Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 17
The Effect of Tirzepatide on Weight, Lipid Metabolism and Blood Pressure in Overweight/Obese Patients with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis
Authors Lv X , Wang H , Chen C, Zhao Y, Li K, Wang Y, Wang L, Fu S, Liu J
Received 2 November 2023
Accepted for publication 11 January 2024
Published 12 February 2024 Volume 2024:17 Pages 701—714
DOI https://doi.org/10.2147/DMSO.S443396
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Antonio Brunetti
Xiaoyu Lv,1 Hui Wang,1 Chongyang Chen,1 Yangting Zhao,1 Kai Li,1 Yawen Wang,1 Liting Wang,1,2 Songbo Fu,1,2 Jingfang Liu1,2
1The First Clinical Medical College, Lanzhou University, Lanzhou, Gansu, People’s Republic of China; 2Department of Endocrinology, The First Hospital of Lanzhou University, Lanzhou, Gansu, People’s Republic of China
Correspondence: Jingfang Liu, Department of Endocrinology, The First Hospital of Lanzhou University, Lanzhou, Gansu, 730000, People’s Republic of China, Tel +86 0931-8356242, Email [email protected]
Aim: To explore the effects of Tirzepatide (TZP), a new hypoglycemic drug, on weight, blood lipids and blood pressure in overweight/obese patients with type 2 diabetes mellitus (T2DM).
Methods: Relevant studies investigating the influence of TZP therapy on weight, lipid profiles and blood pressure in overweight/obese T2DM patients were selected from the PubMed, Embase, Web of Science and Cochrane databases from establishment until November 2022. A systematic review and meta-analysis were conducted to evaluate the effect of TZP on weight, blood lipids and blood pressure in overweight/obese patients with T2DM.
Results: Eight randomized controlled trials (RCTs), comprising 7491 patients with T2DM, were included in the meta-analysis. Results showed that compared with the glucagon-like peptide-1 receptor agonist (GLP-1RA), insulin, and placebo groups, body weight, triglycerides (TG), very low-density lipoprotein cholesterol (VLDL-C), total cholesterol (TC), systolic blood pressure (SBP), diastolic blood pressure (DBP), fasting blood glucose (FBG), and glycosylated hemoglobin (HbA1c) levels were significantly decreased in the TZP-treated groups, while high-density lipoprotein cholesterol (HDL-C) levels increased. With the gradual increase of TZP doses, the proportions of T2DM patients with weight loss > 5% gradually increased. The 10 mg and 15 mg TZP doses had a stronger effect on the levels of TG, VLDL-C, and HDL-C. Moreover, the reduction in SBP levels in the 15 mg TZP-treated group was more pronounced than those in the 10 mg and 5 mg TZP-treated groups [MD=− 2.07, 95% CI (− 2.52, − 1.63) and MD=− 3.14, 95% CI (− 4.42, − 1.87)]. Compared with GLP-1RA, insulin, and placebo groups, the proportions of patients with HbA1c< 7% in 10mg and 15mg TZP-treated groups were significantly higher than in the 5mg TZP-treated group [OR=1.53, 95% CI (1.25, 1.8)], OR=1.7, 95% CI (1.15, 2.50)].There was no significant difference regarding the risk of adverse reactions.
Keywords: tirzepatide, T2DM, weight, lipid metabolism, meta-analysis
Introduction
Type 2 diabetes mellitus (T2DM) is a chronic, progressive metabolic disease that accounts for 90–95% of all diabetes mellitus cases. The global prevalence of T2DM is 6059 cases/100,000 diabetic patients,1 with the incidence being slightly higher among men than women. Obesity, or being overweight, are common causes of T2DM, and are associated with elevated risks of cardiovascular diseases and mortality when compared to normal-weight T2DM patients. Thus, weight control can reduce the occurrence of cardiovascular complications and improve prognosis. Obesity and T2DM are independent factors that influence atherosclerotic cardiovascular diseases.2 As atherosclerotic plaques progressively develop, they can trigger conditions such as angina pectoris or cerebral ischemia attack, ultimately leading to severe complications such as heart failure, myocardial infarction (MI), cerebral infarction, lower limb ischemic amputations, and other life-threatening complications.2
Long-term effective control of blood sugar is of great importance in preventing and reducing diabetic complications; however, there are still many patients with diabetes whose blood glucose levels have not been effectively controlled. With the in-depth study of the pathogenesis of T2DM, there is an increasing demand for hypoglycemic drugs. However, there are no effective and safe drugs for obese diabetic patients, and all have certain side effects, such as weight gain (Thiazolidinediones, Sulfonylureas and insulin), hypoglycemia (Sulfonylureas, Repaglinide and insulin), and gastrointestinal side effects (Metformin and α-Glucosidase Inhibitors), among others.
Recently, a new type of hypoglycemic drug called Tirzepatide(TZP) has been garnering increasing attention. It is a pioneering once-weekly dual glucagon-like peptide-1 (GLP-1) and glucose-dependent insulin-stimulating polypeptide (GIP) receptor agonist, and the first new class of hypoglycemic drug to be approved for the market in nearly ten years. On one hand, by activating GLP-1 receptors, it promotes glucose-dependent insulin secretion, inhibits glucagon release, and delays gastric emptying, thereby achieving hypoglycemic effects similar to those of selective GLP-1 agonists. Simultaneously, it also inhibits food intake and appetite.3 On the other hand, it restores the reactivity of islet β cells to GIP, enabling the role of GIP in promoting first-phase insulin secretion and improving insulin sensitivity, resulting in a more effective and stable hypoglycemic effect.4 Recent research has shown that the combination of GLP-1 and GIP can achieve complementary synergistic effects, resulting in more effective blood sugar control and weight loss. This can greatly benefit patients by providing protection against cardiovascular and cerebrovascular diseases.5
Many randomized controlled trials (RCTs) have been conducted on the efficacy and safety of TZP in foreign countries. However, most studies have focused on its effects on blood glucose and body weight in patients with T2DM, while only a few have examined its impact on lipid metabolism, blood pressure, heart rate, and other aspects. In this study, the effects of TZP on body weight, lipid metabolism, blood pressure, and heart rate in T2DM patients were systematically evaluated using a meta-analysis, thus providing evidence for the clinical application of TZP.
Materials and Methods
Literature Retrieval
The PubMed, Embase, Cochrane Library and Web of Science databases were systematically searched from their inception until December 2022 for literature on TZP treatment for overweight/obese T2DM patients. The literature search included the following keywords: Type 2 diabetes mellitus, Tirzepatide, body weight, lipids, blood pressure, and others. The selected studies were presented and reported in accordance with the PRISMA-P System Evaluation Reporting Guidelines. Predefined schemes were registered with PROSPERO under the registration number CRD42022370543.
All of the included studies were randomized controlled trials (RCTs) that compared the efficacy and safety of TZP with other drugs, such as GLP-1, insulin, or placebo. Duplicates were removed, and the titles and abstracts of the retrieved references were screened using Endnote software. Articles that potentially met the criteria were retrieved for full-text screening. In addition, the reference lists of the included articles were manually filtered to identify additional relevant studies for consideration.
Study Selection
Inclusion criteria: (a) Phase II/III RCTs or crossover trials evaluating TZP intervention; (b) all subjects were overweight or obese T2DM patients aged 18 years or older; (c) the primary outcomes included changes in weight, blood lipids and blood pressure.
Exclusion criteria: (a) non-human studies and in vitro studies, Phase I clinical trials, overlapping data sets, case reports, editorials, conference proceedings, reviews, expert opinions, abstracts-only papers, and reviews; (b) studies involving people with specific types of diabetes; (c) the duration of the study was 12 weeks or less; (d) studies without raw data; (e) non-randomized controlled trials; (f) non-English language publications and duplicative publications; (g) studies lacking control groups; (h) inconsistent doses of TZP.
Data Extraction and Quality Assessment
The authors independently reviewed the primary and secondary outcomes of the study. Data extraction was performed independently using a standardized, predefined table with first author names. The primary outcome was a change in weight (kg). Secondary outcomes were changes in lipid profiles (mg/dL), blood pressure (mmHg), heart rate, and proportion of patients with >5% weight loss.
Information extracted from each study included: 1) study characteristics: author, year, study design, country, sample size, and study duration; 2) personal characteristics: race, sex, age, course of disease, and inclusion and exclusion criteria; 3) characteristics of interventions: type, dose, duration, and control of drugs administered; and 4) measured outcomes: weight, blood lipids, blood pressure, proportion of patients with weight loss >5%, and reports of adverse events during the study.
For quality assessment, “The Cochrane Collaboration of Randomized Controlled Trial Quality Assessment Tool” was used to evaluate the risk of bias among the eligible randomized controlled studies, which mainly included the following: (i) selection bias: random sequence generation and allocation concealment; (ii) detection bias: focusing on the blinding method used for outcome assessment; (iii) wear deviation: incomplete result deviation; (iv) reporting bias: selective reporting of outcomes; and other deviations (such as whether the baseline characteristics of the patients were comparable).
Data Analysis
The mean difference (MD) and standard deviation (SD) or mean standard deviation (SEM) of the weights based on changes in baseline data were extracted from the available literature and entered into the Review Manager software (version 5.3) for meta-analysis. A random-effects model was used to obtain the combined mean differences in the primary outcomes. Continuous variables were expressed as mean differences with 95% confidence intervals (CI). Forest maps were generated from relevant results to evaluate the comprehensive effects of the intervention measures. A subgroup analysis was conducted according to different doses and types of drugs. The heterogeneity of the studies was assessed using I2, and I2>50% and p<0.05 were deemed as significant heterogeneity. Changes in weight, blood pressure, and blood lipid levels compared to baseline data were considered the primary outcomes. All final estimates are shown as averages with 95% CI intervals or P-values.
Results
Research Characteristics
The initial search identified 217 articles; 27 articles were included in PubMed, 58 in Embase, 82 in the Cochrane Library, and 69 in the Web of Science. All the retrieved documents were imported into Endnote software, and 110 duplicate documents were excluded. After a preliminary screening of the titles or abstracts, 126 papers were excluded, including 15 reviews, 12 meta-analyses, 27 conferences, reviews, or letters, and four animal experiments. After reading the abstracts, 50 articles were excluded. After carefully reading the full texts, additional 10 articles were excluded, including 5 repeated reports, 1 article for which we could not obtain the original text, 2 articles with data extraction issues, and 2 articles with inconsistent grouping. Ultimately, 8 studies were selected for inclusion.6–13 The detailed screening process is shown in Figure 1.
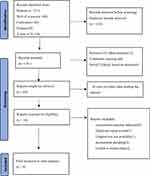 |
Figure 1 Screening process for studies included in this meta-analysis. |
The included studies were multicenter and multi-country studies, conducted mostly in the United States, Europe, and Japan. The duration of drug treatment ranged from 26 to 52 weeks. The mean body mass index (BMI) levels of participants in the TZP-treated or control-treated groups in all RCTs ranged from 28 kg/m2 to 34 kg/m2 respectively. Additionally, the mean HbA1c levels ranged from 7.7% to 8.6%, respectively. Further details and characteristics of the included studies are summarized in Table 1.
 |
Table 1 The Characteristics of Included Studies |
Quality of Deviation Control
The bias risks of the eight RCTs included in this meta-analysis are shown in Figure 2. Random sequence generation, incomplete data, reporting bias, and other biases are considered to have a low risk. Four of the eight studies were at low risk of both performance and detection deviations.
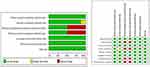 |
Figure 2 Risk of bias of included studies. |
Meta-Analysis
Weight
Among the eight RCTs included, 3530 T2DM patients were treated with three TZP doses (5, 10, and 15 mg). The control group included 2409 T2DM patients who were treated with a placebo, GLP-1RA, or insulin.
Compared to the placebo-treated group, the weight of T2DM patients in the 5 mg, 10 mg and 15 mg TZP-treated groups decreased by 6.17 kg [95% CI (−7.16, −5.17)], 9.20 kg [95% CI (−10.12, −8.29)] and 10.04 kg [95% CI (−11.14, −8.93)], respectively (Figure 3a).
Compared to the GLP-1RA-treated group, the weight of T2DM patients in the 5 mg, 10 mg and 15 mg TZP-treated groups decreased by 3.0 kg[95% CI (−3.67, −2.33)], 5.74 kg[95% CI (−6.41,-5.06)] and 7.4 kg[95% CI (−10.01, −4.81)], respectively (Figure 3b).
Compared to the insulin-treated group, the weight of T2DM patients in the 5 mg, 10 mg, and 15 mg TZP-treated groups decreased by 9.26 kg[95% CI (−9.89,-8.63)], 11.91 kg[95% CI (−12.54,-11.29)] and 14.35 kg[95% CI (−15.91, −12.78)], respectively (Figure 3c).
Weight loss in T2DM patients in the 15 mg TZP-treated group was significantly higher compared to that in the 5 mg [MD=−2.07, 95% CI (−2.52, −1.63)] and 10 mg TZP-treated groups [MD=−4.5, 95% CI (−5.26, 3.74)]. Further, weight loss in the 10 mg TZP-treated group was higher than that in the 5 mg TZP-treated group [MD=2.48, 95% CI (2.92, 2.03)] (Table 2).
 |
Table 2 Effect of the Different Doses of TZP on Metabolism-Related Indicators |
In addition, the percentage of T2DM patients with a weight loss of >5% was extracted from seven trials. The findings indicated that when compared to the GLP-1RA-, insulin-, and placebo-treated groups, the TZP-treated group showed a significantly higher proportion of patients with weight loss >5% [OR=8.39, 95% CI(2.81, 25.00), OR=17.53, 95% CI(5.88, 52.29), OR=22.63, 95% CI(7.97, 27.92)], respectively. Furthermore, there was a gradual increase in the proportion of T2DM patients with weight loss >5% with an escalation in the TZP dose (Figure 4).
 |
Figure 4 Effects of different doses of TZP on weight loss >5% in obese T2DM patients. |
Lipid Parameters
Due to inconsistent data among the eight included RCTs, four were finally selected for meta-analysis.
Compared to the GLP-1RA-treated group, TG levels in the 5 mg, 10 mg and 15 mg TZP-treated groups decreased by 13.5 mg/dL[95% CI (−18.17,-8.84)], 18.91 mg/dL [95% CI (−24.17,-13.65)] and 14.22 mg/dL[95% CI (−26.37,-2.07)], respectively; VLDL-C decreased by 12.33 mg/dL[95% CI (−24.65, 0.00)], 17.98 mg/dL[95% CI (−30.41,-5.56)], and 14.97 mg/dL[95% CI (−18.24,-11.71)], respectively; HDL-C levels increased by 4.54 mg/dL [95% CI (2.98, 6.11)], 7.16 mg/dL [95% CI (5.82, 8.49)], and 6.72 mg/dL [95% CI (5.40, 8.05)], respectively.
Compared to the insulin-treated group, TG levels in 5 mg, 10 mg and 15 mg TZP-treated groups were reduced by 6.83 mg/dL[95% CI (−11.6,-2.06)], 18.91 mg/dL[95% CI (−24.17,-13.65)] and 14.7 mg/dL[95% CI (−23.03,-6.38)] respectively; VLDL-C levels decreased by 6.99 mg/dL[95% CI (−13.25,-0.73)], 14.12 mg/dL[95% CI (−17.43, −10.81)], and 14.97 mg/dL [95% CI (−18.24, −11.71)], respectively; and VLDL-C levels increased by 4.54 mg/dL [95% CI (2.98, 6.11)], 7.56 mg/dL[95% CI (5.90, 9.21)], and 7.43 mg/dL [95% CI (5.77, 9.09)], respectively. In addition, TC levels in the 10 mg and 15 mg TZP-treated groups decreased by 4.29 mg/dL [95% CI [−6.84, −1.74)] and 4.29 mg/dL [95% CI (−7.05, −1.52)], respectively (Table 3).
 |
Table 3 Comparison Between Subgroups of Metabolic Indexes |
Different doses of TZP were found to have different effects on lipid metabolism indices in patients with T2DM under the same conditions. Compared with the 5 mg TZP-treated group, the 10 mg and 15 mg doses had a stronger effect on the decrease of TG levels [MD=−7.65, 95% CI (−10.16,-5.1) and MD=−6.48, 95% CI (−9.00,-3.96)] and VLDL-C [MD=−7.71, 95% CI (−10.17, −5.26) and MD=−6.57, 95% CI (−9.63,-3.5)] as well as stronger effect on the increase of HDL-C levels [MD=2.73, 95% CI (0.51, 4.94) and MD=2.52, 95% CI (1.2, 3.84)]. There were no statistically significant differences in the effects of different TZP treatment doses on TC and LDL-C levels. There was no difference in lipid metabolism indices between the 10 and 15 mg TZP-treated groups (Table 2).
Blood Pressure
Compared to the placebo group, the SBP levels of T2DM patients in the 5 mg, 10 mg, and 15 mg TZP-treated groups were significantly reduced by 3.57 mmHg [95% CI (−5.64, −1.5)], 4.36 mmHg [95% CI (−6.53, −2.4)] and 6.06 mmHg [95% CI (−7.88, −4.24)], and DBP levels in 10 mg and 15 mg TZP treatment groups decreased by 1.37 mmHg [95% CI (−2.7, −0.05)] and 2.48 mmHg[95% CI (−3.72, −1.23)], respectively (Table 3).
Compared to the GLP-1RA group, SBP levels of T2DM patients in 5 mg, 10 mg and 15 mg TZP-treated groups decreased by 3.11 mmHg [95% CI (−5.01, −1.2)], 4.27 mmHg [95% CI (−6.19, −2.35)] and 3.14 mmHg[95% CI (−5.38, −0.9)], DBP levels reduced by 2.19 mmHg [95% CI (−3.45,-0.92)], 2.4 mmHg[95% CI (−3.7,-1.1)], 3.4 mmHg[95% CI (−4.59, −2.21]), respectively.
The effects of the different doses of TZP on SBP differed. The reduction in SBP in the 15 mg TZP-treated group was stronger than that in the 10 mg and 5 mg TZP-treated groups [MD=−2.07, 95% CI (−2.52, −1.63) and MD=−3.14, 95% CI (−4.42,-1.87), respectively]. The effect of 10 mg of TZP was stronger than that of 5 mg of TZP (MD=−1.39, 95% CI[−2.52, −0.25]). There was no statistical difference in the effects of different TZP doses on TZP.
In addition, compared to the GLP-1RA group, the heart rate (HR) of T2DM patients in the 15 mg TZP-treated group was significantly increased (Table 3).
Glycemic Control
Compared to the placebo group, the fast blood glucose (FBG) levels of T2DM patients in the 5 mg, 10 mg and 15 mg TZP groups significantly decreased by 56.43 mg/dL[95% CI (−65.34, −47.51)], 65.87 mg/dL[95% CI (−82.63, −49.12)] and 58.92 mg/dL[95% CI (−73.3, −44.54)], respectively, and HbA1c levels decreased by 1.85%[95% CI (−2.08, 1.61)], 1.98%[95% CI (−2.22, −1.74)] and 2.22%[95% CI (−2.47, −1.98)], respectively.
Compared to the GLP-1RA group, FBG levels in T2DM patients in the 5 mg, 10 mg and 15 mg TZP-treated groups decreased by 25.55 mg/dL [95% CI (−30.17, −20.92)], 33.17 mg/dL [95% CI (−37.84,-28.51)] and 26.37 mg/dL [95% CI (−47.27,-5.47)], respectively. Meanwhile, HbA1c levels decreased by 0.61% [95% CI (−1.2, −0.02)], 0.89%[95% CI (−1.45. −0.34)], and 1.12%[95% CI (−1.79, −0.45)], respectively.
Compared to the insulin-treated group, FBG levels in T2DM patients in the 5 mg, 10 mg and 15 mg TZP-treated groups decreased by 2.82 mg/dL [95% CI (0.18, 5.45)], 1.52 mg/dL[95% CI (−4.96, 1.92)] and 5.93 mg/dL[95% CI (−9.36, −2.51)], respectively. Meanwhile, HbA1c levels decreased by 0.7% [95% CI (−0.91, −0.50)], 0.93%[95% CI (−1.06, −0.80)], and 1.09%[95% CI (−1.20, −0.98)], respectively (Table 3).
Compared to the GLP-1RA, insulin, and placebo groups, the proportion of patients with HbA1c<7% was significantly higher in the TZP-treated group [OR=4.89, 95% CI (2.47, 9.67), OR=8.11, 95% CI (3.85, 17.08), OR=8.20, 95% CI (4.41, 15.25)]. The proportion of patients with HbA1c<7% in the 10 mg and 15 mg TZP-treated groups was significantly higher than that in the 5 mg TZP-treated group [OR=1.53, 95% CI (1.25, 1.8)], OR=1.7, 95% CI (1.15, 2.50)]. However, there was no significant difference in the proportion of patients with HbA1c<7% between the 10 and 15 mg TZP-treated groups (Table 2).
Adverse Events
There was no overall increase in the number of patients who withdrew from TZP treatment. We studied the incidence of adverse reactions to different doses of TZP-treated groups (Table 4). When compared with the 5 mg TZP group, the risks of nausea [OR=1.81, 95% CI (1.51, 2.18)], vomiting [OR=1.87, 95% CI (1.43, 2.43)], discontinuation [OR=1.43, 95% CI (1.12, 1.83)], and hypoglycemia [OR=1.47, 95% CI (1.07, 2.01)] in the 15 mg TZP-treated group, and the 10 mg TZP-treated group [OR=1.46, 95% CI (1.21, 1.77)] were slightly increased. However, the risk of other adverse reactions did not show significant differences among the different dose groups (Table 4).
 |
Table 4 Comparison of the Adverse Effects of the Different Doses of TZP |
Discussion
TZP, as a dual GIP-1/GIP receptor antagonist, represents a new type of hypoglycemic drug. The findings on TZP treatment showed significant weight loss, as well as hypotensive and lipid-regulating effects in overweight and obese patients with T2DM. Importantly, TZP did not increase the risk of severe gastrointestinal reactions, hypoglycemia, pancreatitis, and cholelithiasis when compared to the GLP-1RA, insulin, and placebo controls. In addition, TZP had a dose-dependent effect on blood glucose, body weight, and lipid levels.
TZP is a linear synthetic peptide consisting of 39 amino acids, designed based on the natural GIP sequence. It exhibits agonist activity for both GIP and GLP-1 receptors, making it an unbalanced dual agonist. Compared to endogenous GIP, TZP shows equivalent affinity for the GIP receptor (GIPR), but binds to the GLP-1 receptor (GLP-1R) with approximately 5-fold weaker affinity compared to endogenous GLP-1. A previous meta-analysis showed that TZP has beneficial effects on blood glucose and blood lipid profiles without significantly increasing adverse effects,14 which is consistent with our findings. However, in our study, a subgroup analysis was conducted based on the different types of control drugs, revealing varying degrees of improvement in body weight, blood lipids, and blood pressure levels with TZP treatment compared to other GLP-1RA, insulin, or placebo controls. In addition, changes in FBG, TG, HDL-C, and SBP levels were not dose-dependent, indicating that the degree of index change did not increase with increasing TZP doses.
This meta-analysis showed that TZP demonstrated a unique appeal in reducing body weight, particularly by increasing the proportion of T2DM patients with >5% weight loss. Compared with the GLP-1RA, insulin, and placebo-treated groups, the body weight were increased in the TZP-treated groups of overweight/obese T2DM patient, and compared with the 5 mg TZP-treated group, the 10 mg and 15 mg doses had a stronger effect on the decrease of body weight levels. GIP receptor activation (GIPRA) can lead to weight loss in patients with metabolic diseases. The GLP-1/GIP dual receptor agonists resulted in better weight loss than GLP-1RA alone,15,16 while long-term administration of GIPRA does not reduce body weight,17 Therefore, the weight loss effect may be associated with the simultaneous activation of GIP-1/GIP receptors by TZP and the synergistic effect exerted by the two enterostatins, which can suppress appetite and increase energy expenditure in T2DM patients. For example, in transgenic mice, chronically elevated GIP levels alleviated diet-induced obesity (DIO) and improved insulin sensitivity by reducing caloric intake.15 In addition, the synergistic effects of GIP and GLP-1 receptors may occur in the CNS level.18 Concomitant administration of GLP-1 and GIP increased the expression of pro-opiomelanocortin (POMC) genes in anorexia nervosa, which reduced appetite and food intake. In addition to POMC neurons, there may be a unique population of neurons in the arcuate nucleus of the hypothalamus (ARN) that is only activated by the simultaneous co-administration of GLP-1 and GIP and is not activated when administered alone. These neurons are POMC gene-independent with both GLP-1 and GIP receptors, which function by transmitting a chemical signal to adjacent anaerobic POMC gene-regulated neurons.19 This raises the possibility that GIP may enhance the function of GLP-1 by facilitating its entry into the anorexigenic neuron population in the basal region of the hypothalamus, thereby increasing satiety and reducing the preference for high-fat and sweet foods in patients, acting as an anorexic and thus reducing their body weight. The cellular properties and mechanisms of action of these activated neurons are not yet known and need to be studied in depth.
Firstly, our study found that compared with the GLP-1RA, insulin, and placebo-treated groups, the body weight, TG, VLDL-C, TC levels were significantly decreased, while HDL-C levels were increased in the TZP-treated groups of overweight/obese T2DM patient. Secondly, compared with the 5 mg TZP-treated group, the 10 mg and 15 mg doses had a stronger effect on the decrease of TG levels and VLDL-C as well as stronger effect on the increase of HDL-C levels. There were no statistically significant differences in the effects of different TZP treatment doses on TC and LDL-C levels. There was no difference in lipid metabolism indices between the 10 and 15 mg TZP-treated groups. In combination with GLP-1RA and insulin therapy, TZP also demonstrates significant lipid-regulating effects without increasing serious gastrointestinal adverse effects. This presents a promising development in the treatment of T2DM patients. In peripheral tissues, GIP promotes the storage of triglycerides (TAG) after food intake, either directly through the activation of GIPR on adipocytes, or indirectly through the lipogenic effect of insulin, or both.20,21 Studies on mice injected with large amounts of GIP have revealed down-regulation of several genes encoding proteins involved in mitochondrial function and inflammatory signaling pathways. These include reduced expression of the pro-inflammatory cytokine MCP-1 (Ccl2), fibrinogen activator inhibitor type 1 (Serpine1), and interleukin 4 receptor α (IL4ra), as well as a component of the nuclear factor kB (NF-kB) pathway, inhibitor of nuclear factor kappa B kinase subunit B (Ikbkb).20 In addition to this, GIP can increase insulin sensitivity and improve islet β-cell function in mice,22,23 reduce steatosis24 and promote lipid metabolism in the body, thus resulting in a reduction of free lipid substances in the blood. However, the number of studies addressing the lipid profile changes is scarce, and further studies are needed.
Ongoing randomized controlled trials will confirm the role of TZP in overweight/obese adults or children with non-T2DM, those with liver damage, and those with kidney damage. A 72-week clinical study in obese patient has confirmed25 that once-weekly treatment with 5 mg, 10 mg, or 15 mg TZP results in significant and sustained weight loss. In addition to this, TZP has shown unique appeal in non-alcoholic fatty liver diseases (NAFLD). A clinical study26 demonstrated that TZP significantly reduces the levels of biomarkers associated with non-alcoholic steatohepatitis (NASH) in patients with T2DM, which provides a basis for further evaluation of the role of TZP in NASH patients. It was reported27 that TZP resulted in a reduction in liver fat content, visceral adipose tissue and abdominal subcutaneous adipose tissue volume compared to insulin treatment. These encouraging results suggest that TZP holds great promise for future applications in the treatment of obesity, heart failure and NAFLD.
In this study, we performed a subgroup analysis to explore the source of heterogeneity; however, the results were unsatisfactory. Ethnic differences in the study population, different follow-up times, or different selections, definitions, and interpretations of the results may have contributed to the high heterogeneity observed in this study. In the comparison with GLP-1RA, the reason for high heterogeneity may be related to different drug classes and different doses.
Conclusions
Compared with the GLP-1RA, insulin, and placebo-treated groups, the body weight, TG, VLDL-C, TC, blood pressure, FBG, and HbA1c levels were significantly decreased, while HDL-C levels were increased in the TZP-treated groups of overweight/obese T2DM patient. With the gradual increase of TZP doses, the proportions of T2DM patients with weight loss >5% gradually increased. The 10 mg and 15 mg TZP doses had a stronger effect on the levels of TG, VLDL-C, and HDL-C. Furthermore, there was no significant difference regarding the risk of adverse reactions.
Data Sharing Statement
Data sharing does not apply to this article.
Ethics Statement
Since this article does not involve human trials, ethical statements do not apply to this manuscript.
Funding
This study was funded by the National Natural Science Foundation of China (No.81960155), Natural Science Foundation of Gansu Province (20JR10RA690) and The Hospital Fund of the First Hospital of Lanzhou University (ldyy-2021-01).
Disclosure
The manuscript has no financial relationship with any institution and all authors claim no conflict of interest.
References
1. Association AD. Classification and diagnosis of diabetes: standards of medical care in diabetes—2021. Diabetes Care. 2021;44:S15–S33.
2. Khan MAB, Hashim MJ, King JK, et al. Epidemiology of type 2 diabetes-global burden of disease and forecasted trends. J Epidemiol Glob Health. 2020;10:107–111. doi:10.2991/jegh.k.191028.001
3. Kim KS, Seeley RJ, Sandoval DA, et al. Signalling from the periphery to the brain that regulates energy homeostasis. Nat Rev Neurosci. 2018;19:185–196. doi:10.1038/nrn.2018.8
4. Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131–2157. doi:10.1053/j.gastro.2007.03.054
5. Skow MA, Bergmann NC, Knop FK. Diabetes and obesity treatment based on ual incretin receptor activation: ‘twincretins’. Diabetes Obes Metab. 2016;18(9):847–854. doi:10.1111/dom.12685
6. Dahl D, Onishi Y, Norwood P, et al. Effect of subcutaneous tirzepatide vs placebo added to titrated insulin glargine on glycemic control in patients with type 2 diabetes: the SURPASS-5 randomized clinical trial. JAMA. 2022;327(6):534–545. doi:10.1001/jama.2022.0078
7. Frias JP, Nauck MA, an J V, et al. Efficacy and safety of L Y3298176, a novel dual GIP and GLP-1 receptor agonist, in patients with type 2 diabetes: a randomised, placebo-controlled and active comparator-controlled Phase 2 trial. Lancet. 2018;392(10160):2180–2193. doi:10.1016/S0140-6736(18)32260-8
8. Frias JP, Davies MJ, Rosenstock J, et al. Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. N Engl J Med. 2021;385(6):503–515. doi:10.1056/NEJMoa2107519
9. Ludvik B, Giorgino F, Jódar E, et al. Once-weekly tirzepatide versus once-daily insulin degludec as add-on to metformin with or without SGLT2 inhibitors in patients with type 2 diabetes (SURPASS-3): a randomised, open-label, parallel-group, phase 3 trial. Lancet. 2021;398(10300):583–598. doi:10.1016/S0140-6736(21)01443-4
10. Prato SD, Kahn SE, Pavo I, et al. Tirzepatide versus insulin glargine in type 2 diabetes and increased cardiovascular risk (SURPASS-4): a randomised, open-label, parallel-group, multicentre, phase 3 trial. Lancet. 2021;398(10313):1811–1824. doi:10.1016/S0140-6736(21)02188-7
11. Rosenstock J, Wysham C, Frías JP, et al. Efficacy and safety of a novel dual GIP and GLP-1 receptor agonist tirzepatide in patients with type 2 diabetes (SURPASS-1): a double-blind, randomised, phase 3 trial. Lancet. 2021;398(10295):143–155. doi:10.1016/S0140-6736(21)01324-6
12. Inagaki N, Takeuchi M, Oura T, et al. Efficacy and safety of tirzepatide monotherapy compared with dulaglutide in Japanese patients with type 2 diabetes (SURPASS J-mono): a double-blind, multicentre, randomised, phase 3 trial. Lancet Diabetes Endocrinol. 2022;10(9):623–633.
13. Heise T, Mari A, DeVries JH, et al. Effects of subcutaneous tirzepatide versus placebo or semaglutide on pancreatic islet function and insulin sensitivity in adults with type 2 diabetes: a multicentre, randomised, double-blind, parallel-arm, phase 1 clinical trial. Lancet Diabetes Endocrinol. 2022;10(6):418–429. doi:10.1016/S2213-8587(22)00085-7
14. Bhagavathula AS, Vidyasagar K, Tesfaye W. Efficacy and safety of tirzepatide in patients with type 2 diabetes mellitus: a systematic review and meta-analysis of randomized phase II/III trials. Pharmaceuticals. 2021;14(10):991. doi:10.3390/ph14100991
15. Finan B, Ma T, Ottaway N, et al. Unimolecular dual incretins maximize metabolic benefits in rodents, monkeys, and humans. Sci trans med. 2013;5(209):209ra151. doi:10.1126/scitranslmed.3007218
16. Frias JP, Bastyr EJ, Vignati L, et al. The sustained effects of a dual GIP/GLP-1 receptor agonist, NNC0090-2746, in patients with type 2 diabetes. Cell Metab. 2017;26(2):
17. Norregaard PK, Deryabina MA, Tofteng Shelton P, et al. A novel GIP analogue, ZP 4165, enhances glucagon-like peptide-1-induced body weight loss and improves glycaemic control in rodents. Diabetes Obesity Metab. 2018;20(1):60–68. doi:10.1111/dom.13034
18. NamKoong C, Kim MS, Jang BT, et al. Central administration of GLP-1 and GIP decreases feeding in mice. Biochem Biophys Res Commun. 2017;490(2):247e252.
19. Adriaenssens AE, Biggs EK, Darwish T, et al. Glucose-dependent insulinotropic polypeptide receptor-expressing cells in the hypothalamus regulate food intake. Cell Metab. 2019;30:987–996. doi:10.1016/j.cmet.2019.07.013
20. Kim SJ, Nian C, Karunakaran S, et al. GIPoverexpressing mice demonstrate reduced diet-induced obesity and steatosis, and improved glucose homeostasis. PLoS One. 2012;7:e40156. doi:10.1371/journal.pone.0040156
21. Kaplan AM, Vigna SR. Gastric inhibitory polypeptide (GIP) binding sites in rat brain. Peptides. 1994;15:297–302.
22. Hinke SA, Gelling RW, Pederson RA, et al. Dipeptidyl peptidase IV-resistant [D-Ala(2)]glucose-dependent insulinotropic polypeptide (GIP) improves glucose tolerance in normal and obese diabetic rats. Diabetes. 2002;51:652–661. doi:10.2337/diabetes.51.3.652
23. Gault VA, Porter DW, Irwin N, Flatt PR. Comparison of sub-chronic metabolic effects of stable forms of naturally occurring GIP(1–30) and GIP(1–42)in high-fat fed mice. J Endocrinol. 2011;208:265–271.
24. Widenmaier SB, Kim SJ, Yang GK, et al. AGIP receptor agonist exhibits beta-cell anti-apoptotic actions in rat models of diabetes resulting in improved beta-cell function and glycemic control. PLoS One. 2010;5:e9590. doi:10.1371/journal.pone.0009590
25. Jastreboff AM, Aronne LJ, Ahmad NN, et al. SURMOUNT-1 Investigators. Tirzepatide once weekly for the treatment of obesity. N Engl J Med. 2022;387(3):205–216. doi:10.1056/NEJMoa2206038
26. Hartman ML, Sanyal AJ, Loomba R, et al. Effects of novel dual GIP and GLP-1 receptor agonist tirzepatide on biomarkers of nonalcoholic steatohepatitis in patients with type 2 diabetes. DiabetesCare. 2020;43(6):1352–1355.
27. Gastaldelli A, Cusi K, Fernández Landó L, Bray R, Brouwers B, Rodríguez Á. Effect of tirzepatide versus insulin degludec on liver fat content and abdominal adipose tissue in people with type 2 diabetes (SURPASS-3 MRI): a substudy of the randomised, open-label, parallel-group, phase 3 SURPASS-3 trial. Lancet Diabetes Endocrinol. 2022;10(6):393–406. doi:10.1016/S2213-8587(22)00070-5
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

