve block for ambulatory surgery. Br J Anaesth. 2021;126(4):862–871. doi:10.1016/j.bja.2020.10.035
3. Lavand’homme P. Rebound pain after regional anesthesia in the ambulatory patient. Curr Opin Anaesthesiol. 2018;31(6):679–684. doi:10.1097/ACO.0000000000000651
4. Woo JH, Lee HJ, Oh HW, Lee JW, Baik HJ, Kim YJ. Perineural dexamethasone reduces rebound pain after ropivacaine single injection interscalene block for arthroscopic shoulder surgery: a randomized controlled trial. Reg Anesth Pain Med. 2021;46(11):965–970. doi:10.1136/rapm-2021-102795
5. Williams BA, Bottegal MT, Kentor ML, Irrgang JJ, Williams JP. Rebound pain scores as a function of femoral nerve block duration after anterior cruciate ligament reconstruction: retrospective analysis of a prospective, randomized clinical trial. Reg Anesth Pain Med. 2007;32(3):186–192. doi:10.1016/j.rapm.2006.10.011
6. Henningsen MJ, Sort R, Moller AM, Herling SF. Peripheral nerve block in ankle fracture surgery: a qualitative study of patients’ experiences. Anaesthesia. 2018;73(1):49–58. doi:10.1111/anae.14088
7. Sunderland S, Yarnold CH, Head SJ, et al. Regional versus general anesthesia and the incidence of unplanned health care resource utilization for postoperative pain after wrist fracture surgery: results from a retrospective quality improvement project. Reg Anesth Pain Med. 2016;41(1):22–27. doi:10.1097/AAP.0000000000000325
8. Fang J, Shi Y, Du F, et al. The effect of perineural dexamethasone on rebound pain after ropivacaine single-injection nerve block: a randomized controlled trial. BMC Anesthesiol. 2021;21(1):47. doi:10.1186/s12871-021-01267-z
9. Joshi G, Gandhi K, Shah N, Gadsden J, Corman SL. Peripheral nerve blocks in the management of postoperative pain: challenges and opportunities. J Clin Anesth. 2016;35:524–529. doi:10.1016/j.jclinane.2016.08.041
10. Myles PS, Corcoran T. Benefits and risks of dexamethasone in noncardiac surgery. Anesthesiology. 2021;135(5):895–903. doi:10.1097/ALN.0000000000003898
11. De Oliveira GS, Almeida MD, Benzon HT, McCarthy RJ. Perioperative single dose systemic dexamethasone for postoperative pain: a meta-analysis of randomized controlled trials. Anesthesiology. 2011;115(3):575–588. doi:10.1097/ALN.0b013e31822a24c2
12. Pehora C, Pearson AM, Kaushal A, Crawford MW, Johnston B. Dexamethasone as an adjuvant to peripheral nerve block. Cochrane Database Syst Rev. 2017;11:CD011770. doi:10.1002/14651858.CD011770.pub2
13. Desai N, Kirkham KR, Albrecht E. Local anaesthetic adjuncts for peripheral regional anaesthesia: a narrative review. Anaesthesia. 2021;76(Suppl 1):100–109. doi:10.1111/anae.15245
14. Jen TTH, Ke JXC, Wing KJ, et al. Development and internal validation of a multivariable risk prediction model for severe rebound pain after foot and ankle surgery involving single-shot popliteal sciatic nerve block. Br J Anaesth. 2022;129(1):127–135. doi:10.1016/j.bja.2022.03.030
15. Kim BG, Lee W, Song JH, Yang C, Heo GA, Kim H. Effect of intravenous dexamethasone on the duration of postoperative analgesia for popliteal sciatic nerve block: a randomized, double-blind, placebo-controlled study. Korean J Anesthesiol. 2021;74(4):317–324. doi:10.4097/kja.20640
16. Yun S, Jo Y, Sim S, et al. Comparison of continuous and single interscalene block for quality of recovery score following arthroscopic rotator cuff repair. J Orthop Surg. 2021;29(1):23094990211000142. doi:10.1177/23094990211000142
17. Abdallah FW, Johnson J, Chan V, et al. Intravenous dexamethasone and perineural dexamethasone similarly prolong the duration of analgesia after supraclavicular brachial plexus block: a randomized, triple-arm, double-blind, placebo-controlled trial [published correction appears in Reg Anesth Pain Med. 2015 Jul-Aug;40(4):398]. Reg Anesth Pain Med. 2015;40(2):125–132. doi:10.1097/AAP.0000000000000210
18. Beilin B, Bessler H, Mayburd E, et al. Effects of preemptive analgesia on pain and cytokine production in the postoperative period. Anesthesiology. 2003;98(1):151–155. doi:10.1097/00000542-200301000-00024
19. Ko FC, Rubenstein WJ, Lee EJ, Siu AL, Sean Morrison R. TNF-alpha and sTNF-RII are associated with pain following hip fracture surgery in older adults. Pain Med. 2018;19(1):169–177. doi:10.1093/pm/pnx085
20. El Azab SR, Rosseel PM, de Lange JJ, et al. Dexamethasone decreases the pro- to anti-inflammatory cytokine ratio during cardiac surgery. Br J Anaesth. 2002;88(4):496–501. doi:10.1093/bja/88.4.496
21. Ionescu DC, Hadade AI, Mocan TA, Margarit SD. The influence of a prophylactic dose of dexamethasone for postoperative nausea and vomiting on plasma interleukin concentrations after laparoscopic cholecystectomy: a randomised trial. Eur J Anaesthesiol. 2014;31(4):204–211. doi:10.1097/EJA.0b013e3283642a01
22. Richebe P, Capdevila X, Rivat C. Persistent postsurgical pain: pathophysiology and preventative pharmacologic considerations. Anesthesiology. 2018;129(3):590–607. doi:10.1097/ALN.0000000000002238
23. Oda A, Ohashi H, Komori S, Iida H, Dohi S. Characteristics of ropivacaine block of Na+ channels in rat dorsal root ganglion neurons. Anesth Analg. 2000;91(5):1213–1220. doi:10.1097/00000539-200011000-00031
24. Rahangdale R, Kendall MC, McCarthy RJ, et al. The effects of perineural versus intravenous dexamethasone on sciatic nerve blockade outcomes: a randomized, double-blind, placebo-controlled study. Anesth Analg. 2014;118(5):1113–1119. doi:10.1213/ANE.0000000000000137
25. Sehmbi H, Brull R, Ceballos KR, et al. Perineural and intravenous dexamethasone and dexmedetomidine: network meta-analysis of adjunctive effects on supraclavicular brachial plexus block. Anaesthesia. 2021;76(7):974–990. doi:10.1111/anae.15288
26. Marhofer P, Columb M, Hopkins PM, et al. Dexamethasone as an adjuvant for peripheral nerve blockade: a randomised, triple-blinded crossover study in volunteers. Br J Anaesth. 2019;122(4):525–531. doi:10.1016/j.bja.2019.01.004
27. Bhatia N, Patial A, Ghai B, et al. ESRA19-0119 Comparison of systemic dexamethasone levels following its perineural versus intravenous administration: a randomized, double-blind study. Reg Anesth Pain Med. 2019;44(Suppl 1):A74–A75. doi:10.1136/rapm-2019-ESRAABS2019.65
28. Stark PA, Myles PS, Burke JA. Development and psychometric evaluation of a postoperative quality of recovery score: the QoR-15. Anesthesiology. 2013;118(6):1332–1340. doi:10.1097/ALN.0b013e318289b84b
29. Myles PS, Myles DB, Galagher W, Chew C, MacDonald N, Dennis A. Minimal clinically important difference for three quality of recovery scales. Anesthesiology. 2016;125(1):39–45. doi:10.1097/ALN.0000000000001158
30. Polderman JA, Farhang-Razi V, Van Dieren S, et al. Adverse side effects of dexamethasone in surgical patients. Cochrane Database Syst Rev. 2018;11:CD011940. doi:10.1002/14651858.CD011940.pub3
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
