Back to Journals » Cancer Management and Research » Volume 15
The Circular RNA circFGFR4 Facilitates Resistance to Anti-PD-1 of Triple-Negative Breast Cancer by Targeting the miR-185-5p/CXCR4 Axis
Authors Wang F, Lu Q, Yu H , Zhang XM
Received 10 March 2023
Accepted for publication 10 August 2023
Published 15 August 2023 Volume 2023:15 Pages 825—835
DOI https://doi.org/10.2147/CMAR.S411901
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Harikrishna Nakshatri
Fei Wang,1 Qiong Lu,1,2 Hong Yu,3 Xue-Mei Zhang1
1Department of Oncology, Shanghai East Hospital, Tongji University School of Medicine, Shanghai, People’s Republic of China; 2Department of Oncology, Shanghai Concord Medical Cancer Center, Shanghai, People’s Republic of China; 3Department of Pathology, Taizhou People’s Hospital, Taizhou, Jiangsu, People’s Republic of China
Correspondence: Xue-Mei Zhang; Hong Yu, Email [email protected]; [email protected]
Purpose: One of the most catastrophic malignant tumors is triple negative breast cancer (TNBC). It is characterized by rapid progression in the clinic. CircRNAs are abnormally expressed in almost all cancers and play important roles in tumor immune evasion. Nevertheless, the biological roles of the circular fibroblast growth factor receptor 4 RNA (circFGFR4) in TNBC remain unclear.
Methods: The expression of circFGFR4 in TNBC tissues and paired nontumor tissues was detected using quantitative real-time polymerase chain reaction (qRT-PCR). The role of circFGFR4 in TNBC immune evasion was estimated by analyzing clinical tissues. In vivo circRNA precipitation, RNA immunoprecipitation, and luciferase reporter assays were performed to explore interaction between circFGFR4 and miR-185-5p.
Results: Our results indicated that circFGFR4 was significantly overexpressed in TNBC tissues. Upregulated circFGFR4 expression was correlated with decreased CD8+ T cell infiltration in tumor tissues and resistance to anti-programmed cell death 1 (PD-1) immunotherapy in TNBC patients and mice bearing TNBC tumors. Forced circFGFR4 expression inhibited CD8+ T cell infiltration in tissue sections from TNCB tumor bearing mice. Mechanistically, circFGFR4 competitively sponged miR-185-5p and prevented miR-185-5p from decreasing the levels of C-X-C motif chemokine receptor 4 (CXCR4).
Conclusion: Ultimately, our results indicated that circFGFR4 plays an important role in immune evasion and anti-PD-1 immunotherapy resistance via regulates miR-185-5p/CXCR4 axis in TNBC, thus suggesting that circFGFR4 has significant potential as a biomarker for predicting sensitivity to anti-PD-1 immunotherapy and as an immunotherapeutic target for TNBC.
Keywords: circFGFR4, triple negative breast cancer, TNBC, immune evasion, immunotherapy
Introduction
Breast cancer (BC) mainly occurs in women, and most of BC lesions are malignant tumors. According to Global Cancer Statistics 2020, female BC accounts for an estimated 2.3 million new cancer cases (11.7%) and more than 0.69 million cancer-related deaths (6.9%).1 Triple-negative breast cancer (TNBC) is a BC subtype that is characterized by the absence of the expression of three receptors (ER-/PR-/HER2-).2 TNBC is more invasive and has higher metastatic potential than luminal and HER2+ subtypes.2 Endocrine therapies and targeted therapies are limited for TNBC, and standardized regimens for TNBC treatment are still lacking. To date, chemotherapy is the main systemic treatment, but the efficacy of conventional chemoradiotherapy is poor. Recently, several clinical trial results have indicated the efficacy of anti-programmed cell death 1 (PD-1) immunotherapy plus chemotherapy in metastatic and neoadjuvant settings for TNBC.3–5 However, PD-1/programmed cell death ligand 1 (PD-L1) monoclonal antibody-based immunotherapy seems to be effective in only a small number of TNBC patients. Thus, studies exploring the mechanisms underlying the poor response of TNBC to PD-1/PD-L1 antibody-based immunotherapy are urgently needed.
Circular RNAs are novel noncoding RNAs. They are distinctive and special due to the presence of a covalently closed loop structure and lack of a 5’ to 3’ polyadenylated tail.6,7 Increasing evidence shows that abnormally expressed circRNAs participate in many aspects of tumor progression, including proliferation, chemotherapy resistance, invasion, migration, and immune evasion.8–10 For instance, circTRIM33-12 sponges miRNA-191 and inhibits hepatocellular carcinoma (HCC) cell proliferation.8 The authors’ previous research report showed that circFGFR1 directly sponges miR-381-3p to increase the expression of C-X-C motif chemokine receptor 4 (CXCR4), subsequently promoting non-small lung cancer (NSCLC) progression and resistance to anti-PD-1-based therapy.11 In TNBC, circGFRA1 via sponging miR-34a to regulate GFRA1 expression promote tumor progression.12 In addition, circEZH2 was overexpressed in liver metastases tumor and predicted the worse prognosis in patients with breast cancer.13 However, knowledge of the function of FGFR family gene-derived circRNAs in TNBC is insufficient.Our results indicated that circFGFR4 (hsa_circ_0075147) expression was significantly increased in TNBC tissues compared with the corresponding controls. Experiments assessing its biological function and molecular mechanism indicated that hsa_circ_0075147 functioned as a sponge for miR-185-5p to prevent its inhibition of the CXCR4 mRNA, which promoted immune evasion and resistance to anti-PD-1 immunotherapy in TNBC. Collectively, our findings confirmed that circFGFR4 is a tumor promoter in TNBC and indicate its potential as both a prognostic biomarker and an immunotherapeutic target.
Materials and Methods
Cell Lines
The human TNBC cell line MDA-MB-231 and HEK-293T cells were provided by the American Type Culture Collection. Cells were cultivated in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum and 1% penicillin G and some remaining streptomycin in humidified air containing 5% CO2 at 37°C.
Patients and Follow-Up
TNBC tissues and adjacent nontumor tissues were collected in East Hospital of Tongji University and the Taizhou People’s Hospital. Both the East Hospital of Tongji University and the Taizhou People’s Hospital examined the pathology. Each patient in this study was confirmed to have received curative resection. The research period of this paper was from January 1, 2015, to December 31, 2018, and a large amount of clinical and pathological information was collected and summarized. Ethical approval was provided by the Biomedical Research Department, Shanghai East Hospital, Tongji University School of Medicine. Written informed consent was obtained from all patients. All the methods related to patients in this study were performed in accordance with the principles stated in the Declaration of Helsinki.
Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR), Western Blotting, and Immunohistochemistry (IHC) Assays
qRT‒PCR, Western blotting, and IHC assays were performed as described in previous studies.11 The qRT-PCR primers used in this study are listed in Supplementary Table 1. The antibodies used in this study are listed in Supplementary Table 2.
In vivo circRNA Precipitation (circRIP), RNA Immunoprecipitation (RIP), and Luciferase Reporter Assays
In vivo circRIP, RIP, and luciferase reporter assays were conducted as described in previous studies.8,14,15 Briefly, GeneChem Company synthesized biotin-labeled circFGFR4 and negative control probes. MDA-MB-231 cells were fixed with 1% formaldehyde and lysed in co-IP buffer. The supernatant of lysed MDA-MB-231 cells was cultured with a M280 streptavidin Dynabeads (Invitrogen) mixture and incubated at 30°C for 12 hours. To reverse the formaldehyde crosslinking, the probes-dynabeads-circRNAs mixture was washed and incubated with 200 μL lysis buffer and proteinase K. Certain operations were performed in the presence of TRIzol reagent to extract RNA from the mixture.
RIP was performed using a Magna RIP RNA-binding protein immunoprecipitation kit. Briefly, total RNA was extracted from the cell lysate, incubated with Dynabeads coated with an Argonaute 2 (AGO2) antibody or IgG antibody at 4°C for 12 h, and the enriched circFGFR1 and miRNAs were measured using qRT-PCR.
Using StarBase v3.0, the relevant luciferase reporter was detected, and the possible location of binding sites was predicted. HEK-293T cells were transfected with pGL3-LUC-circFGFR4, pGL3-LUC- CXCR4 3′ UTR or mutant pGL3-LUC-circFGFR4, pGL3-LUC- CXCR4 3′ UTR and miR-185-3p mimics or negative control mimics. After two days, ideal cells were obtained during the experiment. Using a dual-luciferase reporter determination system, luciferase activity was measured.
Transfection Experiment to Overexpress circFGFR4 and miR-185-5p
CircFGFR4-overexpressing and negative control lentiviruses were synthesized by Genomeditech Company (Shanghai, China). The miR-185-5p mimics and negative control were synthesized by GeneChem Company (Shanghai, China). MDA-MB-231 and HEK-293T cell lines were transfected with the circFGFR4-overexpressing lentivirus and miR-185-5p mimics according to the manufacturer’s instructions.
Animals
The experiments in the NSG mice were approved by the Animal Experimentation Ethics Committee of East Hospital, Tongji University and performed in accordance with the NIH guidelines for the care and use of laboratory animals (8th edition, NIH). Hu-PBL-NSG mouse model was established as described as in reference.11,16 In brief, 1.0×107 PBMC derived from healthy donor for intravenous injection in the tail of NSG mouse. A total of 2 × 106 cells MDA-MB-231-circFGFR4 cells or MDA-MB-231-Mock cells were transplanted subcutaneously in the left flank of the Hu-PBL-NSG mice. When tumors reached a size of approximately 100 mm3, the mice were assigned to four groups and treated with a PD-1 monoclonal IgG antibody or isotype control IgG antibody. Animals were euthanized when tumors reached a maximum of 1000 mm3 (n = 6).
Statistical Analysis
The statistical analysis of the data in this paper was performed using SPSS software, as described in previous studies.11 Briefly, the values are reported as the means ± standard deviations (SDs). Student’s t-test was used for scientific comparisons between groups. Correlation analyses between circFGFR4 and CXCR4 expression were performed. The Kaplan‒Meier method was used to analyze the cumulative recurrence and survival rates. P<0.05 indicates statistical significance.
Results
CircFGFR4 is Upregulated in TNBC Tissues and Correlates with a Poor Prognosis
Considering the important biological functions of the FGFR4-related pathway in breast cancer progression, FGFR4 overexpression was determined to be a common event in breast cancer.17,18 First, we analyzed the expression of fgfr4 gene-derived two circRNAs from 30 TNBC tissues and paired adjacent nontumor tissues using qRT-PCR. Of two circRNAs, hsa_circ_0075147 was significantly upregulated in TNBC tumor tissues compared with paired adjacent nontumor breast tissues. However, hsa_circ_0075146 expression was not significantly different between TNBC tissues and paired adjacent nontumor breast tissues (Figure 1A and B). Therefore, the biological function of hsa_circ_0075147 (circFGFR4 in this study indicate hsa_circ_0075147) was analyzed in this study. CircFGFR4 consists of 903 nucleotides and 6 exons (Figure 1C). We analyzed circFGFR4 expression in another 60 paired TNBC tissues and paired adjacent nontumor breast tissues to further clarify the function of circFGFR4 in TNBC and found that circFGFR4 expression was significantly increased (44/60) in TNBC tissues (Figure 1D). Next, we analyzed the relationship between circFGFR4 expression and the clinicopathological characteristics of 60 TNBC patients, as listed in Table 1. The results indicated that TNBC patients with circFGFR4high cells had bigger tumors (P = 0.002). Then, we investigated the relationship between circFGFR4 expression and the clinicopathological features of 60 patients with TNBC. The results suggested that TNBC patients with high circFGFR4 expression had a large tumor size (Figure 1E). Next, we discussed the prognostic significance of circFGFR4 expression in patients with TNBC. Compared with TNBC patients presenting low circFGFR4 expression, TNBC patients with high circFGFR4 expression had a poor prognosis (Figure 1F and G).
 |
Table 1 The Clinicopathological Features of 60 TNBC Patients |
circFGFR4 Expression Correlates with Decreased CD8+ T Cell Infiltration
Recently, several studies reported that dysregulated circRNA expression induces resistance to immunotherapy in various malignant tumors.9,11 In addition, the quantity of infiltrating CD8+ T cells in tumor tissues is considered an indicator of a good prognosis and a good response to a PD-1 antibody for patients with several malignant tumors.19,20 For a deeper understanding of whether there is a relationship between circFGFR4 and resistance to anti-PD-1 immunotherapy, we detected the infiltration of CD8+ T cells in above 60 TNBC tissues (Figure 2A). TNBC patients with high circFGFR4 expression exhibited decreased CD8+ T cell infiltration in tumor tissues (Figure 2A). The scatter plot and analysis showed that circFGFR4 expression in TNBC tissues was negatively correlated with the frequency of CD8+ T cells (Figure 2B).
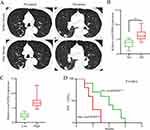
High Levels of circFGFR4 Indicate Resistance to Anti-PD-1 Immunotherapy in TNBC Patients
Because increased circFGFR4 expression correlates with decreased infiltration of CD8+ T cells in TNBC tissues, we hypothesized that circFGFR4 upregulation might limit the therapeutic effect of the PD-1 monoclonal antibody. Therefore, we analyzed retrospective data of 17 TNBC patients with relapsed disease and distant metastasis of TNBC who received anti-PD-1 immunotherapy (pembrolizumab). After four therapy cycles, enhanced CT was used to evaluate the treatment effect. According to the iRECIST criteria, the results indicated that no patients achieved a partial response (PR) or complete response (CR), 5 patients had stable disease (SD), and 12 patients had progressive disease (PD) (Figure 3A). Then, circFGFR4 expression was detected using qRT-PCR. The circFGFR4 in the PD group was higher than that in the SD group (Figure 3B). To further validated that increased circFGFR4 expression limit the therapeutic effect of the anti-PD-1 immunotherapy in TNBC patient. Next, we analyzed another cohort 20 patients with distant metastasis of TNBC who received anti-PD-1 immunotherapy (pembrolizumab). CircFGFR4 expression levels were then detected and divide into circFGFR4High and circFGFR4Low two groups (Figure 3C), and Kaplan-Meier survival analysis indicated that the PFS for the circFGFR4High group was decreased compared to circFGFR4Low group (Figure 3D). Taken together, we conclude that upregulated circFGFR4 lead to TNBC anti-PD-1 resistance.
CircFGFR4 Upregulates CXCR4 by Sponging miR-185-5p
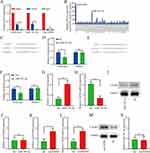
An increasing number of research reports indicate that circRNAs mainly act as competitive endogenous RNAs (ceRNAs) by sponging miRNAs. Therefore, the researchers conducted RIP with antibodies against AGO2 to further explore whether circFGFR4 sponged some miRNAs in MDA-MB-231 TNBC cells. The anti-AGO2 antibody significantly enriched circFGFR4, but circANRIL was not amplified (Figure 4A). These results indicate that circFGFR4 might function as a ceRNA to sponge some miRNAs in TNBC cell lines. Next, StarBase v3.0 was used to predict miRNAs binding to circFGFR4, and 49 candidate miRNAs were identified. By performing circRIP with a circFGFR4 probe, we observed the significant enrichment of circFGFR4 and miR-185-5p, but other miRNAs were not significantly enriched (Figure 4B), indicating that miR-185-5p was the miRNA related to circFGFR4 in MDA-MB-231 cells. We used HEK-293T cells to cotransfect miR-185-5p mimics and a luciferase reporter for the luciferase assay to further validate these results. Compared with the negative control (NC), the miR-185-5p mimics significantly inhibited the luciferase reporter activity in cells expressing the wild-type circFGFR4 sequence but did not inhibit luciferase activity in cells transfected with the miR-185-5p-targeted mutant circFGFR4 sequence (Figure 4C and D). We performed an in-depth investigation of the downstream pathway of circFGFR4/miR-185-5p that promotes TNBC immune escape by applying StarBase v3.0 and PITA to predict the target mRNAs of miR-185-5p. Our results indicated that the CXCR4 mRNA 3’ UTR contained predicted miR-185-5p target sequences. Next, a luciferase reporter gene assay was performed to verify whether the 3’UTR of the CXCR4 mRNA was the binding site of miR-185-5p in HER-293T cells. With the negative control mimics (NC) serving as the reference, miR-185-5p mimics suppressed luciferase reporter activity in cells transfected with the wild-type CXCR4 sequence but did not inhibit luciferase activity in cells transfected with the miR-185-5p-targeted mutant CXCR4 gene sequence (Figure 4E and F). Importantly, after the forced expression of miR-185-5p expression in MDA-MB-231 cells, CXCR4 mRNA and protein levels were significantly reduced (Figure 4G–I). However, after elevated expression of miR-185-5p in MDA-MB-231 cells, circFGFR4 expression did not change significantly (Figure 4J). Moreover, after the forced expression of circFGFR4 in MDA-MB-231 cells, CXCR4 mRNA and protein expression increased significantly (Figure 4K–M). Additionally, no significant change in miR-185-5p expression was observed after the forced expression of circFGFR4 in MDA-MB-231 cells (Figure 4N).
Increased circFGFR4 Expression are Correlated with Resistance to Anti-PD-1 Immunotherapy in TNBC
To further determine the effects of circFGFR4 expression on anti-PD-1 therapy resistance, therefore, we examine the anti-tumor effects of the PD-1 antibody in Hu-PBL-NSG mice that transplanted MDA-MB-231-circFGFR4 cells or mock cells. Compared to that of the mock cell group, the tumor growth in the MDA-MB-231-circFGFR4 cells transplant xenograft mice showed an obvious phenotype of resistance to PD-1 monoclonal antibody, and the xenograft mice had a shorter survival time (Figure 5A–C). In addition, the number of CD8-positive cells in tumor tissues derived by MDA-MB-231-circFGFR4 cells was significantly decreased compared with that in tumors derived by MDA-MB-231-Mock cells (Figure 5D).
Elevated CXCR4 Expression Correlates with Resistance to Anti-PD-1 Immunotherapy in TNBC
We next determined whether forced CXCR4 expression impeded the therapeutic effect of anti-PD-1 immunotherapy by combining the retrospective data from the aforementioned 17 TNBC patients with relapsed disease and distant metastasis of TNBC who received anti-PD-1 immunotherapy. Then, CXCR4 expression levels were detected using IHC (Figure 6A). The PD group displayed higher CXCR4 expression than the SD group (Figure 6B). These results indicate that CXCR4 upregulation might be involved in the resistance of TNBC patients to PD-1 immunotherapy. The results from the scatter plot analysis proved the positive correlation between the expression of circFGFR4 and CXCR4 in TNBC tissues (Figure 6C). All the results suggested that circFGFR4 may play an immunosuppressive role by sponging miR-185-5p to upregulate CXCR4 expression.
Discussion
Although anti-PD-1-based immunotherapy appears to provide advantages to patients with TNBC, not all these patients effectively respond to anti-PD-1 therapy.21,22 The low response rate is partially due to the decreased infiltration of tumor-infiltrating lymphocytes in tumor tissues and eventual inability to control tumor progression.23 We learned from this study that circFGFR4 forced TNBC immune evasion by upregulating CXCR4 expression. The present study validated that circFGFR4 inhibited miR-381-3p binding to CXCR4 to promote immune evasion in TNBC patients. Importantly, our results demonstrated that circFGFR4 plays a vital role in immune evasion and as a promising immunotherapeutic target for TNBC.
An increasing number of studies have reported that miRNAs decrease target gene expression by binding to the 3’ UTR of target mRNAs, leading to the degradation or inhibition of the translation of target mRNAs.24 Recently, studies have confirmed that circRNAs sponge miRNAs as endogenous competing RNAs, subsequently releasing target mRNAs from the repression of miRNAs.8,11 In addition, miRNA-185-5p has been proven to be a tumor suppressor involved in inhibiting cancer progression. For example, miRNA-185-5p inhibits the proliferation, migration, and invasion of non-small cell lung cancer cells by targeting RAB35.25 In glioma, circPOSTN promotes tumor progression by acting as a miR-185-5p sponge to upregulate KIF1B expression.26 In TNBC, the expression of circRNA-CREIT was decreased in doxorubicin resistant TNBC cells and associated with a poor prognosis.27 Here, we verified that forced circFGFR4 expression promotes CXCR4 expression and immune evasion in TNBC patients. Therefore, CXCR4 is regulated by circFGFR4 at the posttranscriptional level in TNBC cells.CXCR4 is expressed at higher levels in multiple human malignant tumors than in control tissues and promotes the progression and immune evasion of these cancers, including TNBC.11,28–30 For example, the rate of distant metastasis was significantly higher in CXCR4-positive TNBC.31 Increased CXCR4 expression is also significantly related to unfavorable clinical characteristics and a poor prognosis of TNBC patients.31 In patients with colorectal cancer, the upregulation of CXCR4 in cancer cells is associated with a higher recurrence rate and shorter survival. In addition, an increasing number of studies have reported that circRNAs play a key role in cancer progression by regulating CXCR4 expression.32 For example, circFGFR1 upregulates CXCR4 to promote NSCLC immune escape and resistance to PD-1 immunotherapy.11 The circPVT1 family promotes CXCR4 expression by sponging miR-455-5p to drive the growth and metastasis of medullary thyroid carcinoma.33 In TNBC, circBACH2 acts as an oncogenic circRNA that promotes proliferation, invasion, and migration by regulating the miR-186-5p/miR-548c-3p/CXCR4 axis.34 In the present study, we detected significant overexpression of circFGFR4 in TNBC tissues. Moreover, increased circFGFR4 expression limited the curative effect of anti-PD-1 on TNBC. Therefore, this study revealed the biological role of circFGFR4 in TNBC immune evasion for the first time.
Conclusion
Overall, the research results show that circFGFR4 promotes TNBC immune evasion and acts as a biomarker to predict sensitivity to anti-PD-1 immunotherapy. Mechanistically, when overexpressed, circFGFR4 competitively sponges miR-185-5p and prevents miR-185-5p from suppressing CXCR4 expression, subsequently inhibiting the infiltration of CD8+ T cells into TNBC tissues. Furthermore, circFGFR4 might represent a therapeutic target in patients with TNBC, especially those receiving anti-PD-1 immunotherapy.
Abbreviations
circRNA, circular RNA; PD-1, programmed cell death 1; CXCR4, C-X-C motif chemokine receptor 4; TNBC, triple negative breast cancer; PD-L1, programmed cell death ligand 1; NSCLC, non-small lung cancer; HCC, hepatocellular carcinoma; IHC, immunohistochemistry; qRT‒PCR, quantitative real-time polymerase chain reaction; RIP, RNA immunoprecipitation.
Data Sharing Statement
Each dataset generated or analyzed during this experiment is included in this paper.
Ethical Approval and Consent to Participate
Ethical approval was provided by the Shanghai East Hospital Research Ethics Committee. Written informed consent was provided by all patients.
Funding
This study was recognized and financed by grants from the National Natural Science Foundation of China (Grant No. 82172696).
Disclosure
The authors have no competing interests to declare.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Yin L, Duan JJ, Bian XW, et al. Triple-negative breast cancer molecular subtyping and treatment progress. Breast Cancer Res. 2020;22(1):61. doi:10.1186/s13058-020-01296-5
3. Schmid P, Adams S, Rugo HS, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2018;379(22):2108–2121. doi:10.1056/NEJMoa1809615
4. Schmid P, Cortes J, Pusztai L, et al. Pembrolizumab for early triple-negative breast cancer. N Engl J Med. 2020;382(9):810–821. doi:10.1056/NEJMoa1910549
5. Mittendorf EA, Zhang H, Barrios CH, et al. Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): a randomised, double-blind, Phase 3 trial. Lancet. 2020;396(10257):1090–1100. doi:10.1016/S0140-6736(20)31953-X
6. Zhou WY, Cai ZR, Liu J, et al. Circular RNA: metabolism, functions and interactions with proteins. Mol Cancer. 2020;19(1):172. doi:10.1186/s12943-020-01286-3
7. Ma S, Kong S, Wang F, et al. CircRNAs: biogenesis, functions, and role in drug-resistant tumours. Mol Cancer. 2020;19(1):119. doi:10.1186/s12943-020-01231-4
8. Zhang PF, Wei CY, Huang XY, et al. Circular RNA circTRIM33-12 acts as the sponge of MicroRNA-191 to suppress hepatocellular carcinoma progression. Mol Cancer. 2019;18(1):105. doi:10.1186/s12943-019-1031-1
9. Zhang PF, Gao C, Huang XY, et al. Cancer cell-derived exosomal circUHRF1 induces natural killer cell exhaustion and may cause resistance to anti-PD1 therapy in hepatocellular carcinoma. Mol Cancer. 2020;19(1):110. doi:10.1186/s12943-020-01222-5
10. Liu Z, Gu S, Wu K, et al. CircRNA-DOPEY2 enhances the chemosensitivity of esophageal cancer cells by inhibiting CPEB4-mediated Mcl-1 translation. J Exp Clin Cancer Res. 2021;40(1):361. doi:10.1186/s13046-021-02149-5
11. Zhang PF, Pei X, Li KS, et al. Circular RNA circFGFR1 promotes progression and anti-PD-1 resistance by sponging miR-381-3p in non-small cell lung cancer cells. Mol Cancer. 2019;18(1):179. doi:10.1186/s12943-019-1111-2
12. He R, Liu P, Xie X, et al. circGFRA1 and GFRA1 act as ceRNAs in triple negative breast cancer by regulating miR-34a. J Exp Clin Cancer Res. 2017;36(1):145. doi:10.1186/s13046-017-0614-1
13. Liu P, Wang Z, Ou X, et al. The FUS/circEZH2/KLF5/ feedback loop contributes to CXCR4-induced liver metastasis of breast cancer by enhancing epithelial-mesenchymal transition. Mol Cancer. 2022;21(1):198. doi:10.1186/s12943-022-01653-2
14. Yu J, Xu QG, Wang ZG, et al. Circular RNA cSMARCA5 inhibits growth and metastasis in hepatocellular carcinoma. J Hepatol. 2018;68(6):1214–1227. doi:10.1016/j.jhep.2018.01.012
15. Wang R, Zhang S, Chen X, et al. CircNT5E acts as a sponge of miR-422a to promote glioblastoma tumorigenesis. Cancer Res. 2018;78(17):4812–4825. doi:10.1158/0008-5472.CAN-18-0532
16. Pyo KH, Kim JH, Lee JM, et al. Promising preclinical platform for evaluation of immuno-oncology drugs using Hu-PBL-NSG lung cancer models. Lung Cancer. 2019;127:112–121. doi:10.1016/j.lungcan.2018.11.035
17. Gu W, Yang J, Wang Y, et al. Comprehensive identification of FGFR1-4 alterations in 5 557 Chinese patients with solid tumors by next-generation sequencing. Am J Cancer Res. 2021;11(8):3893–3906.
18. Levine KM, Ding K, Chen L, et al. FGFR4: a promising therapeutic target for breast cancer and other solid tumors. Pharmacol Ther. 2020;214:107590. doi:10.1016/j.pharmthera.2020.107590
19. Tian T, Li Z. Targeting tim-3 in cancer with resistance to PD-1/PD-L1 blockade. Front Oncol. 2021;11:731175. doi:10.3389/fonc.2021.731175
20. Liu J, Chen Z, Li Y, et al. PD-1/PD-L1 checkpoint inhibitors in tumor immunotherapy. Front Pharmacol. 2021;12:731798. doi:10.3389/fphar.2021.731798
21. Voorwerk L, Slagter M, Horlings HM, et al. Immune induction strategies in metastatic triple-negative breast cancer to enhance the sensitivity to PD-1 blockade: the TONIC trial. Nat Med. 2019;25(6):920–928. doi:10.1038/s41591-019-0432-4
22. Li Q, Wang Y, Jia W, et al. Low-dose anti-angiogenic therapy sensitizes breast cancer to PD-1 blockade. Clin Cancer Res. 2020;26(7):1712–1724. doi:10.1158/1078-0432.CCR-19-2179
23. Peng S, Hu P, Xiao YT, et al. Single-cell analysis reveals EP4 as a target for restoring T cell infiltration and sensitizing prostate cancer to immunotherapy. Clin Cancer Res. 2021. doi:10.1158/1078-0432.CCR-21-0299
24. Bartel DP. Metazoan MicroRNAs. Cell. 2018;173(1):20–51. doi:10.1016/j.cell.2018.03.006
25. Wen H, Liu Z, Tang J, et al. MiR-185-5p targets RAB35 gene to regulate tumor cell-derived exosomes-mediated proliferation, migration and invasion of non-small cell lung cancer cells. Aging. 2021;13(17):21435–21450. doi:10.18632/aging.203483
26. Guan Y, Yang W, Zhang F, et al. CircPOSTN competes with KIF1B for miR-185-5p binding sites to promote the tumorigenesis of glioma. Brain Res Bull. 2022;180:86–96. doi:10.1016/j.brainresbull.2021.12.014
27. Wang X, Chen T, Li C, et al. CircRNA-CREIT inhibits stress granule assembly and overcomes doxorubicin resistance in TNBC by destabilizing PKR. J Hematol Oncol. 2022;15(1):122. doi:10.1186/s13045-022-01345-w
28. Majidpoor J, Mortezaee K. The efficacy of PD-1/PD-L1 blockade in cold cancers and future perspectives. Clin Immunol. 2021;226:108707. doi:10.1016/j.clim.2021.108707
29. Zhou M, Luo C, Zhou Z, et al. Improving anti-PD-L1 therapy in triple negative breast cancer by polymer-enhanced immunogenic cell death and CXCR4 blockade. J Control Release. 2021;334:248–262. doi:10.1016/j.jconrel.2021.04.029
30. Lin Q, Fang X, Liang G, et al. Silencing CTNND1 mediates triple-negative breast cancer bone metastasis via upregulating CXCR4/CXCL12 axis and neutrophils infiltration in bone. Cancers. 2021;13(22):5703. doi:10.3390/cancers13225703
31. Chen HW, Du CW, Wei XL, et al. Cytoplasmic CXCR4 high-expression exhibits distinct poor clinicopathological characteristics and predicts poor prognosis in triple-negative breast cancer. Curr Mol Med. 2013;13(3):410–416.
32. Khare T, Bissonnette M, Khare S. CXCL12-CXCR4/CXCR7 axis in colorectal cancer: therapeutic target in preclinical and clinical studies. Int J Mol Sci. 2021;22(14):7371. doi:10.3390/ijms22147371
33. Zheng X, Rui S, Wang XF, et al. circPVT1 regulates medullary thyroid cancer growth and metastasis by targeting miR-455-5p to activate CXCL12/CXCR4 signaling. J Exp Clin Cancer Res. 2021;40(1):157. doi:10.1186/s13046-021-01964-0
34. Wang X, Xue B, Zhang Y, et al. Up-regulated circBACH2 contributes to cell proliferation, invasion, and migration of triple-negative breast cancer. Cell Death Dis. 2021;12(5):412. doi:10.1038/s41419-021-03684-x
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.