Back to Journals » OncoTargets and Therapy » Volume 14
The Controversial Role of Polyploidy in Hepatocellular Carcinoma
Authors Wang N , Hao F, Shi Y, Wang J
Received 23 September 2021
Accepted for publication 16 November 2021
Published 27 November 2021 Volume 2021:14 Pages 5335—5344
DOI https://doi.org/10.2147/OTT.S340435
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Takuya Aoki
Nan Wang,1,* Fengjie Hao,1,* Yan Shi,2 Junqing Wang1
1Department of General Surgery, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, People’s Republic of China; 2Department of Oncology, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yan Shi; Junqing Wang Email [email protected]; [email protected]
Abstract: Polyploidy, a physiological phenomenon in which cells contain more than two sets of homologous chromosomes, commonly exists in plants, fish, and amphibians but is rare in mammals. In humans, polyploid cells are detected commonly in specific organs or tissues including the heart, marrow, and liver. As the largest solid organ in the body, the liver is responsible for a myriad of functions, most of which are closely related to polyploid hepatocytes. It has been confirmed that polyploid hepatocytes are related to liver regeneration, homeostasis, terminal differentiation, and aging. Polyploid hepatocytes accumulate during the aging process as well as in chronically injured livers. The relationship between polyploid hepatocytes and hepatocellular carcinoma, the endpoint of most chronic liver diseases, is not yet fully understood. Recently, accumulated evidence has revealed that polyploid involves in the process of tumorigenesis and development. The study of the correlation and relationship between polyploidy hepatocytes and the development of hepatocellular carcinoma can potentially promote the prevention, early diagnosis, and treatment of hepatocellular carcinoma. In this review, we conclude the potential mechanisms of polyploid hepatocytes formation, focusing on the specific biological significance of polyploid hepatocytes. In addition, we examine recent discoveries that have begun to clarify the relevance between polyploid hepatocytes and hepatocellular carcinoma and discuss recent excellent findings that reveal the role of polyploid hepatocytes as resisters of hepatocellular carcinoma or as promoters of hepatocarcinogenesis.
Keywords: polyploidy, liver, hepatocyte, cancer, cell cycle
Introduction
Polyploid is one of the characteristics of hepatocytes,1 which shows essential relationship between liver regeneration and physiological features.2–6 Generally, in mammals, up to 90% of rodent livers and about 50% of human livers are polyploid.7–9 Polyploid hepatocytes are raised during postnatal development, and as for rodents, polyploid hepatocytes appears around weaning (postnatal day 14 [P14] to P21) and increases with age.4,10 In the liver, hepatocytes vary considerably in cell and nuclear size, number of nuclei per cell and DNA content per nucleus. The cellular ploidy of hepatocytes depends on the DNA content of each nucleus, plus the number of nuclei per cell.7,11 Tetraploid hepatocytes, for example, can be binucleated cells with two nuclei or mononucleated tetraploid cells.
Primary liver cancer is the most common malignancy of the digestive system worldwide. According to new data released by Globocan 2020,12 Primary liver cancer ranks the 6th most common malignancy in the world with 906,000 new cases per year pathogenesis and the 3rd most common malignancy with 830,000 deaths per year. Polyploidy is a hallmark of cancer. Within the latest decade, accumulated studies have suggested that polyploid cells may be involved in the development and progression of tumors, including the precancerous changes and malignant transformation of many human tumors.13 Intriguingly, liver presents to be a special polyploid organ during its development, and the relationship between polyploid hepatocytes and liver cancer remains ambiguous. Recently, Zhang et al10 proposed that polyploidy in liver behavior as protector against cancer via inhibiting heterozygosity loss. While, many of the others gave out the opinion that polyploidization and its subsequent reduction promotes tumors via facilitating aneuploidy and chromosomal instability.14–16 This review introduces the formation mechanism and physiological function of polyploid hepatocytes and focuses on the relationship between polyploid hepatocytes and liver cancer, which provides a potential pivotal research section in oncology and tumor control.
Molecular Mechanisms Lead to the Polyploidy
Except for germ cells, human chromosomes usually exist in diploid form and perform biological functions. Depending on different physiological or pathological contexts, diploid organisms can generate polyploid cells in specific tissues or organs through various pathways and mechanisms. Generally, the formation of polyploid cells by diploid organisms mainly follows the three classic forms: cell fusion, endoreplication, and cytokinesis failure.6,17,18 (Figure 1)
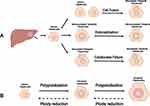 |
Figure 1 Mechanisms of polyploidization in the liver. (A) The formation of polyploid hepatocytes mainly follows the three classic mechanisms: cell fusion, endoreplication, and cytokinesis failure. (B) Ploidy in hepatocytes is well balanced by the “ploidy conveyor”. Diploid hepatocytes can generate tetraploid hepatocyte via polyploidization. Similarly, octoploid hepatocytes can give rise to tetraploid hepatocytes via ploidy reduction. Notes: Created with BioRender.com. |
Cell Fusion
Cell fusion in the liver is defined as the fusion of two mononuclear diploid hepatocytes (2n) to produce a dikaryotic tetraploid hepatocyte (2×2n). Interestingly, dikaryotic tetraploid hepatocytes retain the ability to enter the division cycle typically, giving rise to two mononuclear tetraploid hepatocytes (4n). On this basis, mononuclear tetraploid hepatocytes can form binuclear tetraploid (2×4n) or mononuclear octoploid hepatocytes (8n).19 Studies showed that hepatocyte fusion had been observed in chimeric mouse transplantation models.20 However, the existence of hepatocyte fusion in the liver is still controversial due to the possible formation of artifacts of extracellular vesicles that interfere with the observation of cell ploidy, which needs further investigation.21
Endoreplication
Endoreplication is a cytological phenomenon by which cells turn to polyploid through multiple DNA duplications with mitosis absence. Under physiological conditions, the mammalian cell cycle consists of four successive phases, including G1, S, G2, and M phases, strictly regulated by cyclin-dependent kinases (CDKs).22,23 In contrast, endoreplication only replicates in the G and S phases without undergoing cell mitosis (M phase), resulting in polyploid cells characterized with multiple sets of chromosomes. Several studies have shown that hepatocyte polyploidy can be affected during the corresponding cell cycle process by knocking out specific cell cycle regulatory genes, and different cytology hepatocyte mouse models can be established according to this. For example, it was possible to induce the formation of polyploid hepatocytes in mice at different stages of the cell cycle by establishing P53,24P21,25 and Rb25,26 knockout mouse models. Sustained DNA damage can trigger Endoreplication in the liver and promote the generation of polyploid hepatocytes.27,28 Studies have reported that liver cells cultured in vitro exhibit high phosphorylation of ATR (ataxia telangiectasia mutated and RAD3-related protein) and oxidative stress response when the cells were exposed to ultraviolet light for a certain period during the S phase and G2 phase. Simultaneously, it promotes the high expression of cell cycle suppressor p53 and pRb proteins through the ATR/p53/pRb pathway, consequentially inhibited the cell cycle and generating polyploid liver cells.27,29
Cytokinesis Failure
Cytokinesis is the last stage of cell division, including establishing the division plane, contraction of the actomyosin ring, ingression of the cleavage furrow, and formation of the intracellular bridge.17,30 In liver tissue, cytokinesis failure dominates the mechanism of polyploid hepatocyte formation. Some latest studies have elegantly described the factors affecting cytokinesis and the molecular mechanism of cytokinesis failure, playing a guiding role in the subsequent research. Margall-Ducos et al31,32 showed that cytokinesis is interrupted by impaired re-organization of the actin cytoskeleton at the division plane during the anaphase-telophase transition, resulting in loss of cell extension. Meanwhile, astral microtubules failed to contact the equatorial cortex and to deliver their molecular signal, which prevents activation of the RhoA pathway and leads to the generation of binucleate progenies. Desdouets et al reported that the insulin-PI3K-Akt signaling pathway primarily regulates incomplete cytoplasmic division, and their study definitely showed a decrease of circulating insulin levels, impairing the formation of binucleate tetraploid hepatocytes. On the contrary, increase of insulin level increased the binucleate tetraploid hepatocytes. Meanwhile, inhibition of PI3K/Akt phosphorylation prevents cytokinesis failure and promotes actin cytoskeletal polarization, cytoskeletal recombination, and RhoA recruitment.19,33–35 Hsu et al36 found that miRNA is closely related to the formation of dikaryotic polyploid hepatocytes. In mouse models with miR-122 knocked out, the total amount of polyploid hepatocytes was reduced from 60% to 70%. On this basis, further studies35,36 showed that miR-122 antagonized the expression of cytokinetic effect factors like Cux1,37 RhoA,38 Mapre1, Iqgap1, Nedd41, and Slc25a34,36 and led to cytokinesis failure with an expansion of binuclear hepatocytes amount. Additionally, miR-122 also promotes the tumorigenesis of hepatocellular carcinoma (HCC), suggesting that miR-122 as a potential target for the therapeutic treatment of hepatocellular carcinoma, and the associated changes in hepatocellular polyploidy may be closely associated with the occurrence and development of hepatocellular carcinoma.39 The molecular events leading to polyploidy remain elusive, and how different signals control liver ploidy remains to be determined.
Function of Polyploidy in the Liver
Under the normal physiological conditions, polyploid hepatocyte is not only a common physiological manifestation, but also presents biological significance certainly. Combining with the current hypotheses put forward, the specific biological significance of polyploid hepatocytes has been theoretically supported.
Polyploidy and Terminal Differentiation of Hepatocytes
As reported, polyploid hepatocytes were associated with hepatocyte terminal differentiation, cell proliferation, division and senescence in both rodents and humans.40 Oppositely, some of the recent studies have shown that polyploid hepatocyte retains the ability to divide and proliferate, which no longer belongs to the terminal form of cell differentiation. Importantly, Pandit et al41 proposed that E2f8-/- mouse liver was mainly composed of diploid hepatocytes, and its regenerative ability was not different from that of wild-type mouse liver with a large number of polyploid cells, which strongly supported the above view. Similarly, Miyaoka et al42 and Kreutz et al43 found that after partial hepatectomy, the polyploid hepatocytes behave the same degree of cell division as that of diploid hepatocytes. Duncan et al40 transplanted diploid and octoploid hepatocytes enriched from the adult mice into the livers of FAH-deficient mice through a xenograft manner, and found that diploid and octoploid hepatocytes showed the similar proliferation potential, which indicates polyploid hepatocytes had a fairly high proliferation ability on the whole.
Aneuploidy and Ploidy Conveyor in the Liver
Duncan et al7,8,12,40 demonstrated that hepatocytes from mice and human were highly aneuploid when examined using traditional karyotyping and fluorescence in situ hybridization. Furthermore, image studies revealed multipolar spindle and chromosome segregation defects in human hepatocyte division, suggesting that aneuploidy does not necessarily make hepatocytes susceptible to transformation, but may promote genetic diversity among hepatocytes. Hepatocyte polyploidization can increase DNA content or decrease it through a process of ploidy reversal. The random addition or loss of entire chromosomes resulting from the division of polyploid hepatocytes increases the probability of aneuploidy. Ploidy conveyor, defined as a dynamic model of hepatocyte polyploidization, ploidy reversal and aneuploidy, is an essential mechanism evolved to generate genetic diversity. However, Knouse et al44 used single-cell sequencing to find aneuploidy levels in mouse and human hepatocytes to be around 5%, so the extent of aneuploidy in healthy livers remains controversial. Mostly, the liver injuries in real life are chronic injuries, leading to multiple rounds of cell proliferation. Whether large numbers of aneuploid cells occur after chronic liver injury and whether this contributes to a higher risk of cancer are important questions that need to be addressed.
Biological Significance of Hepatocytes Polyploidized
Polyploid hepatocytes contain multiple sets of genomes. Recently, the corresponding genes in polyploid cells were reported to multiply during the transcription process, leading to specific metabolic functional enhancement.45,46 Miettinen et al45 establish a mononuclear polyploid hepatocytes mice model by using cyclin-dependent kinase-1 knockout method for studying the relationship between high nuclear ploidy number and liver cell metabolism. The results showed that hepatocytes with higher ploidy could induce decreased expression levels of mitochondrial and lipid de novo synthesis pathway-related genes and increased expression levels of cytoskeleton and glycolysis genes, suggesting that polyploid hepatocytes play a promoting role in liver metabolism process. Moreover, Kreutz et al43 demonstrate that mouse hepatocytes with diploid nuclei have distinct metabolic characteristics compared to mouse hepatocytes with polyploid nuclei. In addition to strong differences in gene expression, comprising metabolic as well as signaling compounds, Kreutz et al found a strongly decreased insulin binding of nuclear polyploid cells. These observations were related to nuclear ploidy but not with total ploidy within a cell. Another elegant study by Diril et al22 generated a conditional-knockout mouse model to study the functions of cyclin-dependent kinase-1 in vivo. The investigators found that liver regeneration was unaffected after partial hepatectomy by liver-specific ablation of Cdk1, suggesting that liver regeneration can be driven by driven by cell growth without cell division. Interestingly, unlike other Cdks, the investigators also found that deletion of Cdk1 in the liver resists tumorigenesis induced by activated Ras as well as p53 silencing.
As the liver inquires high energy requirements, the enhanced function of cells via polyploidy helps to effectively adapt the biological stress caused by micro-environmental changes. Based on the single molecule fluorescence in situ hybridization, Halpern et al47 found that in the process of gene transcription, the promoter random switching between open and closed states, leading to gene expression variation in different cells. That is, transcription noise is formed. Worth mentioning, the transcriptional noise from polyploid hepatocytes is tiny, which indicates that liver polyploidy is involved in the strict regulation and effective buffering of transcriptional noise generation, making gene expression tend to be stable, and on this basis, maintaining the stable physiological function of liver cells.17,46
Polyploid Hepatocytes Promote Liver Tissue Regeneration and Resist Genotoxic Injury
The liver is the most important regenerative organ in the human body, and it is also one of the few organs in which polyploid cells make up more than half of the total cell population. Studies have shown that polyploid hepatocytes proliferating significantly and participating in liver tissue regeneration after partial liver resection in mice. Zhang et al10,21 established a diploid liver mouse model with E2F7 and E2F8 double knockout for partial hepatectomy to compare the regeneration ability of liver tissue post-operational treatment in with wild-type mice’s liver. The finding illustrated that the polyploid hepatocytes presented much more significant ability of liver tissue reparation ability than that of the diploid ones. Wild-type mice and E2f7\E2f8 double gene knockout mice were treated by intraperitoneal injection of carbon tetrachloride toxin for establishing the mouse liver injury model. Similar results were obtained by calculating and comparing the volume and weight of the liver between the two treated mice, which indicate polyploid hepatocytes induced the ability of resisting genotoxic injury. These findings above suggest that the polyploidy of hepatocytes has a significant effect on liver regeneration.48 Another elegant study designed by Matsumoto et al3 developed a multicolor reporter allele system to genetically label and trace polyploid cells in situ. With this system, the researchers clearly indicated that polyploid hepatocytes continuously proliferate and serve as an important source of regeneration in chronic liver injuries, which brought more direct evidence.
Polyploid Hepatocytes and Hepatocellular Carcinoma
Around 30% of solid human tumors, confirmed by genomics, such as colorectal cancer,49 pancreatic cancer,50 and lung cancer,51 have a large number of polyploid cells.52 Clinicopathologically, polyploid tumor cells are associated with strong aggressiveness, a high degree of malignancy, and poor prognosis. Some of the researchers prompted that polyploidy involves with the dynamic process of transformation from precancerous lesions to malignant tumors, and polyploidization is probably one of the essential driving factors of tumorigenesis.47 Ganem et al53 found that transplantation of TP53-/- tetraploid breast epithelial cells into immunodeficient nude mice caused malignant tumors, while transplantation of TP53-/- diploid breast epithelial cells did not, suggesting tetraploid breast epithelial cells contribute in tumor formation. Due to the abundance of polyploid cells in normal adult liver physiology, the role of polyploid cells in liver tumorigenesis is ambiguous. Discussion on whether polyploid cells promote or inhibit hepatocellular carcinoma transformation in liver lays out controversial opinions.
Polyploid Hepatocytes Restrict the Development of Hepatocellular Carcinoma
According to the points mentioned above, polyploidy tends to be a key factor in tumor development and progression. However, physiologically, 50% of the cells in normal adult liver are polyploid. Obviously, no adequate evidence directly supports a relationship between polyploid hepatocytes and the occurrence or development of liver cancer. On contrast with most of the human malignancies, polyploid hepatocytes seem to show an antagonistic role in liver cancer initiation and progress. Cells in diploid tissues lead to malignancy when classical heterozygosity loss occurs. One or both alleles in two pairs of tumor suppressor genes have different genomic changes, resulting in the loss of their ability to inhibit cell transformation into cancer cells (Figure 2).
 |
Figure 2 Ploidy and loss of heterozygosity. Compared with diploid hepatocytes, polyploid hepatocytes prevent tumor initiation through the possession of multiple copies of each chromosome. After first mutations (lightning) occur, the remaining wild-type alleles in polyploid hepatocytes provide additional tumor suppressor gene copies. In diploid cells, by contrast, a second mutation give rise to loss of heterozygosity. In addition, unexpected reduction of hepatocyte ploidy (red arrow) may have a undesirable consequence, leading to carcinogenesis. Notes: Created with BioRender.com. |
On the contrary, polyploid hepatocytes provide a strong buffer against loss or mutation of tumor suppressor genes, probably due to adequate tumor suppressor genes reserve up to 16 allelic. At present, the findings of Zhang et al4,5,10,48 are the most widely accepted hypotheses that polyploid hepatocytes inhibit hepatocellular carcinoma genesis. Simultaneously, by controlling the weaning time and the Anln gene and E2f8 gene levels, the Anln gene knockout mouse model (mostly polyploid liver cells) and E2f8 gene knockout mouse model (mostly diploid liver cells) were established, followed the treatment with diethyl nitrosamine to induce a hepatocellular carcinoma model. The results showed that the predominance of polyploid cells in the composition of hepatocytes showed tumor inhibition in various hepatocellular carcinoma models. Further studies showed that the tumor suppressor effect of polyploid hepatocytes was related to the mutation or deletion of the buffer tumor suppressor gene rather than the inhibition of abnormal cell proliferation of hepatocytes after the action of carcinogenic factors.
As theoretical support for the above hypothesis, furtherly, Chen et al54 demonstrated that ablation of E2F7 and E2F8, which coordinate the expression of a unique G2/M transcriptional program that is critical for mitosis, karyokinesis and cytokinesis, resulting in a higher proportion of diploid hepatocytes in the liver. Knouse et al44 performed partial hepatectomy on E2F8 knockout mice and wild-type mice, respectively. Diploid E2f7/E2f8 knockout mice demonstrated a higher recovery rate of liver quality compared with wild-type mice. Wilkinson et al5 directly extracted hepatocytes from mouse liver for primary cell culture and found that the proliferation rate of E2f7/E2f8 knockout hepatocytes dominated by diploids was significantly higher than that of wild-type polyploid hepatocytes. These results suggest that diploid hepatocytes have more proliferative ability than polyploid hepatocytes, and polyploid hepatocytes may have multiple replication opportunities due to their tumor suppressor genes and have a more apparent inhibitory effect on liver cancer.
Recently, in another significant study, Sladky et al2,55 found that PIDDosome controlled the hepatocyte polyploidization, and PIDDosome promoted the increase of the proportion of diploid hepatocytes, aggravating the number and burden of tumors. PIDDosome, composed of PIDD1, RAIDD, and Casp2, is a protein complex activated by excess centrosomes, induces p53, and restricts the proliferation of polyploid cells.56 Meaningfully, Sladky et al found that the protein loss of the PIDDosome protein complex destroyed the typical ploidy ratio of the liver, which promotes the increase of 8n hepatocytes and 16n hepatocyte population. Thus, the activity of PIDDosome limits hyperpolyploidy. Additionally, Sladky used the DEN-induced mouse model to explore the influence of PIDDosome gene knockout and liver tumor formation, and found that the PIDDosome gene knockout mice did not develop liver tumors due to the high proportion of hyperpolyploid hepatocytes, implying the primary formation and progression of liver tumors driven by diploid hepatocytes. Meanwhile, it also provided the evidence of the inhibitory effect of polyploid hepatocytes on the occurrence and progression of liver tumors. Subsequently, the researchers validated that transcription factor E2F1 has binding sites in the upstream promoters of CASP2 and PIDD1, and suggested that the increased expression levels of CASP2 and PIDD1 promote proliferation. This may provide a new idea for medical treatment to prevent the formation of PIDDosome protein complex, such as CASP2 deletion, simulates liver polyploidy and restricts the occurrence and development potential of liver cancer.
Polyploid Hepatocytes in Human Hepatocellular Carcinoma and Pathological Liver
Different nuclear states of polyploid hepatocytes are associated with unequal prognosis and outcome of HCC patients.2019, Bou-Nader et al57 took advantage of the tumor cells in situ imaging methods, such as the analysis of the liver tissue cell ploidy spectrum post-surgical resection between HCC patients and the healthy controls. The result shows that in the process of human hepatocellular carcinoma, dual-core polyploid ratio and cell ploidy were remarkably reduced (about 15% in normal tissue and 5% in tumor tissue). In contrast, the percentage of mononuclear polyploidy and cytology was increased significantly in HCC (about 12% in normal tissue and 33% in tumor tissue). Mononuclear polyploid hepatocytes may be associated with low differentiation, high proliferation rate, and poor prognosis of hepatocellular carcinoma. During the development of mammals after birth, the vast majority of hepatocytes give rise to dikaryotic polyploid cells in the form of cytokinesis failure. Bou-Nader et al found that dikaryotic polyploid hepatocytes almost did not exist in human hepatocellular carcinoma, which strongly suggested no cytoplasmic defect malignant hepatocellular division, which provided a direction for future studies.
Polyploid hepatocytes accumulate during aging process as well as in chronically injured livers. Both aging and chronic liver injuries are important risk factors of hepatocellular carcinoma, Gentric et al58 focus on nonalcoholic fatty liver disease (NAFLD), a widespread hepatic metabolic disorder that is believed to be a risk factor for hepatocellular carcinoma. The researchers cleverly constructed a mouse model of NAFLD and found an increased proportion of mononuclear polyploid hepatocytes, which was confirmed in patients with nonalcoholic steatohepatitis (NASH). In addition, the researchers demonstrated the relationship between oxidative stress and polyploid cells by treating NAFLD hepatocytes with antioxidants and found that these hepatocytes underwent normal cell division, which in turn verified that oxidative stress promotes the formation of “pathological polyploids”, which may promote the development of hepatocellular carcinoma.
Depolyploidy Increases the Risk of Precancerous Lesions
Recently, several groups have proposed a provocative idea that the ploidy reduction of liver cells after polyploidization increases the incidence of liver cancer. Compared with previous experiments, Lin et al14 focused on the process of reduced ploidy following the polyploidy of hepatocytes. Hepatocyte depolyploidization is one of the mechanisms of rapid regeneration of hepatocytes.42
Lin et al14 focused on the study of polyploidy hepatocytes at the initial stage of hepatocellular carcinoma, using exogenous substances such as diethyl nitrosamine (DEN) to induce polyploidy of centrilobular (CL) hepatocytes, revealing the relationship between pathological polyploidy of CL hepatocytes in mice. The formation of hepatocellular carcinoma induced by DEN and proving that the upregulation of AURKB is closely related to the polyploidy hepatocytes. In addition, Lin et al observed multipolar mitosis in CL-derived tumor cells, leading to the generation of aneuploidy, which may contribute to the loss of heterozygosity of tumor suppressor genes. Thus, the occurrence of hepatocellular carcinoma may have occurred. These phenomena differ from previous studies by researchers in that “physiological polyploidy” reduces LOH and the loss of tumor suppressor genes through the presence of additional genomes. In “pathological polyploidy”, however, the opposite may be true, as hyperpolyploid hepatocytes undergo multipolar mitosis to transform into diploid hepatocytes with low DNA content, which are the primary source of precancerous lesions.
Another excellent team discovered that although polyploid hepatocytes can “buffer” the mutation of tumor suppressor genes by reducing the loss of heterozygosity by using in vivo lineage tracing. However, frequent hepatocyte ploidy decline is prone to chromosomal aberrations, which promote the formation of liver tumors. Matsumoto et al3,15 observed bicolored tumors in livers with the coexistence of multiple cell ploidy by using the novel multicolor lineage tracing system directly, which directly suggested that reduced polyploidy of hepatocytes was more likely to produce liver tumors. These researchers believe that enhanced chromosomal instability (CIN) is one of the mechanisms by which ploidy reduction of polyploid hepatocytes increases the susceptibility to cancer. Moreover, there are other studies also supporting CIN.59,60
Generally, previous studies have used drug-induced models to promote the occurrence of hepatic carcinoma. However, the process of hepatocellular depolyploidization and the failure of polyploid hepatocytes to protect against loss of heterozygosity are not the only factors contributing to liver carcinogenesis caused by drug injury. Notably, it is necessary to determine whether ploidy reduction affects the process of oncogene-induced carcinogenesis.
Conclusion
Cell polyploidization is a unique cellular functional mechanism, and cytokinesis failure is the main reason for the polyploid hepatocytes. Polyploid cells are essential in the physiological development of the human body. As the largest polyploid organ in the human body, polyploid hepatocytes run through the whole process of liver development, growth, regeneration, and aging. In recent years, the relationship between polyploid hepatocytes and hepatocellular carcinoma has attracted the attention of many experts and scholars. The most significant point is to clarify the relationship between polyploid hepatocytes and hepatocellular carcinoma, which will provide important theoretical support for the prevention, diagnosis, and treatment of hepatocellular carcinoma in clinical practice. Moreover, in the field of precision medicine, fully revealing the specific mechanism of polyploid hepatocytes inhibiting the occurrence and development of liver cancer will promote the development of precision treatment of liver cancer. Remarkably, another recent research challenges previous theories by expounding the dynamic process of ploidy reduction after polyploidization of hepatocytes accelerates the formation of hepatocellular carcinoma, which needs more experimental and theoretical support. Great progress has been made in the relationship between polyploid hepatocytes and liver tumors recently, but there are still many questions left: 1) Do mononuclear polyploid hepatocytes and multinuclear polyploid hepatocytes have advantages and limitations in tissue function? 2) How to use “physiological polyploidy” for the prevention and treatment of human liver tumors? 3) How do polyploid hepatocytes solve problems such as multipolar spindles formed during mitosis?17,44
Disclosure
The authors report no conflicts of interest in this work.
References
1. Epstein CJ. Cell size, nuclear content, and the development of polyploidy in the mammalian liver. Proc Natl Acad Sci U S A. 1967;57(2):327–334. doi:10.1073/pnas.57.2.327
2. Sladky VC, Knapp K, Soratroi C, et al. E2F-family members engage the PIDDosome to limit hepatocyte ploidy in liver development and regeneration. Dev Cell. 2020;52(3):335–349.e7. doi:10.1016/j.devcel.2019.12.016
3. Matsumoto T, Wakefield L, Tarlow BD, Grompe M. In vivo lineage tracing of polyploid hepatocytes reveals extensive proliferation during liver regeneration. Cell Stem Cell. 2020;26(1):34–47.e3. doi:10.1016/j.stem.2019.11.014
4. Zhang S, Nguyen LH, Zhou K, et al. Knockdown of anillin actin binding protein blocks cytokinesis in hepatocytes and reduces liver tumor development in mice without affecting regeneration. Gastroenterology. 2018;154(5):1421–1434. doi:10.1053/j.gastro.2017.12.013
5. Wilkinson PD, Delgado ER, Alencastro F, et al. The polyploid state restricts hepatocyte proliferation and liver regeneration in mice. Hepatology. 2019;69(3):1242–1258. doi:10.1002/hep.30286
6. Øvrebø JI, Edgar BA. Polyploidy in tissue homeostasis and regeneration. Development. 2018;145(14). doi:10.1242/dev.156034
7. Duncan AW. Aneuploidy, polyploidy and ploidy reversal in the liver. Semin Cell Dev Biol. 2013;24(4):347–356. doi:10.1016/j.semcdb.2013.01.003
8. Duncan AW, Hanlon Newell AE, Smith L, et al. Frequent aneuploidy among normal human hepatocytes. Gastroenterology. 2012;142(1):25–28. doi:10.1053/j.gastro.2011.10.029
9. Si-Tayeb K, Lemaigre FP, Duncan SA. Organogenesis and development of the liver. Dev Cell. 2010;18(2):175–189. doi:10.1016/j.devcel.2010.01.011
10. Zhang S, Zhou K, Luo X, et al. The polyploid state plays a tumor-suppressive role in the liver. Dev Cell. 2018;44(4):447–459.e5. doi:10.1016/j.devcel.2018.01.010
11. Guidotti JE, Bregerie O, Robert A, Debey P, Brechot C, Desdouets C. Liver cell polyploidization: a pivotal role for binuclear hepatocytes. J Biol Chem. 2003;278(21):19095–19101. doi:10.1074/jbc.M300982200
12. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
13. Davoli T, de Lange T. The causes and consequences of polyploidy in normal development and cancer. Annu Rev Cell Dev Biol. 2011;27:585–610. doi:10.1146/annurev-cellbio-092910-154234
14. Lin H, Huang YS, Fustin JM, et al. Hyperpolyploidization of hepatocyte initiates preneoplastic lesion formation in the liver. Nat Commun. 2021;12(1):645. doi:10.1038/s41467-020-20572-8
15. Matsumoto T, Wakefield L, Peters A, Peto M, Spellman P, Grompe M. Proliferative polyploid cells give rise to tumors via ploidy reduction. Nat Commun. 2021;12(1):646. doi:10.1038/s41467-021-20916-y
16. Muller M, May S, Bird TG. Ploidy dynamics increase the risk of liver cancer initiation. Nat Commun. 2021;12(1):1896. doi:10.1038/s41467-021-21897-8
17. Donne R, Saroul-Ainama M, Cordier P, Celton-Morizur S, Desdouets C. Polyploidy in liver development, homeostasis and disease. Nat Rev Gastroenterol Hepatol. 2020;17(7):391–405. doi:10.1038/s41575-020-0284-x
18. Gentric G, Desdouets C. Polyploidization in liver tissue. Am J Pathol. 2014;184(2):322–331. doi:10.1016/j.ajpath.2013.06.035
19. Celton-Morizur S, Desdouets C. Polyploidization of liver cells. Adv Exp Med Biol. 2010;676:123–135. doi:10.1007/978-1-4419-6199-0_8
20. Faggioli F, Sacco MG, Susani L, Montagna C, Vezzoni P. Cell fusion is a physiological process in mouse liver. Hepatology. 2008;48(5):1655–1664. doi:10.1002/hep.22488
21. Zhang S, Lin YH, Tarlow B, Zhu H. The origins and functions of hepatic polyploidy. Cell Cycle. 2019;18(12):1302–1315. doi:10.1080/15384101.2019.1618123
22. Diril MK, Ratnacaram CK, Padmakumar VC, et al. Cyclin-dependent kinase 1 (Cdk1) is essential for cell division and suppression of DNA re-replication but not for liver regeneration. Proc Natl Acad Sci U S A. 2012;109(10):3826–3831. doi:10.1073/pnas.1115201109
23. Hartwell LH, Weinert TA. Checkpoints: controls that ensure the order of cell cycle events. Science. 1989;246(4930):629–634. doi:10.1126/science.2683079
24. Kurinna S, Stratton SA, Coban Z, et al. p53 regulates a mitotic transcription program and determines ploidy in normal mouse liver. Hepatology. 2013;57(5):2004–2013. doi:10.1002/hep.26233
25. Sheahan S, Bellamy CO, Treanor L, Harrison DJ, Prost S. Additive effect of p53, p21 and Rb deletion in triple knockout primary hepatocytes. Oncogene. 2004;23(8):1489–1497. doi:10.1038/sj.onc.1207280
26. Mayhew CN, Bosco EE, Fox SR, et al. Liver-specific pRB loss results in ectopic cell cycle entry and aberrant ploidy. Cancer Res. 2005;65(11):4568–4577. doi:10.1158/0008-5472.Can-04-4221
27. Edgar BA, Zielke N, Gutierrez C. Endocycles: a recurrent evolutionary innovation for post-mitotic cell growth. Nat Rev Mol Cell Biol. 2014;15(3):197–210. doi:10.1038/nrm3756
28. Radziejwoski A, Vlieghe K, Lammens T, et al. Atypical E2F activity coordinates PHR1 photolyase gene transcription with endoreduplication onset. EMBO j. 2011;30(2):355–363. doi:10.1038/emboj.2010.313
29. Davoli T, de Lange T. Telomere-driven tetraploidization occurs in human cells undergoing crisis and promotes transformation of mouse cells. Cancer Cell. 2012;21(6):765–776. doi:10.1016/j.ccr.2012.03.044
30. D’Avino PP, Giansanti MG, Petronczki M. Cytokinesis in animal cells. Cold Spring Harb Perspect Biol. 2015;7(4):a015834. doi:10.1101/cshperspect.a015834
31. Donne R, Sangouard F, Celton-Morizur S, Desdouets C. Hepatocyte polyploidy: driver or gatekeeper of chronic liver diseases. Cancers (Basel). 2021;13(20):5151. doi:10.3390/cancers13205151
32. Margall-Ducos G, Celton-Morizur S, Couton D, Brégerie O, Desdouets C. Liver tetraploidization is controlled by a new process of incomplete cytokinesis. J Cell Sci. 2007;120(Pt 20):3633–3639. doi:10.1242/jcs.016907
33. Celton-Morizur S, Merlen G, Couton D, Desdouets C. Polyploidy and liver proliferation: central role of insulin signaling. Cell Cycle. 2010;9(3):460–466. doi:10.4161/cc.9.3.10542
34. Celton-Morizur S, Merlen G, Couton D, Margall-Ducos G, Desdouets C. The insulin/Akt pathway controls a specific cell division program that leads to generation of binucleated tetraploid liver cells in rodents. J Clin Invest. 2009;119(7):1880–1887. doi:10.1172/jci38677
35. Wang MJ, Chen F, Lau JTY, Hu YP. Hepatocyte polyploidization and its association with pathophysiological processes. Cell Death Dis. 2017;8(5):e2805. doi:10.1038/cddis.2017.167
36. Hsu SH, Delgado ER, Otero PA, et al. MicroRNA-122 regulates polyploidization in the murine liver. Hepatology. 2016;64(2):599–615. doi:10.1002/hep.28573
37. Xu H, He JH, Xu SJ, et al. A group of tissue-specific microRNAs contribute to the silencing of CUX1 in different cell lineages during development. J Cell Biochem. 2018;119(7):6238–6248. doi:10.1002/jcb.26852
38. Ghafouri-Fard S, Noroozi R, Abak A, Taheri M, Salimi A. Emerging role of lncRNAs in the regulation of Rho GTPase pathway. Biomed Pharmacother. 2021;140:111731. doi:10.1016/j.biopha.2021.111731
39. Luna JM, Barajas JM, Teng KY, et al. Argonaute CLIP defines a deregulated miR-122-bound transcriptome that correlates with patient survival in human liver cancer. Mol Cell. 2017;67(3):400–410.e7. doi:10.1016/j.molcel.2017.06.025
40. Duncan AW, Taylor MH, Hickey RD, et al. The ploidy conveyor of mature hepatocytes as a source of genetic variation. Nature. 2010;467(7316):707–710. doi:10.1038/nature09414
41. Pandit SK, Westendorp B, Nantasanti S, et al. E2F8 is essential for polyploidization in mammalian cells. Nat Cell Biol. 2012;14(11):1181–1191. doi:10.1038/ncb2585
42. Miyaoka Y, Ebato K, Kato H, Arakawa S, Shimizu S, Miyajima A. Hypertrophy and unconventional cell division of hepatocytes underlie liver regeneration. Curr Biol. 2012;22(13):1166–1175. doi:10.1016/j.cub.2012.05.016
43. Kreutz C, MacNelly S, Follo M, et al. Hepatocyte ploidy is a diversity factor for liver homeostasis. Front Physiol. 2017;8:862. doi:10.3389/fphys.2017.00862
44. Knouse KA, Lopez KE, Bachofner M, Amon A. Chromosome segregation fidelity in epithelia requires tissue architecture. Cell. 2018;175(1):200–211.e13. doi:10.1016/j.cell.2018.07.042
45. Miettinen TP, Pessa HK, Caldez MJ, et al. Identification of transcriptional and metabolic programs related to mammalian cell size. Curr Biol. 2014;24(6):598–608. doi:10.1016/j.cub.2014.01.071
46. Schoenfelder KP, Fox DT. The expanding implications of polyploidy. J Cell Biol. 2015;209(4):485–491. doi:10.1083/jcb.201502016
47. Halpern KB, Tanami S, Landen S, et al. Bursty gene expression in the intact mammalian liver. Mol Cell. 2015;58(1):147–156. doi:10.1016/j.molcel.2015.01.027
48. Lin YH, Zhang S, Zhu M, et al. Mice with increased numbers of polyploid hepatocytes maintain regenerative capacity but develop fewer hepatocellular carcinomas following chronic liver injury. Gastroenterology. 2020;158(6):1698–1712 e14. doi:10.1053/j.gastro.2020.01.026
49. Hamada S, Itoh R, Fujita S. DNA distribution pattern of the so-called severe dysplasias and small carcinomas of the colon and rectum and its possible significance in the tumor progression. Cancer. 1988;61(8):1555–1562. doi:10.1002/1097-0142(19880415)61:8<1555::aid-cncr2820610812>3.0.co;2-w
50. Sato N, Mizumoto K, Nakamura M, et al. Centrosome abnormalities in pancreatic ductal carcinoma. Clin Cancer Res. 1999;5(5):963–970.
51. Lothschütz D, Jennewein M, Pahl S, et al. Polyploidization and centrosome hyperamplification in inflammatory bronchi. Inflamm Res. 2002;51(8):416–422. doi:10.1007/pl00000323
52. Bielski CM, Zehir A, Penson AV, et al. Genome doubling shapes the evolution and prognosis of advanced cancers. Nat Genet. 2018;50(8):1189–1195. doi:10.1038/s41588-018-0165-1
53. Ganem NJ, Godinho SA, Pellman D. A mechanism linking extra centrosomes to chromosomal instability. Nature. 2009;460(7252):278–282. doi:10.1038/nature08136
54. Chen HZ, Ouseph MM, Li J, et al. Canonical and atypical E2Fs regulate the mammalian endocycle. Nat Cell Biol. 2012;14(11):1192–1202. doi:10.1038/ncb2595
55. Sladky VC, Knapp K, Szabo TG, et al. PIDDosome-induced p53-dependent ploidy restriction facilitates hepatocarcinogenesis. EMBO Rep. 2020;21(12):e50893. doi:10.15252/embr.202050893
56. Fava LL, Schuler F, Sladky V, et al. The PIDDosome activates p53 in response to supernumerary centrosomes. Genes Dev. 2017;31(1):34–45. doi:10.1101/gad.289728.116
57. Bou-Nader M, Caruso S, Donne R, et al. Polyploidy spectrum: a new marker in HCC classification. Gut. 2020;69(2):355–364. doi:10.1136/gutjnl-2018-318021
58. Gentric G, Maillet V, Paradis V, et al. Oxidative stress promotes pathologic polyploidization in nonalcoholic fatty liver disease. J Clin Invest. 2015;125(3):981–992. doi:10.1172/JCI73957
59. Sansregret L, Vanhaesebroeck B, Swanton C. Determinants and clinical implications of chromosomal instability in cancer. Nat Rev Clin Oncol. 2018;15(3):139–150. doi:10.1038/nrclinonc.2017.198
60. Targa A, Rancati G. Cancer: a CINful evolution. Curr Opin Cell Biol. 2018;52:136–144. doi:10.1016/j.ceb.2018.03.007
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
