Back to Journals » Clinical Epidemiology » Volume 15
The Danish Atrial Fibrillation Registry: A Multidisciplinary National Pragmatic Initiative for Monitoring and Supporting Quality of Care Based on Data Retrieved from Administrative Registries
Authors Frost L , Joensen AM, Dam-Schmidt U, Qvist I , Brinck M, Brandes A , Davidsen U, Pedersen OD, Damgaard D, Mølgaard I, Bedsted R , Damgaard Møller Schlünsen A, Grijota Chousa M, Andersen J, Pedersen AR, Johnsen SP, Vinter N
Received 6 October 2023
Accepted for publication 9 December 2023
Published 22 December 2023 Volume 2023:15 Pages 1259—1272
DOI https://doi.org/10.2147/CLEP.S443473
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Laura Horsfall
Lars Frost,1,2 Albert Marni Joensen,3 Ulla Dam-Schmidt,4 Ina Qvist,5 Margit Brinck,6 Axel Brandes,7,8 Ulla Davidsen,4 Ole Dyg Pedersen,9 Dorte Damgaard,10 Inge Mølgaard,11 Robert Bedsted,11 Anders Damgaard Møller Schlünsen,12 Miriam Grijota Chousa,12 Julie Andersen,12 Asger Roer Pedersen,1 Søren Paaske Johnsen,13 Nicklas Vinter1,13
1Diagnostic Centre, Silkeborg Regional Hospital, Silkeborg, Denmark; 2Department of Clinical Medicine, Aarhus University, Aarhus, Denmark; 3Department of Cardiology, North Denmark Regional Hospital, Hjørring, Denmark; 4Department of Cardiology, Bispebjerg Hospital, Copenhagen University Hospitals, Copenhagen, Denmark; 5Department of Cardiology, Aarhus University Hospital, Aarhus, Denmark; 6Department of Cardiology, Odense University Hospital, Odense, Denmark; 7Department of Cardiology, Esbjerg Hospital – University Hospital of Southern Denmark, Esbjerg, Denmark; 8Department of Regional Health Research, University of Southern Denmark, Odense, Denmark; 9Department of Cardiology, Zealand University Hospital, Roskilde, Denmark; 10Department of Neurology, Aarhus University Hospital, Aarhus, Denmark; 11Patient Representative, Aalborg and Roskilde, Denmark; 12The Danish Clinical Quality Program – National Clinical Registries (RKKP), Aarhus, Denmark; 13Danish Center for Health Services Research, Department of Clinical Medicine, Aalborg University, Aalborg, Denmark
Correspondence: Lars Frost, Diagnostic Centre, University Clinic for Development of Innovative Patient Pathways, Silkeborg Regional Hospital; Department of Clinical Medicine, Aarhus University, Falkevej 3, Silkeborg, 8600, Denmark, Tel +45 23988590, Email [email protected]
Aim: The Danish Atrial Fibrillation (AF) Registry monitors and supports improvement of quality of care for all AF patients in Denmark. This report describes the registry’s administrative and organizational structure, data sources, data flow, data analyses, annual reporting, and feedback between the registry, clinicians, and the administrative system. We also report the selection process of the quality indicators and the temporal trends in results from 2017– 2021.
Methods and Results: The Danish AF Registry aims for complete registration and monitoring of care for all patients diagnosed with AF in Denmark. Administrative registries provide data on contacts to general practice, contacts to private cardiology practice, hospital contacts, medication prescriptions, updated vital status information, and biochemical test results. The Danish Stroke Registry provides information on stroke events. From 2017 to 2021, the proportion with a reported echocardiography among incident AF patients increased from 39.9% (95% CI: 39.3– 40.6) to 82.6% (95% CI: 82.1– 83.1). The initiation of oral anticoagulant therapy among patients with incident AF and a CHA2DS2-VASc score of ≥ 1 in men and ≥ 2 in women increased from 85.3% (95% CI: 84.6– 85.9) to 90.4% (95% CI: 89.9– 91.0). The 1-year and 2-year persistence increased from 85.2% (95% CI: 84.5– 85.9) to 88.7% (95% CI: 88.0– 89.3), and from 85.4% (95% CI: 84.7– 86.2) to 88.2% (95% CI: 87.5– 88.8), respectively. The 1-year risk of ischemic stroke among prevalent patients with AF decreased from 0.88% (95% CI: 0.83– 0.93) to 0.71% (95% CI: 0.66– 0.75). Variation in clinical performance between the five administrative Danish regions was reduced.
Conclusion: Continuous nationwide monitoring of quality indicators for AF originating from administrative registries is feasible and supportive of improvements of quality of care.
Keywords: atrial fibrillation, quality indicators, quality of care
Introduction
Atrial fibrillation (AF) is a natural candidate condition for quality-of-care monitoring and improvement because of the increasing prevalence, the associated poor prognosis, and the high health care and societal costs.1–4 The 2020 European Society of Cardiology (ESC) Guidelines for the diagnosis and management of AF recommends, as a class IIa recommendation, that practitioners and institutions consider tools to measure quality of care and to identify opportunities for improvement of treatment quality and patient outcomes.5,6 The ESC guidelines for AF suggest surveillance in six domains: patient assessment, anticoagulation, rate control, rhythm control, risk factor management, and outcomes.5,6 The American College of Cardiology and the American Heart Association have jointly developed clinical performance and quality measures for patients with AF.7,8 The European and American guidelines on quality indicators for the care and outcome of adults with AF are similar, although their conceptualization may differ. However, none of these guidelines have practical recommendations for long-term follow-up in primary or secondary care, nor do they suggest a common data platform covering all health care sectors. The European Unified Registries for Heart Care Evaluation and Randomized Trials (EuroHeart) recently published data standards for AF/flutter and catheter ablation.9
For more than 20 years, clinical quality databases have constituted a central tool for quality improvement work in the Danish healthcare system. The activities of the clinical quality databases have since 2011 been coordinated through the umbrella organization of the Danish Clinical Registries. There are currently 85 approved national clinical quality databases in Denmark. The majority cover specific disease entities, ie, heart failure, stroke, or breast cancer. Other databases cover a specific disease-related procedure (eg, bariatric surgery or hip replacement), while a third category of databases covers a broader range of procedures such as intensive care treatment or anaesthesia procedures.
Danish nationwide data documented regional inequities in treatment and outcomes in patients with AF from 2007–2014,10 and AF was therefore included as a disease of interest under the Danish Clinical Registries in 2016. The prerequisite of the inclusion was that the Danish AF Registry should adhere to Danish guidelines for AF established by the Danish Society of Cardiology by endorsement of the ESC AF guidelines, and be based exclusively on existing data retrieved from administrative data sources, including the Danish National Registry of Patients and the Civil Registration System.11 The impetus to let the Danish AF Registry originate from already existing information was double; there should be no excess work for clinicians concerning the registration of data, and the Danish AF Registry should be complete; ie, all patients diagnosed with AF at Danish hospitals, general practice, and private cardiology practices should be included.
We aim to report the administrative and organizational structure of The Danish AF Registry, data sources, data flow, data analyses, annual reporting, and feedback between The Danish AF Registry, clinicians, and the administrative system. We also describe the selection process of the quality indicators and the temporal trends in results over the five years of operation from 2017–2021.
Materials and Methods
Organization
The multi-disciplinary steering committee of The Danish AF Registry was established in 2015 and included one hospital-employed cardiologist from each of five Danish administrative regions, three hospital-employed specialist nurses, one cardiologist from private practice, one general practitioner, one neurologist, two patient representatives, one epidemiologist, one statistician, and one public health expert. All healthcare professionals are delegated from their professional organizations. The epidemiologist, the statistician, and the public healthcare experts are all employed at Danish Clinical Registries. The clinical registries are founded on a national initiative, mandated by law and regulated by the national government, but financed and owned by the regional governments.
Information Flow and Data Sources
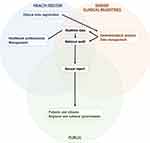
Figure 1 shows the information flow between the steering committee, clinicians, and stakeholders, including hospital management, regional administrators, regional politicians, the National Board of Health, and the national government. Table 1 shows the data sources and the information retrieved from each. A description of the data sources follows below.
 |
Table 1 Data Sources and Retrieved Information. The Danish Atrial Fibrillation Database |
The Danish National Patient Registry was established in 1977 and collects prospectively registered data on all inpatients, and after 1995 also all outpatients. Data include individual-level information on dates of admission and discharge, surgical procedures performed, and one primary and several secondary diagnoses per discharge. Coding of diagnoses followed the International Classification of Diseases 8th Revision before 1994 and the 10th revision (ICD-10) from 1994 and onwards. The physician who discharged a patient coded all diagnoses for that patient. We used a standardized look-back period of 10 years to differentiate incident from prevalent AF.
The Danish National Prescription Registry (from 2016 to 2022) and Register of Medicinal Product Statistics (from 2022) contain individual-level data on all dispensed prescriptions since 1994 and provide information on oral anticoagulants. Coding of medications followed the Anatomical Therapeutic Chemical Classification System.
The Danish Civil Registration System contains individual-level information on sex, date of birth, vital status and migration. All Danish citizens are assigned a unique 10-digit Civil Registration number, which enabled unambiguous linkages of data between registries.
The Register of Laboratory Results for Research was established in 2013 and contains information about all tests performed in hospital departments of clinical biochemistry.
The Danish Stroke Registry provides information about all patients with acute stroke (from 2003) or transient ischemic attack (from 2013) treated at Danish hospitals. Reporting is mandatory by law for all hospital departments treating these patients.
Data retrieved from private cardiology practices and general practices are currently pending but will commence during 2023 via data capture from commercial software houses servicing practices.
Selection of Quality Indicators
The quality indicators are based on national and international clinical recommendations. The national steering committee defined and selected the indicators based on systematic literature reviews and consensus. Requirements for the selection of indicators were that they should reflect the quality of the diagnostic process, treatment, safety, and a good patient outcome, and that information about the indicators could be retrieved from administrative registries. The indicators included clinical evaluation (echocardiography, patient education, measurement of thyroid stimulating hormone (TSH), measurement of plasma creatinine in patients treated with direct oral anticoagulants (DOACs), any oral anticoagulation (OAC) at AF diagnosis, long-term use of OAC, and clinical outcomes including ischemic stroke, intracranial bleeding, and major bleedings. The indicators and their specific quality targets are shown in Table 2. Every third year a report addresses the evidence base concerning the management of AF to evaluate whether the indicators used in the database are consistent with the current scientific literature and guidelines. The report also determines whether the targets for quality of care should be adjusted, whether indicators should be considered outdated, and new indicators should be introduced. A description of key variables and codes needed for calculating indicator results is provided in Supplementary Table 1.
 |
Table 2 Quality Indicators for Management of Atrial Fibrillation |
Statistical Analyses
For each quality indicator, the yearly number of patients (national or within regions) fulfilling the indicator was considered as the outcome of a binomial trial with size equal to the number of patients at the start of each year. Hence, 95%-confidence intervals for the yearly percentages were calculated by exact methods for the binomial distribution, and test for trends in proportions were conducted as statistical tests of the systematic effect of year in binomial regressions. National and regional data were tested for linear trend (linearity of proportions over years), as non-linear trends (systematic difference between proportions over years) would be analysed for all indicators unless all exhibited linear trend. Non-linear trend was tested on national data and partially quantified by the absolute difference in proportions between the last and the first year. Difference in regional non-linear trends (null hypothesis: same non-linear trend in all regions) was tested and partially quantified by the absolute difference in proportions between the last and the first year. For indicators with statistically significant different non-linear trends between regions, the absolute difference in proportions between last and first year was quantified per region and compared statistically to the regional average absolute difference. Statistical tests were done with a significance level of 5%. Data were analysed using Stata version 17.
Ethics
The Danish AF Registry was approved by the Danish Health Data Authority, and the Danish Data Protection Agency. Monitoring of quality of care does not require approval from an ethical committee according to Danish law.
Results
Echocardiography, Patient Education, and Blood Testing
Figure 2 and Supplementary Tables 2–4 show temporal trends in use of echocardiography, patient education and measurement of TSH among patients with incident AF. The quality target of echocardiography of more than 80% of all patients with incident AF has been fulfilled since 2020 at a national level, but with variation between regions. Patient education was suboptimal; less than one third of patients had a recording of having received education (quality target: ≥50% of incident patients). TSH measurement was suboptimal; only three out of four patients with incident AF were tested (quality target: ≥ 95%). Annual measurement of plasma creatinine among patients treated with a DOAC was performed in ∼93% of patients between 2017 and 2021 (Figure 2 and Supplementary Table 5), which is close to the quality target of ≥95%.
Oral Anticoagulation
Figure 3 and Supplementary Tables 6–8 show temporal trends in the initiation of and persistence to OAC. At the national level, the proportion of patients initiating OAC gradually increased from 85.3% (95% CI: 84.6–85.9) in 2017 to 90.4% (95% CI: 89.9–91.0) in 2021 (quality target ≥ 90%). The one-year persistence rate to OAC increased over the study period from 85.2% (95% CI: 84.5–85.9) to 88.7% (95% CI: 88.0–89.3), and the two-year persistence to OAC increased from 85.4% (95% CI: 84.7–86.2) to 88.2% (95% CI: 87.5–88.8) (Figure 3 and Supplementary Tables 7 and 8).
Outcomes
Figure 4 and Supplementary Tables 9–11 show temporal trends in the one-year risk of stroke, intracranial bleeding, and major bleeding among prevalent patients with AF. The one-year risk of stroke among prevalent patients with AF declined over the 6-year period from 0.88% (95% CI: 0.83–0.93) to 0.71% (95% CI: 0.66–0.75). The one-year risk of intracranial bleeding among prevalent patients with AF was ∼0.6% and did not change over the period (Supplementary Table 10), and the one-year risk of major bleeding also remained unchanged, ∼2.2%.
Regional Variation
The national performance improved over time in relation to all quality indicators except for intracranial bleeding and other major bleeding, which remained constant. We observed less variation between regions over calendar years (Tables 3 and 4, and Figures 2–4).
 |
Table 3 Trends in National Performance Measures (2021 versus 2017) |
 |
Table 4 Trend in Regional Quality Performance Measures |
Discussion
We have documented that it is feasible to establish and maintain a multi-disciplinary nationwide quality of care initiative including the selection of quality targeted monitoring of standards of care for patients with incident and prevalent AF based on data retrieved from administrative registries. Over the first five years, we observed a gradual increase in use of OAC, and a reduction in risk of stroke without imposing an increased risk of intracranial and other major bleedings. We also observed that variation between regions decreased. The increased uptake of OAC correlating to a secular decrease in risk of stroke among patients with AF has been reported from many countries.12–21
Contrasting our findings of an initiation rate of OAC close to 90%, a systematic review and meta-analysis of worldwide trends in oral OAC use in patients with AF from 2010 to 2018 concluded that the proportion of OAC users worldwide almost doubled, but one-quarter of OAC eligible patients with AF were not anticoagulated.22
Less than one of three had patient education. A recent survey of AF service at Danish hospitals reported that under-use of patient education was explained by a lack of nursing resources, uncertainty in relation to what and how to deliver education, and low management priority.23
Strengths and Limitations
The use of data capture from administrative data sources has strengths and limitations. The strengths are that data can be considered almost complete and that it is possible to follow all incident patients with AF from diagnosis until death. This prevents selection and follow-up bias. There may be errors in the clinical coding practice resulting in misclassification. However, validity studies have demonstrated a high positive predictive value for a hospital diagnosis of AF.24,25 The positive predictive value of a stroke diagnosis recorded in The Danish Stroke Registry has been reported to be > 90%.26
Performance data were analysed at the national level, and at regional, hospital, and municipality level. Direct comparison of performance between regions, hospitals, and municipalities should be made with caution due to limited statistical power, and because there may be differences in lifestyle factors, socioeconomic conditions, and comorbidities, which are not accounted for.
We had no information about patient preference for anticoagulation, absolute contraindications for anticoagulation, active bleeding, use of low molecular heparin, and end-of- life conditions. Therefore, we could not set any quality targets at 100%. Based on validation samples, we determined by consensus that the standard for OAC is an initiation rate of ≥90% and a persistence rate of ≥90% at 1, 2, and 5 years. Validation samples in relation to echocardiography demonstrated incomplete registration of echocardiography, in particular in the Capital Region and in Region Zealand. This is thought to be caused by software-related obstacles in relation to registration of echocardiography in these regions.
At present we cannot retrieve complete information from private cardiology practices and general practices, but this will be possible in 2023–2024. Information from the primary sector will shorten the delay from AF being diagnosed until it is recorded in the registry. In addition, flow of information from both the primary and secondary health care sectors will be mandatory to fully monitor the care and outcome of a chronic disease.
The near-complete inclusion by data capture of all patients with AF comes with a price, because information cannot be detailed. At present, we cannot monitor body weight, exercise, smoking, alcohol consumption, and blood pressure. However, at a later stage, it may be possible to retrieve detailed information from data sources used in general practice.
We have not included use of antiarrhythmic drugs or ablation for AF as quality indicators. The evidence base is not yet considered mature, because randomized studies in relation to rhythm control versus rate control included a limited number of selected patients. However, the evidence base for early rhythm control and catheter ablation is improving, and early rhythm control may be anticipated to be recommended in future clinical guidelines.27,28 We did not include information about quality of treatment of comorbidities, such as hypertension, diabetes, and chronic obstructive pulmonary disease. However, as both diabetes and chronic obstructive pulmonary disease are covered by other quality management databases by Danish Clinical Registries it is possible to cross-link the Danish AF Database to these databases. Information about risk factor management is currently not included, but updated information in relation to risk factor management may be included in future versions, given recognition that risk factor management is a life-long process and given that general practice and authorities accept that information about, for example, smoking history, current smoking, alcohol consumption, body mass index, and leisure time exercise should be recorded and updated in an administrative data base. This could ultimately lead to a fully monitored version of the “Atrial Fibrillation Better Care pathway.29
Perspectives
Improvements in health care and clinical outcomes usually advance gradually and may require persistence in a quality management organization, especially when treatments and outcomes are approaching the maximally obtainable. There is a hope for further improvements in care and outcomes derived from monitoring and from the implementation of new and better treatments and care pathways. The concept is not static, so it is very likely that quality targets may be adjusted and that future registry-based mapping of clinical performance and quality measures can include updated information in relation to lifestyle components, and whether a patient has been assigned to a heart rhythm or a heart rate control strategy including cross over between these strategies.
Conclusion
Continuous nationwide monitoring of clinical performance and quality measures in patients with AF is feasible based on data retrieved from administrative registries. We observed a gradual improvement in clinical performance and quality measures related to patients with AF, and less variation between regions in Denmark. However, we cannot conclude that the improvements were a direct result of the activities of the Danish AF Registry.
Data Sharing Statement
Individual-level data cannot be shared in public due to national legislation. Data can be accessed by application to the Danish Health Data Authority.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
L.F reports being supported by a research grant from the Health Research Foundation of Central Denmark Region. Consultant for BMS/Pfizer and AstraZeneca. A.B reports lecture fees from Boehringer-Ingelheim and Bristol-Myers Squibb; research grants from Theravance, The Zealand Region, the Canadian Institutes of Health Research, and the Danish Heart Foundation, grants from Independent Research Fund Denmark outside this work. DD reports lecture fees from BMS and Pfizer. SPJ reports grants from EU, institutional research grant from BMS/Pfizer. Consultant work for BMS/Pfizer. NV reports being supported by a research grant from the Danish Cardiovascular Academy (PD2Y-2022002-DCA). Consultant for AstraZenaca. No fees were received personally. The authors report no other conflicts of interest in this work.
References
1. Odutayo A, Wong CX, Hsiao AJ, Hopewell S, Altman DG, Emdin CA. Atrial fibrillation and risks of cardiovascular disease, renal disease, and death: systematic review and meta-analysis. BMJ. 2016;354:i4482. doi:10.1136/bmj.i4482
2. Vinter N, Huang Q, Fenger-Gron M, Frost L, Benjamin EJ, Trinquart L. Trends in excess mortality associated with atrial fibrillation over 45 years (Framingham heart study): community based cohort study. BMJ. 2020;370:m2724.
3. Burdett P, Lip GYH. Atrial fibrillation in the UK: predicting costs of an emerging epidemic recognizing and forecasting the cost drivers of atrial fibrillation-related costs. Eur Heart J Qual Care Clin Outcomes. 2022;8:187–194. doi:10.1093/ehjqcco/qcaa093
4. Johnsen SP, Dalby LW, Tackstrom T, Olsen J, Fraschke A. Cost of illness of atrial fibrillation: a nationwide study of societal impact. BMC Health Serv Res. 2017;17:714. doi:10.1186/s12913-017-2652-y
5. Hindricks G, Potpara T, Dagres N, et al. ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42(5):373–498. doi:10.1093/eurheartj/ehaa612
6. Arbelo E, Aktaa S, Bollmann A, et al. Quality indicators for the care and outcomes of adults with atrial fibrillation. Europace. 2021;23(4):494–495. doi:10.1093/europace/euaa253
7. Heidenreich PA, Solis P, Mark ENA, et al. ACC/AHA clinical performance and quality measures for adults with atrial fibrillation or atrial flutter: a report of the American college of cardiology/American heart association task force on performance measures. Circ Cardiovasc Qual Outcomes. 2016;9:443–488. doi:10.1161/HCQ.0000000000000018
8. Heidenreich PA, Estes NAM, Fonarow GC, et al. Update to the 2016 ACC/aha clinical performance and quality measures for adults with atrial fibrillation or atrial flutter: a report of the American college of cardiology/American heart association task force on performance measures. J Am Coll Cardiol. 2021;77:326–341. doi:10.1016/j.jacc.2020.08.037
9. Batra G, Aktaa S, Camm AJ, et al. Data standards for atrial fibrillation/flutter and catheter ablation: the European unified registries for heart care evaluation and randomized trials (EuroHeart). Eur Heart J Qual Care Clin Outcomes. 2022.9:609–620.
10. Christesen AMS, Vinter N, Mortensen LS, Fenger-Gron M, Johnsen SP, Frost L. Inequality in oral anticoagulation use and clinical outcomes in atrial fibrillation: a Danish nationwide perspective. Eur Heart J Qual Care Clin Outcomes. 2018;4(3):189–199. doi:10.1093/ehjqcco/qcy011
11. Schmidt M, Schmidt SAJ, Adelborg K, et al. The Danish health care system and epidemiological research: from health care contacts to database records. Clin Epidemiol. 2019;11:563–591. doi:10.2147/CLEP.S179083
12. Shroff GR, Solid CA, Herzog CA. Atrial fibrillation, stroke, and anticoagulation in medicare beneficiaries: trends by age, sex, and race, 1992–2010. J Am Heart Assoc. 2014;3(3):e000756. doi:10.1161/JAHA.113.000756
13. Hansen PW, Sehested TSG, Fosbol EL, et al. Trends in warfarin use and its associations with thromboembolic and bleeding rates in a population with atrial fibrillation between 1996 and 2011. PLoS One. 2018;13:e0194295.
14. Forslund T, Komen JJ, Andersen M, et al. Improved stroke Prevention in Atrial fibrillation after the introduction of non-vitamin k antagonist oral anticoagulants. Stroke. 2018;49(9):2122–2128. doi:10.1161/STROKEAHA.118.021990
15. Hohnloser SH, Basic E, Nabauer M. Uptake in antithrombotic treatment and its association with stroke incidence in atrial fibrillation: insights from a large German claims database. Clin Res Cardiol. 2019;108(9):1042–1052. doi:10.1007/s00392-019-01437-7
16. Lee SR, Choi EK, Kwon S, et al. Effectiveness and safety of direct oral anticoagulants in relation to temporal changes in their use. Circ Cardiovasc Qual Outcomes. 2020;13(3):e005894. doi:10.1161/CIRCOUTCOMES.119.005894
17. Maggioni AP, Dondi L, Andreotti F, et al. Four-year trends in oral anticoagulant use and declining rates of ischemic stroke among 194,030 atrial fibrillation patients drawn from a sample of 12 million people. Am Heart J. 2020;220:12–19. doi:10.1016/j.ahj.2019.10.017
18. Cowan JC, Wu J, Hall M, Orlowski A, West RM, Gale CP. A 10 year study of hospitalized atrial fibrillation-related stroke in England and its association with uptake of oral anticoagulation. Eur Heart J. 2018;39(32):2975–2983. doi:10.1093/eurheartj/ehy411
19. Akao M, Ogawa H, Masunaga N, et al. 10-year trends of antithrombotic therapy status and outcomes in Japanese atrial fibrillation patients- the Fushimi AF registry. Circ J. 2022;86:726–736. doi:10.1253/circj.CJ-22-0023
20. Freixa-Pamias R, Blanch Gracia P, Rodriguez Latre ML, et al. Impact of prescription patterns of antithrombotic treatment on atrial fibrillation-related ischemic stroke. Curr Med Res Opin. 2021;37(3):357–365. doi:10.1080/03007995.2020.1865892
21. Kennedy C, Gabr A, McCormack J, Collins R, Barry M, Harbison J. The association between increasing oral anticoagulant prescribing and atrial fibrillation related stroke in Ireland. Br J Clin Pharmacol. 2022;88(1):178–186. doi:10.1111/bcp.14938
22. Grymonprez M, Simoens C, Steurbaut S, De Backer TL, Lahousse L. Worldwide trends in oral anticoagulant use in patients with atrial fibrillation from 2010 to 2018: a systematic review and meta-analysis. Europace. 2022;24(6):887–898. doi:10.1093/europace/euab303
23. Qvist I, Lane DA, Risom SS, et al. Implementation of atrial fibrillation patient education. Eur J Cardiovasc Nurs. 2023;zvad066. doi:10.1093/eurjcn/zvad066
24. Rix TA, Riahi S, Overvad K, Lundbye-Christensen S, Schmidt EB, Joensen AM. Validity of the diagnoses atrial fibrillation and atrial flutter in a Danish patient registry. Scand Cardiovasc J. 2012;46(3):149–153. doi:10.3109/14017431.2012.673728
25. Sundboll J, Adelborg K, Munch T, et al. Positive predictive value of cardiovascular diagnoses in the Danish national patient registry: a validation study. BMJ Open. 2016;6:e012832.
26. Wildenschild C, Mehnert F, Thomsen RW, et al. Registration of acute stroke: validity in the Danish stroke registry and the Danish national registry of patients. Clin Epidemiol. 2014;6:27–36. doi:10.2147/CLEP.S50449
27. Han S, Jia R, Cen Z, et al. Early rhythm control vs. rate control in atrial fibrillation: a systematic review and meta-analysis. Front Cardiovasc Med. 2023;10:978637. doi:10.3389/fcvm.2023.978637
28. Simader FA, Howard JP, Ahmad Y, et al. Catheter ablation improves cardiovascular outcomes in patients with atrial fibrillation and heart failure: a meta-analysis of randomized controlled trials. Europace. 2023;25(2):341–350. doi:10.1093/europace/euac173
29. Romiti GF, Pastori D, Rivera-Caravaca JM, et al. Adherence to the ‘atrial fibrillation better care’ pathway in patients with atrial fibrillation: impact on clinical outcomes-a systematic review and meta-analysis of 285,000 patients. Thromb Haemost. 2022;122:406–414. doi:10.1055/a-1515-9630
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.