Back to Journals » Journal of Pain Research » Volume 16
The Effects and Potential Mechanisms of Moxibustion for Rheumatoid Arthritis-Related Pain: A Randomized, Controlled Trial
Authors Liao C , Tao S, Xiong Y, Dai J, Bai Y, Wang X, Li Y, Wu P
Received 15 March 2023
Accepted for publication 9 May 2023
Published 26 May 2023 Volume 2023:16 Pages 1739—1749
DOI https://doi.org/10.2147/JPR.S408814
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Houman Danesh
Chenxi Liao,1,* Siyu Tao,1,* Yan Xiong,2,* Jingyang Dai,3 Yu Bai,4 Xue Wang,1 Yuan Li,5 Ping Wu1
1Acupuncture and Tuina School, Chengdu University of Traditional Chinese Medicine, Chengdu, People’s Republic of China; 2Department of Osteoporosis, Huaxi Fourth Hospital, Sichuan University, Chengdu, People’s Republic of China; 3Department of Traditional Chinese Medicine, Chengdu Fifth People’s Hospital, Chengdu, People’s Republic of China; 4Department of Traditional Chinese Medicine, Chengdu Second People’s Hospital, Chengdu, People’s Republic of China; 5Department of Rheumatology, The Affiliated Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Ping Wu, Acupuncture and Tuina School, Chengdu University of Traditional Chinese Medicine, Chengdu, Sichuan, People’s Republic of China, Email [email protected]
Purpose: To investigate the effects of moxibustion in relieving pain, and other clinical symptoms for patients with rheumatoid arthritis (RA), and explore the potential mechanism of moxibustion treatment for RA.
Patients and Methods: Seventy qualified RA patients were randomly assigned in a 1:1 ratio to the moxibustion group or the routine group. The routine group only took oral methotrexate tablets and folic acid tablets. The moxibustion group was treated with moxibustion based on oral pharmaceutical. Moxibustion was performed two times weekly for 8 weeks, a total of 16 sessions. Patients scored their pain on a visual analog scale (VAS). The American College of Rheumatology improvement criteria of 20%, 50% and 70% (ACR20, ACR50 and ACR70) after treatment were investigated. Clinical symptoms, a disease activity score using 28 joint counts (DAS28), simplified disease activity index (SDAI), clinical disease activity index (CDAI), health assessment questionnaire (HAQ), interleukin 1β (IL-1β), tumor necrosis factor-alpha (TNF-α), and vascular endothelial growth factor (VEGF) of RA patients were analyzed before and after treatment.
Results: After treatment, the VAS scores, tender and swollen joint counts, morning stiffness scores, disease activity scores (DAS28, SDAI, CDAI), HAQ scores in the two groups were both improved, and the effects of moxibustion group were more obvious (P < 0.05). The ACR20 and ACR50 of the moxibustion group were greater than that of the routine group (P < 0.05), no significant difference of the ACR70 existed between the two groups (P > 0.05). In addition, the decreases of IL-1β, TNF-α, VEGF of the moxibustion group were better than that of the routine group (P < 0.05).
Conclusion: Moxibustion could effectively relieve pain, ameliorate the clinical symptoms, and decrease the disease activity of RA. The potential mechanism may be the decrease in the level of serum inflammatory factors.
Keywords: moxibustion, rheumatoid arthritis, pain, inflammatory factor
Introduction
As a chronic inflammatory disease related to autoimmunity, rheumatoid arthritis (RA) can cause pain, swelling, stiffness of multiple joints, damage of synovium and cartilage.1,2 RA affects about 0.5% to 1.0% of the population worldwide. The peak age of incidence is 45–55 years, the prevalence ratio of female and male is about 2–3:1 for RA.3 Because of daily pain, stiffness, and physical disability, RA leads to the decrease of quality of life (QoL) and the increase of psychological burden in patients.4–6 Recent study showed approximately 13.5% of RA patients accompanied by anxiety,7 which was related to the increase of disease activity and worse QoL.8 In addition, RA also has many extra-articular manifestations and complications, such as pulmonary involvement, peripheral neuropathy, cardiovascular disease.9–12 RA has proved to be a significant public health-care and social issue because of its lingering disease course, high disability rate, and substantial burden.13
RA is mainly treated with pharmacological therapy at present, including disease-modifying antirheumatic drugs (DMARDs), non-steroidal anti-inflammatory drugs (NSAIDs), glucocorticoids. Although the response for most patients is favorable in joint pain and inflammation, many are still suboptimal for the current therapies. Besides, long-term use of these medications is easy to cause adverse effects of liver, kidney, gastrointestinal tract, and cardiovascular system.14–17 Accordingly, a complementary and alternative therapy with effective and few adverse events is urgently required.
Moxibustion, as a widely used traditional Chinese medicine therapy, has been applied for thousands of years as non-pharmacological therapy for pain relief in clinical. Several animal experiments have validated the effects of moxibustion for anti-inflammatory, analgesic and detumescence, including regulating inflammatory cytokines, alleviating the cartilage degradation and bone destruction, improving the synovial inflammation hyperemia and edema in RA.18–20 Moxibustion on “Zusanli (ST 36)” and “Shenshu (BL 23)” could diminish swelling, reduce the metatarsal circumference, relieve multiple arthritis, lower tumor necrosis factor (TNF), interleukin 1 (IL-1) and nitric oxide (NO) content in adjuvant arthritis rats.21 A clinical trial also found that spreading moxibustion had a significant therapeutic effect on RA.22 In two recent meta-analyses, moxibustion combined with western medicine therapy could effectively improve pain in patients with RA.23,24 However, there are still few relevant clinical studies comprehensively evaluating the efficacy of moxibustion in treating RA, and the effects of moxibustion on inflammatory factors in RA patients are unclear. We conducted a 9-week two-arm parallel pilot randomized clinical trial to observe the effectiveness and safety for moxibustion, further to explore the potential mechanism of moxibustion treatment for RA.
Patients and Methods
This was a randomized controlled trial. RA patients were recruited from the outpatient department of the Affiliated Hospital of Chengdu University of Traditional Chinese Medicine (CDUTCM) from March 2017 to January 2019. All subjects signed the informed consent.
Participants
Patients were enrolled if they fulfilled all of the following criteria: (1) matched the diagnosis criteria for RA based on the 2010 American College of Rheumatology and the European League Against Rheumatism classification criteria25; (2) aged 18 to 65 years; (3) were with DAS28 >3.2; (4) did not use glucocorticoid drugs and other antirheumatic pharmaceutical within 24 weeks; (5) had not participated in any other clinical trials.
Patients were excluded if they had any following conditions: (1) were with unclear conscious and unable to cooperate with the investigator to complete the study or diagnosed with mental disorders; (2) were in an advanced stage with IV joint function; (3) suffered from other autoimmune diseases; (4) complicated by malignant tumors or severe disorders in heart, brain, liver, kidney, lung; (5) were pregnant or lactating; (6) had contraindications to moxibustion (eg, scar or allergic constitution).
Randomization and Blinding
After a 1-week baseline period, the qualified RA patients were randomly assigned in a 1:1 ratio to the moxibustion group or the routine group, with a computer-generated randomization sequence generated by SPSS 21.0 statistical software package. The sealed and opaque envelopes were used to seal the randomization sequence. The entire randomization process was performed by an individual coworker. Because of the nature of the intervention, acupuncturists could not be blinded. Data collection and data analysis was performed blinded.
Interventions
The Routine Group
Patients only received conventional pharmaceutical therapy for 8 weeks as recommended by the guidelines,26 including 7.5mg methotrexate and 10 mg folic acid (took orally, once a week).
The Moxibustion Group
Patients received moxibustion treatment in addition to pharmaceutical therapy. Moxibustion was performed on bilateral ST36, bilateral BL23 and A-Shi points (Figure 1). The acupoints were localized according to the WHO Standard Acupuncture Locations. ST36 located 3 cun directly below ST35 (Dubi), and one-digit lateral to the anterior margin of the tibia. BL23 located the same level of the second subspinous of lumbar vertebra, and 1.5 cun lateral to the posterior midline on the back. A-Shi points in this study were the evident tender points of the pain joints. These acupoints selection was based on some experimental study,19,20 experience from clinical experts and our previous study.27 Wheat-grain moxibustion, one of the direct moxibustion, was applied on ST36 and A-Shi points at small joints of limbs. BL23 was treated with salt-separated moxibustion. A-Shi points at large joints was treated with moxibustion on ginger.
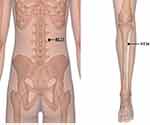 |
Figure 1 The acupoints used in this trial. |
Acupuncturists used moxa wool (Yilejia Moxa Co., Nanyang, China) to process small moxa cone (3mm of diameter and height) and large moxa cone (10mm of diameter and height) (Figure 2A), then labelled acupoints with markers. The acupuncturists applied Vaseline above ST36 and A-Shi points in small joints of limbs to stick the small moxa cones, then light the small moxa cones by line incenses. When patients felt hot, the acupuncturists removed the small moxa cone immediately to avoid burn. Five small moxa cones were burnt at per acupoint for each time (Figures 2B and 2C). A-Shi points at large joints were applied with moxibustion on ginger (Figures 2D and 2E), the acupuncturists lighted the moxa cone and then put it at a cut ginger on A-Shi points. Once the moxa cone was burned out and the patients had a sense of hot, replaced another moxa cone, three large moxa cones were burnt at per acupoint for each time. For BL23, the acupuncturists put a piece of sterile gauze with an appropriate amount of salt on the BL23 points area, then placed the large moxa cones and lit them. When patients felt hot, replaced the moxa cones, six large moxa cones were burnt every time (Figure 2F).
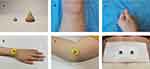 |
Figure 2 Schematic diagram of the moxibustion. (A) Samples of moxa cones; (B and C) Wheat-grain moxibustion; (D and E) Moxibustion on ginger; (F) Salt-separated moxibustion. |
Patients in the moxibustion group received 16 sessions of moxibustion treatment (twice per week, a total of 8 weeks). All moxibustion treatments were operated by licensed acupuncturists, who were trained with how to locate acupoints, make moxa cone, and complete the moxibustion operation according to the standard.
Outcome Measurements
The pain of joints was measured by a horizontal visual analog scale (VAS) ranging from 0 to 10, where higher scores indicate greater pain.28,29 The other outcomes included tender and swollen joint counts, morning stiffness scores (evaluated the morning stiffness duration, 0 point: asymptomatic, 2 points: <1 hour, 4 points: 1–2 hours, 6 points: >2 hours). In addition, 28 joint counts along with other components (DAS28), simplified disease activity index (SDAI) and clinical disease activity index (CDAI) reflected the disease activity (higher is worse).30–34 The QoL of patients was evaluated through health assessment questionnaire (HAQ).35 The clinical response was measured using the American College of Rheumatology (ACR) improvement criteria, including improvements of 20%, 50% and 70% (ACR20, ACR50 and ACR70 responses),36 the ACR evaluation system was accepted as an international standard for evaluating clinical therapeutic effect of RA. The levels of serum inflammatory factors including interleukin 1β (IL-1β), tumor necrosis factor-alpha (TNF-α), vascular endothelial growth factor (VEGF), C-reactive protein (CRP), erythrocyte sedimentation rate (ESR) were measured before and after treatment.
Calculation of Sample Size and Statistical Analysis
According to our previous pilot study, after treatment, the mean VAS score of RA patients in the moxibustion group was 4.0 points and the standard deviation was 1.28 points, while the mean VAS score of RA patients in the routine group was 5.4 points and the standard deviation was 1.9 points. This study was set up according to the ratio of 1:1 to test the level α=0.05, inspection efficiency 1-β=0.95. The sample size was estimated using t tests in G*Power (version 3.1.7, Franz Faul, University Kiel, Germany). Through calculation, the comprehensive effect size was 0.864. It was estimated that the total number of samples required in the two groups was 60. To compensate for a 15% attrition rate, the sample size expanded to 35 patients in each group, a total of 70 cases were required.
Statistical analysis was calculated with SPSS 21.0 by a blinded evaluator. Categorical data were described as number and percentages (n%), and compared using the chi-squared (χ2) test. T-test and nonparametric tests were used for normally distributed quantitative variables and skewed quantitative variables, respectively. Pearson correlation coefficients were employed for nonnormal distribution data for correlation analysis. P value <0.05 was considered statistically significant.
Results
Baseline Characteristics
A total of 101 RA patients were screened, 70 patients were eligible and randomized, but only 66 patients completed 8 weeks of treatment (Figure 3). Baseline data included gender, age, family history, education level, body mass index (BMI), resting blood pressure, and duration of illness. No remarkable differences in baseline data were found between the two groups (Table 1).
 |
Table 1 The Baseline Characteristics in the Two Groups |
 |
Figure 3 Flowchart of screening, enrollment, randomization, and treatment. |
The Therapeutic Effects in the Two Groups
Significant improvements were observed for most clinical outcome measurements after treatment between the two groups (Table 2). The VAS scores, tender and swollen joint counts, morning stiffness scores of the moxibustion group were significantly less than those in the routine group at week 8 (P<0.05). The changes of SDAI, CDAI and DAS28 in the moxibustion group from baseline to week 8 were greater compared with the routine group (P<0.05). At baseline, mean HAQ scores were similar for patients between the two groups. At week 8, mean HAQ scores in the moxibustion group were lower than that in the routine group (P<0.05).
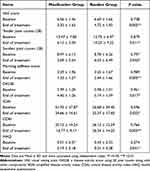 |
Table 2 Comparison of the Therapeutic Effects Between the Two Groups |
The ACR20 response rate was 79.4% (95% CI, 65.1–93.7%) in the moxibustion group and 53.1% (95% CI, 34.8–71.4%) in the routine group. The ACR20 response rate in the moxibustion group was higher than the routine group (P = 0.024; Table 3). The ACR50 and ACR70 response rates of the moxibustion group were reached by 41.2% and 14.7% at week 8. In the routine group, the ACR50 and ACR70 response rates were 15.6% and 3.1%, respectively. The proportion of patients achieving ACR50 response rates in moxibustion group was significantly higher than that in the routine group (P<0.05). No significant difference in ACR70 response rates was observed between the two groups (P>0.05; Table 3).
 |
Table 3 Treatment Response Rates of RA Patients in the Two Groups |
RA-Associated Serum Inflammation Markers
No significant between-group differences were observed in ESR and CRP levels after treatment (P>0.05). The moxibustion group had greater improvement in mean IL-1β, TNF-α, and VEGF levels at week 8 (P<0.05; Table 4).
 |
Table 4 Change in Serum Inflammatory Factor Level of the Two Groups |
Correlation Among the Different Indicators After Treatment
IL-1β showed a positive correlation with HAQ, TNF-α, and VEGF. TNF-α had a positive correlation with DAS28, HAQ, and VEGF. VEGF showed a positive correlation with VAS score, DAS28, and HAQ (P<0.05; Table 5).
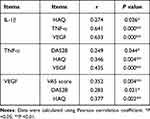 |
Table 5 Correlation Among the Different Indicators After Treatment |
Safety
Two patients in the moxibustion group were reported for moxibustion-related adverse reaction, including burn and blister. All AEs were reported as mild, and none of them required for special medical interventions. The two patients fully recovered from the AEs and remained in the trial.
Discussion
RA is an inflammatory disease involving the autoimmune system with complicated etiology and pathogenesis, which cannot be cured, and even brings heavy economic and medical burden. Moxibustion, as one of the important integral parts of TCM, has been used in treating arthralgia for thousands of years. It contains direct moxibustion (applied the moxa cone directly on the skin) and indirect moxibustion (applied the moxa cone indirectly on the skin through insulating materials). A variety of studies found that both the two moxibustion treatments had significant anti-inflammatory effects on rabbits with RA.37,38 Our study combined the two methods of moxibustion, which could effectively relieve local pain of the joints and regulate systemic immunity. At the same time, the direct moxibustion on ST36 and the salt-separated moxibustion on BL23 can also replenish qi, nourish blood, reinforce kidney and warm Yang. Previous studies indicated that moxibustion on ST36 and BL23 had promising antiarthritic effects,18 and stimulating A-Shi points could play an analgesic role.39,40 Therefore, the three acupoints were selected in this study, embodied the principle of treating both symptoms and root causes.
In terms of improving VAS score, moxibustion group had better effect than routine group, and similar results were also observed in the tender and swollen joint counts. The warm stimulation of moxibustion can dilate blood vessel, increase local blood flow, improve microcirculation to relieve swelling and pain.41,42 Except for pain, morning stiffness is also one of the main symptoms that accompany the progression of RA. The longer morning stiffness and the more severe pain are associated with the worse condition.43 In this study, moxibustion combined with pharmaceutical had a good effect on improving morning stiffness of RA patients. Moreover, compared with routine group, moxibustion group had greater improvements in DAS28, SDAI, and CDAI, indicating that the combination was more effective than drug therapy alone in reduction of disease activity. In addition, improvements of QoL were also found, based on the HAQ scores, which was related to relief of symptoms and signs. Although moxibustion methods are different, other studies have also shown that moxibustion combined with western medication can effectively decrease the VAS, HAQ and DAS28 scores.44–46 The ACR20 and ACR50 also reflected that the overall effectiveness of moxibustion group was better than routine group. The results of this study suggested that moxibustion plus conventional pharmaceutical therapies were more effective than the use of drugs alone. Our study was congruent with a previous meta-analysis that demonstrated moxibustion plus pharmaceutical therapy were more effective than the pharmaceutical therapy alone in the treatment of RA, especially in relieving pain.23 Thus, results of this study demonstrated that moxibustion could improve the degree of the curative effect, and had a clinically meaningful benefit in decreasing pain, swelling, morning stiffness in RA patients.
Proinflammatory cytokines (TNF-α, IL-1β) and VEGF contribute to inflammatory pain.47–49 In addition, IL-1β and TNF-α can cause joint synovial hyperplasia and hypertrophy, aggravate synovial inflammation, erode cartilage, and joint damage.50,51 VEGF promotes angiogenesis and proliferation of inflammatory synovial tissue, affects joint function.52 Some studies have identified high levels of TNF-α, IL-1β and VEGF expression in synovial fluid and plasma of RA patients, and have a positive correlation with ESR, CRP.53–56 Therefore, decreasing the level of inflammatory factors is crucial in treating RA. In this study, significantly decreases in the levels of serum IL-1β, TNF-α and VEGF of RA patients after moxibustion were found. The correlation analysis showed that IL-1β, TNF-α and VEGF were correlated with VAS score, HAQ, DAS28. With the reduction of IL-1β, TNF-α and VEGF, pain and disease activity also decreased, the patient’s physical function improved. Therefore, the decrease of the level of serum inflammatory factors may be the mechanism for analgesia and improvement the clinical symptoms of moxibustion. The results were consistent with a systematic review which indicates that moxibustion can protect the synovium of joint in animal models with RA by downregulation of the level of proinflammatory cytokines.57 Moxibustion has a suppressive action on the mediators of inflammation.
The findings of the current study demonstrated that moxibustion combined with pharmaceutical therapy on RA was more effective than monotherapy. Moxibustion showed an advantage in the treatment of RA, not only significantly alleviated the joint pain but also improved patients’ QoL. In addition, the incidence of adverse events was low, suggesting that moxibustion was an operable, convenient, effective and safe adjunctive treatment of RA.
Limitations
There were some limitations in this study. Firstly, the sample size is relatively small. Secondly, due to the lack of follow-up, the long-term efficacy remains unclear. In the future studies, we will focus on the long-term effects of the moxibustion on inflammatory mechanisms of RA-related pain.
Conclusion
After a comprehensive comparison of the outcome indicators of the two groups, moxibustion combined with pharmaceutical therapy could significantly relieve pain, decrease the disease activity, ameliorate the clinical symptoms, improve the QoL and overall effective rate of RA patients. Moreover, moxibustion could significantly decrease the level of serum inflammatory factors to achieve anti-inflammatory and analgesic effect.
Abbreviations
RA, rheumatoid arthritis; QoL, quality of life; DMARDs, disease-modifying antirheumatic drugs; NSAIDs, non-steroidal anti-inflammatory drugs; CDUTCM, Chengdu University of Traditional Chinese Medicine; DAS28, a disease activity score using 28 joint counts along with other components; VAS, visual analog scale; ACR20, ACR50 and ACR70, the American College of Rheumatology improvements criteria of 20%, 50% and 70%; SDAI, simplified disease activity index; CDAI, clinical disease activity index; HAQ, health assessment questionnaire; IL-1β, interleukin 1β; TNF-α, tumor necrosis factor-alpha; VEGF, vascular endothelial growth factor; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; BMI, body mass index.
Data Sharing Statement
All data generated or used during the study appear in the submitted article. No further data will be shared.
Ethics Approval and Informed Consent
The study was performed according to the principles of the Declaration of Helsinki, approved by the Ethics Committee of the Affiliated Hospital of CDUTCM (NO.2015KL-015), and registered at the Clinical Trial Registry (registration number: ChiCTR-IOR-17012282). Only patients signed the informed consent form were included.
Consent for Publication
Written informed consent for publication was obtained from all participants.
Acknowledgments
The authors would like to thank all study participants. The authors were grateful their colleagues from CDUTCM and The Affiliated Hospital of CDUTCM, who provided insight and expertise that greatly assisted the research.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by The National Natural Science Foundation of China (81373738) and the Education Department of Sichuan Province (2017JY0016 and 2022YFS0386).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet. 2016;388(10055):2023–2038. doi:10.1016/S0140-6736(16)30173-8
2. Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423(6937):356–361. doi:10.1038/nature01661
3. Tedeschi SK, Bermas B, Costenbader KH. Sexual disparities in the incidence and course of SLE and RA. Clin Immunol. 2013;149(2):211–218. doi:10.1016/j.clim.2013.03.003
4. Kwiatkowska B, Klak A, Raciborski F, et al. The prevalence of depression and insomnia symptoms among patients with rheumatoid arthritis and osteoarthritis in Poland: a case control study. Psychol Health Med. 2019;24(3):333–343. doi:10.1080/13548506.2018.1529325
5. Uguz F, Kucuk A, Cicek E, et al. Quality of life in rheumatological patients: the impact of personality disorders. Int J Psychiatry Med. 2015;49(3):199–207. doi:10.1177/0091217415582183
6. Ovayolu N, Ovayolu O, Karadag G. Health-related quality of life in ankylosing spondylitis, fibromyalgia syndrome, and rheumatoid arthritis: a comparison with a selected sample of healthy individuals. Clin Immunol. 2011;30(5):655–664. doi:10.1007/s10067-010-1604-2
7. Covic T, Cumming SR, Pallant JF, et al. Depression and anxiety in patients with rheumatoid arthritis: prevalence rates based on a comparison of the Depression, Anxiety and Stress Scale (DASS) and the hospital, Anxiety and Depression Scale (Hads). BMC Psychiatry. 2012;12:6. doi:10.1186/1471-244X-12-6
8. Machin AR, Babatunde O, Haththotuwa R, et al. The association between anxiety and disease activity and quality of life in rheumatoid arthritis: a systematic review and meta-analysis. Clin Rheumatol. 2020;39(5):1471–1482. doi:10.1007/s10067-019-04900-y
9. Rawla P. Cardiac and vascular complications in rheumatoid arthritis. Reumatologia. 2019;57(1):27–36. doi:10.5114/reum.2019.83236
10. Xing L, Yufeng Y, Bin M, et al. Clinical study of 157 rheumatoid arthritis patients’combination and complication. Chin J Dis Control Prevention. 2016;20(02):201–203.
11. Spagnolo P, Lee JS, Sverzellati N, et al. The lung in rheumatoid arthritis: focus on interstitial lung disease. Arthritis Rheumatol. 2018;70(10):1544–1554. doi:10.1002/art.40574
12. Kaeley N, Ahmad S, Pathania M, et al. Prevalence and patterns of peripheral neuropathy in patients of rheumatoid arthritis. J Family Med Primary Care. 2019;8(1):22–26. doi:10.4103/jfmpc.jfmpc_260_18
13. Drosos AA, Pelechas E, Kaltsonoudis E, et al. Therapeutic options and cost-effectiveness for rheumatoid arthritis treatment. Curr Rheumatol Rep. 2020;22(8):44. doi:10.1007/s11926-020-00921-8
14. Scheiman JM. NSAID-induced gastrointestinal injury: a focused update for clinicians. J Clin Gastroenterol. 2016;50(1):5–10. doi:10.1097/MCG.0000000000000432
15. Koike T, Harigai M, Inokuma S, et al. Effectiveness and safety of tocilizumab: postmarketing surveillance of 7901 patients with rheumatoid arthritis in Japan. J Rheumatol. 2014;41(1):15–23. doi:10.3899/jrheum.130466
16. Hwang YG, Saag K. The safety of low-dose glucocorticoids in rheumatic diseases: results from observational studies. Neuroimmunomodulation. 2015;22(1–2):72–82. doi:10.1159/000362727
17. Negrei C, Bojinca V, Balanescu A, et al. Management of rheumatoid arthritis: impact and risks of various therapeutic approaches. Exp Ther Med. 2016;11(4):1177–1183. doi:10.3892/etm.2016.3045
18. Chen Y, Li H, Luo X, et al. Moxibustion of Zusanli (ST36) and Shenshu (BL23) alleviates cartilage degradation through RANKL/OPG signaling in a rabbit model of rheumatoid arthritis. Evid Based Complement Alternat Med. 2019;2019:6436420. doi:10.1155/2019/6436420
19. Zhidan L, Xiaoyan L, Chuang Z, et al. Effects of moxibustion on Treg/Th17 cell and its signal pathway in mice with rheumatoid arthritis. Chinese Acupuncture & Moxibustion. 2017;37(10):1083–1092. doi:10.13703/j.0255-2930.2017.10.015
20. Jigang R, Xuguang L, Xiao L, et al. Study on the anti-inflammatory effect and the expression of mPD-1 in synovial tissue of RA model with moxibustion therapy. Lishizhen Med Materia Med Res. 2017;28(12):3048–3050.
21. Zhang C, Tang Z. Progress of mechanism study on rheumatoid arthritis treated by moxibustion. J Acupuncture Tuina Sci. 2009;7(2):65–70. doi:10.1007/s11726-009-0065-0
22. Xie X, Lei Q. Observation on therapeutic effect of the spreading moxibustion on rheumatoid arthritis. Chinese Acupuncture & Moxibustion. 2008;1(10):730–732.
23. Shen B, Sun Q, Chen H, et al. Effects of moxibustion on pain behaviors in patients with rheumatoid arthritis: a meta-analysis. Medicine. 2019;98(30):e16413. doi:10.1097/MD.0000000000016413
24. Wan R, Fan Y, Zhao A, et al. Comparison of efficacy of acupuncture-related therapy in the treatment of rheumatoid arthritis: a network meta-analysis of randomized controlled trials. Front Immunol. 2022;13:829409. doi:10.3389/fimmu.2022.829409
25. Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis. 2010;62(9):2569–2581. doi:10.1002/art.27584
26. Smolen JS, Landewe R, Bijlsma J, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis. 2017;76(6):960–977. doi:10.1136/annrheumdis-2016-210715
27. Gong Y, Yu Z, Wang Y, et al. Effect of moxibustion on HIF-1 alpha and VEGF levels in patients with rheumatoid arthritis. Pain Res Management. 2019;2019:4705247. doi:10.1155/2019/4705247
28. Katz J, Melzack R. Measurement of pain. Surg Clin North Am. 1999;79(2):231–252. doi:10.1016/s0039-6109(05)70381-9
29. Jensen MP, Chen C, Brugger AM. Interpretation of visual analog scale ratings and change scores: a reanalysis of two clinical trials of postoperative pain. J Pain. 2003;4(7):407–414. doi:10.1016/s1526-5900(03)00716-8
30. Prevoo ML, Kuper HH. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheumatism. 1995;38(1):44–48. doi:10.1002/art.1780380107
31. Fransen J, Creemers MC, Van Riel PL. Remission in rheumatoid arthritis: agreement of the disease activity score (DAS28) with the ARA preliminary remission criteria. Rheumatology. 2004;43(10):1252–1255. doi:10.1093/rheumatology/keh297
32. Smolen JS, Breedveld FC, Schiff MH, et al. A simplified disease activity index for rheumatoid arthritis for use in clinical practice. Rheumatology. 2003;42(2):244–257. doi:10.1093/rheumatology/keg072
33. Aletaha D, Nell VP, Stamm T, et al. Acute phase reactants add little to composite disease activity indices for rheumatoid arthritis: validation of a clinical activity score. Arthritis Res Ther. 2005;7(4):R796–806. doi:10.1186/ar1740
34. Aletaha D, Martinez-Avila J, Kvien TK, et al. Definition of treatment response in rheumatoid arthritis based on the simplified and the clinical disease activity index. Ann Rheum Dis. 2012;71(7):1190–1196. doi:10.1136/annrheumdis-2012-201491
35. Bruce B, Fries JF. The Stanford Health Assessment Questionnaire: a review of its history, issues, progress, and documentation. J Rheumatol. 2003;30(1):167–178.
36. Felson DT, Anderson JJ, Boers M, et al. American College of Rheumatology. Preliminary definition of improvement in rheumatoid arthritis. Arthritis Rheum. 1995;38(6):727–735. doi:10.1002/art.1780380602
37. Yang H, Liu X, Yang X, et al. Effect of different types of moxibustion intervention on expression of inflammatory cytokines IL-1 and TNF-α in rabbits with rheumatoid arthritis. Acupuncture Res. 2013;38(02):134–139. doi:10.13702/j.1000-0607.2013.02.009
38. Gao X, Wang X, Liu X, et al. Effects of different moxibustion on the function of HPAA in rabbits with rheumatoid arthritis. J Basic Chine Med. 2015;21(09):1140–1142. doi:10.19945/j.cnki.issn.1006-3250.2015.09.036
39. Yu X, Zhang F, Zhang J. Effect of transcutaneous electrical acupuncture point stimulation on expression of p-JNK in the dorsal root ganglion in a rat model of myofascial pain syndrome. Acupunct Med. 2019;37(5):312–318. doi:10.1136/acupmed-2017-011536
40. Mitidieri AMS, Baltazar M, da Silva APM, et al. Ashi acupuncture versus local anesthetic trigger point injections in the treatment of abdominal myofascial pain syndrome: a randomized clinical trial. Pain Physician. 2020;23(5):507–518.
41. Wei J, Shen X, Ding G, et al. Investigation of pathway and mechanism of heat stimulating action of sandwiched moxibustion. Chinese Acupuncture & Moxibustion. 2007;3(5):391–393.
42. Xu S, Zhang H, Gu Y. Progress of research on mechanisms of moxibustion intervention underlying improvement of blood circulation. Acupuncture Res. 2018;43(11):738–743. doi:10.13702/j.1000-0607.170309
43. Orange DE, Blachere NE, DiCarlo EF, et al. Rheumatoid arthritis morning stiffness is associated with synovial fibrin and neutrophils. Arthritis Rheumatol. 2020;72(4):557–564. doi:10.1002/art.41141
44. Zhang M, Zhao C, Jiang L, et al. Clinical effect and mechanism of moxibustion combined with western medication for rheumatoid arthritis of liver-kidney deficiency. Chinese Acupuncture & Moxibustion. 2021;41(5):489–92+524. doi:10.13703/j.0255-2930.20200519-k0005
45. Wang S, Ye G, Zhang Y, et al. Flipping moxibustion of Hui medicine combined with western medication for rheumatoid arthritis with cold dampness bi syndrome. Chinese Acupuncture & Moxibustion. 2017;37(10):1047–1051. doi:10.13703/j.0255-2930.2017.10.006
46. Yu H, Zhu Y, Pan Y, et al. Clinical efficacy of moxibustion as supplement on rheumatoid arthritis and the exploration on its mechanism. Chinese Acupuncture & Moxibustion. 2016;36(01):17–20. doi:10.13703/j.0255-2930.2016.01.005
47. Gao F, Xiang HC, Li HP, et al. Electroacupuncture inhibits NLRP3 inflammasome activation through CB2 receptors in inflammatory pain. Brain Behav Immun. 2018;67:91–100. doi:10.1016/j.bbi.2017.08.004
48. Su TF, Zhao YQ, Zhang LH, et al. Electroacupuncture reduces the expression of proinflammatory cytokines in inflamed skin tissues through activation of cannabinoid CB2 receptors. Eur J Pain. 2012;16(5):624–635. doi:10.1002/j.1532-2149.2011.00055.x
49. Takano S, Uchida K, Inoue G, et al. Vascular endothelial growth factor expression and their action in the synovial membranes of patients with painful knee osteoarthritis. BMC Musculoskelet Disord. 2018;19(1):204. doi:10.1186/s12891-018-2127-2
50. Juarez M, Filer A, Buckley CD. Fibroblasts as therapeutic targets in rheumatoid arthritis and cancer. Swiss Med Wkly. 2012;142:w13529. doi:10.4414/smw.2012.13529
51. Moelants EA, Mortier A, Van Damme J, et al. Regulation of TNF-alpha with a focus on rheumatoid arthritis. Immunol Cell Biol. 2013;91(6):393–401. doi:10.1038/icb.2013.15
52. Kim HR, Kim KW, Kim BM, et al. The effect of vascular endothelial growth factor on osteoclastogenesis in rheumatoid arthritis. PLoS One. 2015;10(4):e0124909. doi:10.1371/journal.pone.0124909
53. Alshevskaya AA, Lopatnikova JA, Shkaruba NS, et al. Differences of IL-1 beta receptors expression by immunocompetent cells subsets in rheumatoid arthritis. Mediators Inflamm. 2015;2015:948393. doi:10.1155/2015/948393
54. Zheng Y, Sun L, Jiang T, et al. TNFalpha promotes Th17 cell differentiation through IL-6 and IL-1 beta produced by monocytes in rheumatoid arthritis. J Immunol Res. 2014;2014:385352. doi:10.1155/2014/385352
55. Paradowska-Gorycka A, Pawlik A, Romanowska-Prochnicka K, et al. Relationship between VEGF gene polymorphisms and serum VEGF protein levels in patients with rheumatoid arthritis. PLoS One. 2016;11(8):e0160769. doi:10.1371/journal.pone.0160769
56. Clavel G, Bessis N, Lemeiter D, et al. Angiogenesis markers (VEGF, soluble receptor of VEGF and angiopoietin-1) in very early arthritis and their association with inflammation and joint destruction. Clin Immunol. 2007;124(2):158–164. doi:10.1016/j.clim.2007.04.014
57. Zhong YM, Cheng B, Zhang LL, et al. Effect of moxibustion on inflammatory cytokines in animals with rheumatoid arthritis: a systematic review and meta-analysis. Evid Based Complement Alternat Med. 2020;2020:6108619. doi:10.1155/2020/6108619
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
