Back to Journals » Pragmatic and Observational Research » Volume 11
The CHRONICLE Study of US Adults with Subspecialist-Treated Severe Asthma: Objectives, Design, and Initial Results
Authors Ambrose CS , Chipps BE, Moore WC , Soong W, Trevor J, Ledford DK, Carr WW, Lugogo N, Trudo F, Tran TN, Panettieri RA Jr
Received 26 February 2020
Accepted for publication 31 May 2020
Published 16 July 2020 Volume 2020:11 Pages 77—90
DOI https://doi.org/10.2147/POR.S251120
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor David Price
Christopher S Ambrose,1 Bradley E Chipps,2 Wendy C Moore,3 Weily Soong,4 Jennifer Trevor,5 Dennis K Ledford,6 Warner W Carr,7 Njira Lugogo,8 Frank Trudo,9 Trung N Tran,10 Reynold A Panettieri Jr11
1US Medical Affairs, AstraZeneca, Gaithersburg, MD, USA; 2Capital Allergy & Respiratory Disease Center, Sacramento, CA, USA; 3Pulmonary, Critical Care, Allergy, and Immunologic Medicine, Wake Forest School of Medicine, Winston-Salem, NC, USA; 4Alabama Allergy & Asthma Center, Birmingham, AL, USA; 5Pulmonary, Allergy, & Critical Care Medicine, University of Alabama at Birmingham, Birmingham, AL, USA; 6Division of Allergy and Immunology, University of South Florida, Tampa, FL, USA; 7Allergy & Asthma Associates of Southern California, Mission Viejo, CA, USA; 8Pulmonary & Critical Care Medicine, University of Michigan, Ann Arbor, MI, USA; 9US Medical Affairs, AstraZeneca, Wilmington, DE, USA; 10Biopharmaceuticals Medical, Respiratory and Immunology, AstraZeneca, Gaithersburg, MD, USA; 11Institute for Translational Medicine and Science, Rutgers, The State University of New Jersey, New Brunswick, NJ, USA
Correspondence: Christopher S Ambrose Tel +1 (301) 398-4454
Fax +1 301-398-9454
Email [email protected]
Background: Approximately 5– 10% of patients with asthma have severe disease. High-quality real-world studies are needed to identify areas for improved management.
Objective: Aligned with the International Severe Asthma Registry, the CHRONICLE study (ClinicalTrials.gov: NCT03373045) was developed to address this need in the US.
Study Design: Learnings from prior studies were applied to develop a real-world, prospective, noninterventional study of US patients with confirmed severe asthma who are treated by subspecialist physicians and require biologic or maintenance systemic immunosuppressant therapy or who are uncontrolled by high-dosage inhaled corticosteroids and additional controllers. Target enrollment is 4000 patients, with patient observation for ≥ 3 years. A geographically diverse sample of allergist/immunologist and pulmonologist sites approach all eligible patients under their care and report patient characteristics, treatment, and health outcomes every 6 months. Patients complete online surveys every 1– 6 months.
Initial Results: From February 2018 to February 2019, 102 sites screened 1428 eligible patients; 936 patients enrolled. Study sites (40% allergist/immunologist, 42% pulmonologist, 18% both) were similar to other US asthma subspecialist samples. Enrolled patients were 67% female with median ages at enrollment and diagnosis of 55 (range: 18– 89) and 26 (0– 80) years, respectively. Median body mass index was 31 kg/m2; 3% and 29% were current or former smokers, respectively, and > 60% reported ≥ 1 exacerbation in the prior year and suboptimal symptom control.
Conclusion: CHRONICLE will provide high-quality provider- and patient-reported data from a large, real-world cohort of US adults with subspecialist-treated severe asthma.
Keywords: asthma exacerbations, longitudinal studies, allergists, pulmonologists, biologic therapy
Introduction
Asthma is a heterogeneous disease characterized by variable phenotypes of airway inflammation, reversible airflow obstruction, and airway hyperresponsiveness.1 Approximately 5% to 10% of patients with asthma have severe disease, defined by the American Thoracic Society and European Respiratory Society (ATS/ERS) as needing high-dosage inhaled corticosteroids (ICS) plus additional controllers to maintain control, remaining uncontrolled even when using these treatments, or requiring systemic corticosteroids for ≥50% of the previous year to maintain control.2,3 Control of asthma is independent of severity, but patients with severe asthma are at higher risk for poor control, resulting in severe exacerbations, emergency department visits, hospitalizations, lost productivity, and reduced quality of life compared with patients with nonsevere asthma.4–8 Although severe asthma affects only a small percentage of patients, it is a major contributor to the overall societal and economic burden of the disease.9,10
Statements from the ATS/ERS2 and the Global Initiative for Asthma (GINA)3 support the proper management of severe asthma; however, the level of adoption of these guidelines is unknown and likely incomplete. Additionally, newer treatment approaches need to be understood in real-world samples to inform future guidelines and recommendations. In particular, several monoclonal antibody therapies have been approved for the treatment of severe asthma;9 however, few high-quality data are available to describe the real-world use of and outcomes associated with these agents.
There is a clear need to enhance our understanding of severe asthma with contemporary data from large, real-world, longitudinal, observational studies. Global randomized controlled trials (RCTs) are the gold standard for establishing treatment safety and efficacy, but RCTs in severe asthma do not represent the overall patient population due to study inclusion/exclusion criteria and limited country-specific samples.11 Analyses of secondary data sources (eg, health care utilization records or insurance claims) may not allow for accurate identification and characterization of patients with severe asthma due to incomplete data, inaccuracies in data entry, and an inability to document subspecialist (allergist/immunologist [AI] or pulmonologist [P]) confirmation of a severe asthma diagnosis. These data sources also often lack data on environmental and social risk factors, laboratory testing, pulmonary function, and subjective assessments by health care providers (HCPs) and patients. In contrast, standardized, longitudinal, observational registry studies are an ideal approach to better understand real-world national and regional variations in the care of patients with severe asthma12 and to address challenges in clinical trial design and patient selection, particularly given the variable nature of asthma.11,12
The Epidemiology and Natural History of Asthma: Outcomes and Treatment Regimens (TENOR) study, initiated in 2001, was a real-world, 3-year, multicenter, prospective cohort study of 4756 patients aged ≥6 years with severe (48% of patients) or difficult-to-treat (96% of patients) asthma. The TENOR study provided valuable insights into the natural history and medical management of severe or difficult-to-treat asthma in the United States at that time.13,14 Despite substantial changes to the treatment landscape in recent years, no large, real-world, observational study of US patients with severe asthma has been undertaken since the TENOR study.
The International Severe Asthma Registry (ISAR) was initiated in 2017 to address this need at the global level by combining retrospective and prospective data from existing national severe asthma registries (and supporting the development of new national severe asthma registries) in more than 14 countries.15 A panel of 27 international asthma experts used a modified Delphi process to reach a consensus on a common set of 95 core variables for standardized collection across all participating countries.16 ISAR chose to focus on the population of adult patients receiving monoclonal antibody or maintenance systemic corticosteroid treatment, or those uncontrolled while receiving high-dosage ICS with additional controllers. At present, only a single US site is involved in ISAR.
The CHRONICLE study was developed to align with ISAR and provide contemporary real-world data describing a large, geographically diverse cohort of US adults with subspecialist-treated severe asthma that is not controlled by high-dosage inhaled therapies. The ultimate goal is to help improve the US standard of care for severe asthma, including optimal use of monoclonal antibody therapies recently approved by the US Food and Drug Administration (FDA), by better characterizing the disease burden and treatment gaps associated with US severe asthma care. The goal of this manuscript is to describe the CHRONICLE study design and present initial observations from the first year of the study, which demonstrate that the study has been effective in enrolling a diverse, real-world sample of US patients with severe asthma treated by subspecialists.
Patients and Methods
Study Design
The CHRONICLE study is designed to be a real-world, prospective, noninterventional cohort study that collects primary HCP-reported and patient-reported data on patient characteristics, comorbidities, laboratory and imaging assessments, medical treatments, health care utilization, and health outcomes from a large, geographically diverse cohort of US patients and providers. The study is sponsored by AstraZeneca and led by AstraZeneca staff and a scientific advisory committee composed of 8 US clinical experts in severe asthma care and research (authors WWC, BEC, DKL, NL, WCM, RAP, WS, JT). This committee provides scientific leadership of the study objectives, protocol, conduct, and analysis.
Study Participants
Inclusion and exclusion criteria are outlined in Table 1. To enable pooled analyses of CHRONICLE and ISAR data, it was essential to align the patient population with that of ISAR; specifically, patients receiving subspecialist care who are either not controlled on high-dosage ICS with additional controllers and/or require monoclonal antibody or maintenance systemic corticosteroid (mSCS)/immunosuppressant therapy. Confirmation of severe asthma by subspecialists was considered essential given the high prevalence of conditions that mimic severe asthma.17–19 To ensure accuracy of diagnosis, patients are required to have a diagnosis of severe asthma for ≥12 months prior to enrollment. To ensure accurate data collection and enable capture of the provider’s subjective assessments of the patient’s disease, enrollment is limited to patients currently receiving care from subspecialist physicians at the investigator’s site.
 |
Table 1 Patient Inclusion and Exclusion Criteria in the CHRONICLE Study |
The study is designed to minimize participation bias. The protocol requires sites to approach every eligible patient (defined as patients meeting all inclusion criteria) under their care, with only 1 exception. When the original protocol was written, there was concern that the number of patients qualifying under inclusion criterion 4a (uncontrolled severe asthma treated with high-dosage ICS with additional controllers; Table 1) would greatly exceed those qualifying under 4b or 4c (use of monoclonal agents or systemic corticosteroids/immunosuppressants, respectively). As a result, the CHRONICLE protocol directed sites to approach only every third patient who was eligible for enrollment according to inclusion criterion 4a but did not qualify under 4b or 4c. Following the first year of enrollment, it was clear that the restricted enrollment of these patients was not necessary. As a result, this “every third” enrollment restriction for patients meeting only inclusion criterion 4a was eliminated in February 2019.
Among all study-eligible patients, basic de-identified characteristics are collected on all patients, including those not approached, those approached but not enrolled, and those enrolled, to enable a direct assessment of any participation biases. A signed informed consent form is obtained at enrollment for study participation and to acquire medical records from other providers, including pharmacy records. Once consented and enrolled, patients are followed until study discontinuation, withdrawal, or death. As with ISAR, there is no required testing or imaging, as all data collection is meant to observe real-life clinical practices, and required testing, imaging, or other intervention could alter provider behavior and/or patient treatment. Moreover, no study visits are required following the initial enrollment. Patients are expected to continue follow-up for ≥3 years. A study termination date has not yet been determined.
Study Conduct
The CHRONICLE study is being performed in accordance with ethical principles that are consistent with the Declaration of Helsinki, International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) Good Clinical Practices, Good Pharmacoepidemiology Practices, the Health Insurance Portability and Accountability Act (HIPAA), and applicable legislation for observational studies. HCP-reported data and patient-reported outcomes (PROs) recorded from the date of enrollment are summarized in Table 2. Upon enrollment, HCPs document patient information such as demographic characteristics and asthma history. They further document the patient’s environmental and social milieu, health care insurance status, smoking history, asthma events, treatment characteristics, health care utilization, comorbidities, major medical events, and HCP assessments of patient status; these data are updated every 6 months. A variety of PROs are recorded at enrollment. Of these, asthma symptom control, exacerbations, and adherence are assessed monthly; asthma-related health care resource utilization, work productivity, and treatment effectiveness are evaluated every 3 months; and information regarding health-related quality of life, prescription medication source(s), and presence of an asthma treatment plan are collected every 6 months. This schedule was selected based on the relevant time periods for validated surveys and to reduce patient survey burden.
 |
Table 2 Data Collection in the CHRONICLE Study |
CHRONICLE data collection was aligned with ISAR core data collection16 to capture core asthma-related variables and ensure that ISAR and CHRONICLE data can be merged to create a larger global dataset. However, additional data elements collected in CHRONICLE enable a more complete characterization of HCPs and patients, including information on important potential confounders of patient health outcomes. These include social and environmental risk factors (eg, marital status, education level, employment, potential air quality risk), comorbidities and major medical events that can impact a patient’s overall health status, relevant nonasthma treatments, and adverse conditions associated with systemic corticosteroid therapy (see Table 2).
Because there are no specified study visits following the enrollment visit, longitudinal collection of HCP-reported information occurs with the extraction of information from medical records and investigator assessments every 6 months. Data quality is optimized through programmed data quality checks that automatically detect anomalous data, as well as a data management team that routinely reviews site-entered data and issues additional queries as necessary. In order to collect patient-reported data, patients are sent online surveys via email for completion outside of the study site, which minimizes influence from HCP opinions. To optimize data accuracy and limit missing data, data collection from HCPs is limited to the 12 months prior to enrollment for most items. However, the surveillance period prior to enrollment was extended up to 3 years for prior laboratory and pulmonary function testing, and to any time prior to enrollment for more serious or rare events (eg, asthma hospitalizations, prior monoclonal or systemic immunosuppressant therapy, diagnosed malignancies). For all data elements, dates of events and changes in patient status are recorded. As a result, sites provide updates to each patient’s data on a feasible schedule of every 6 months, but a comprehensive longitudinal record of the patient’s medical history is constructed.
Because the CHRONICLE study is not designed to test a specific hypothesis, the target study population size was not determined by statistical power calculations. The initial target sample size of ≥1500 patients was increased to 4000 patients based on the sample deemed necessary to enable robust subgroup analyses, similar to that achieved by the TENOR study.20 Site feasibility survey results suggested that enrollment of 4000 patients would require 125 study sites enrolling subjects for approximately 3 years. Study sites were selected to represent a geographically diverse mix of academic and community-based medical centers, as well as hospital-based and office-based sites. The only site selection criteria were requirements that the HCP(s) be a relevant US subspecialist (AI and/or P who treats severe asthma), that the site was able to complete the study procedures, and that the overall site sample was geographically dispersed across the United States.
Data Analysis
The study uses a hypothesis-free approach. Hypothesis-testing analyses may be conducted following initial descriptive results. All data will be reported in a manner aligned with Good Publication Practice (GPP3) guidelines,21 recommendations of the International Committee of Medical Journal Editors,22 the STrengthening the Reporting of OBservational studies in Epidemiology Initiative checklist for cohort studies (STROBE 2012),23 and study sponsor (AstraZeneca) policies. Annual descriptive analyses of eligible and enrolled patients are planned. The current manuscript presents baseline site and patient characteristics from the first year of data collection. More detailed analyses will be shared in future publications.
Initial Results
A comparison of the CHRONICLE study and other severe asthma registry-style cohort studies is presented in Table 3. Compared with the TENOR study, CHRONICLE has fewer sites but a similar number of treating physicians, focuses exclusively on adults with severe asthma, has a longer duration of follow-up planned, and assesses more patient-reported data, which are collected more frequently.20 By design, the characteristics of ISAR and CHRONICLE are similar. Two primary differences are that CHRONICLE has a robust set of PROs collected every 1 to 6 months and that HCP-reported data collection in CHRONICLE occurs every 6 months based on site medical records to provide a comprehensive longitudinal record for each patient. In ISAR, longitudinal data collection is intermittent, as it is driven by the timing of patient visits to specialist centers. In the sections below, we present baseline characteristics for the study sites and patients in CHRONICLE. These initial observations are included to show that the study design has effectively enrolled a diverse sample of patients with severe asthma treated by US subspecialists; detailed results from the first interim analysis will be presented in a subsequent report.
 |
Table 3 Comparison of Asthma Registries: TENOR, ISAR, and CHRONICLE |
CHRONICLE Study Sites
As of February 2019, a total of 102 sites were active in CHRONICLE; characteristics of included study sites are summarized in Table 4. Among the first 102 sites, 40% were AI practices, 42% were P practices, and 18% reported having both AIs and Ps in their severe asthma practice. Principal treating physicians were predominantly male (83%), had practiced in the specialty for a median of 22 years (range: 3–43), were in single-specialty practices (64%) with a median of 2 physicians and 2 nonphysicians treating severe asthma, and were in nonhospital (83%) and urban/suburban (49%/44%) settings. Practices reported treating a median of 200 patients per year with severe asthma, ranging from 7 to 6000. The presence of a severe asthma clinic was reported by 37% of sites, and 88% reported administering biologics onsite. Overall, the physician characteristics of the first 102 sites in CHRONICLE were similar to those of asthma specialists in the Centers for Disease Control and Prevention National Asthma Survey of Physicians (CDC NAS)24 and AIs and Ps overall in the American Medical Association (AMA) subspecialist characteristics (Table 5).25 Additionally, the geographic distribution of CHRONICLE sites was generally aligned with that of the CDC NAS and AMA subspecialist characteristics, but with moderate underrepresentation of providers in the Midwest and Pacific regions.
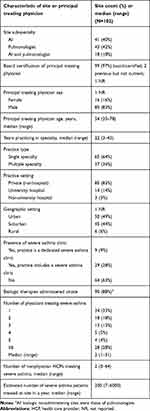 |
Table 4 CHRONICLE Study Site Characteristics |
Eligible and Enrolled Patient Characteristics
Between February 2018 and February 2019, a total of 1428 eligible patients were screened for enrollment in CHRONICLE, and 936 patients had enrolled, of whom 796 had all baseline forms completed (see Figure E1 in the Online Repository). Table 6 summarizes the general patient characteristics of the eligible and enrolled patient populations. Eligible patients had median ages at enrollment and diagnosis of 56 and 27 years, respectively; 68% were female, and 60% had commercial health care insurance. By asthma treatment category, 58% of all eligible patients were receiving biologics, 12% were receiving mSCS therapy, and 37% were receiving high-dosage ICS therapy with additional controllers without biologics or mSCS. Asthma exacerbations were common, with 61% reporting ≥1 exacerbation in the 12 months prior to screening, for an overall mean of 1.9 exacerbations in the prior year. Characteristics of the enrolled population were similar, with the exception of the expected underrepresentation of patients receiving high-dosage ICS therapy with additional controllers without biologics or mSCS (due to the protocol-mandated “every-third” enrollment scheme). Additional data collected from enrolled patients demonstrated a median body mass index of 31 kg/m2; most patients were non-Hispanic White (71%) and 50% lived in a suburban setting; and 3% and 29% were current or former smokers with median pack-year histories of 7.5 and 6.8 pack-years, respectively. As of enrollment, patients first received subspecialty care for asthma a median of 12.2 years previously, with a median of 4.6 years since the first visit with their current subspecialist. Among those receiving ongoing therapy with biologics, 54% were receiving omalizumab, 24% mepolizumab, 18% benralizumab, 5% reslizumab, and 1% dupilumab. Based on HCP report and as of their most recent visit prior to enrollment, ≥50% of all enrolled patients had suboptimal asthma symptom control based on daytime symptoms, asthma reliever use, and activity limitation, with 40% reporting nocturnal asthma awakenings/symptoms and 12% reporting an asthma hospitalization in the prior 12 months. Spirometry or complete pulmonary function testing was reported for 88% of enrolled patients. For the subset with confirmed bronchodilator withhold, median FEV1 and FVC were 70% and 78% predicted, respectively. Mean and median blood eosinophil counts were 287 and 152 cells/µL, which varied significantly by treatment group: 370 and 263 cells/µL for those receiving high-dosage ICS with additional controllers without biologic or mSCS use, 236 and 106 cells/µL for those receiving biologics, and 206 and 74 cells/µL for those receiving mSCS.
 |
Table 5 Site-Level Characteristics in the CHRONICLE Study Compared with the CDC NAS and AMA Subspecialist Data |
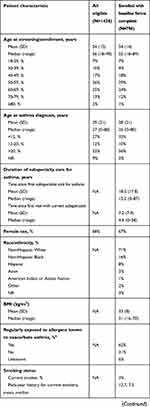 | 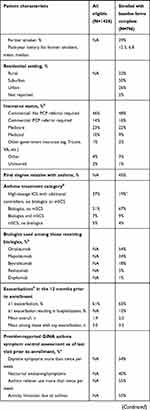 |  |
Table 6 Characteristics of the Initial Eligible and Enrolled Patients |
Discussion
The CHRONICLE study will provide high-quality, longitudinal, real-world data regarding US adult patients with severe asthma treated by subspecialists. The noninterventional design has no mandated study visits following enrollment so as not to affect the natural history of the disease or treatment. Several strategies to minimize bias have been incorporated into the study design, including systematic enrollment, minimal exclusion criteria for sites and patients, standardization of data collection at the study sites with routine monitoring of data entry, and PROs collected directly from patients using a web-based interface on a regular schedule and not during HCP visits. Study sites represent a geographically diverse mix of US academic and community-based medical centers, with both hospital-based and office-based sites. Characteristics of the first 102 study sites are similar to other samples and suggest that there is no significant bias with site selection. Additionally, a comparison of the characteristics of the initial eligible and enrolled patients demonstrate no substantial recruitment or enrollment biases, other than the protocol-mandated under-enrollment of patients not on biologics or mSCS therapy, which has been eliminated as of February 2019.
After 1 year of operation, the CHRONICLE study has provided novel insights into the characteristics of US adults who suffer from severe asthma as well as the subspecialist physicians and HCPs who care for them. Despite subspecialist assessment and treatment for a median of 12 years, >60% of patients reported ≥1 exacerbation within the past year and suboptimal asthma symptom control, and approximately 1 in 8 patients were receiving mSCS therapy, which can have significant associated toxicity26,27 and indirect cost.27,ccc28 These observations underscore the need for novel therapies and further improvements in the standard of care for this population. Additionally, the initial observations demonstrate that one-third of patients with severe asthma in the United States are current or former smokers, many with a significant pack-year history, which is a population rarely studied in randomized controlled trials for severe asthma treatments.11 With regard to the ongoing use of biologics, newer anti-interleukin (IL)-5/IL-5 receptor therapies had a cumulative prevalence similar to anti-immunoglobulin E therapy (omalizumab), which demonstrates a significant shift in US severe asthma biologic therapy following the approvals of mepolizumab, reslizumab, and benralizumab. Dupilumab received FDA approval for moderate to severe asthma in October 2018; as a result, its use was limited in this analysis of patients enrolled between February 2018 and February 2019. Future analyses of CHRONICLE will provide insights into the evolving use of biologics among US subspecialists.
The first-year patient cohort from the CHRONICLE study already represents the largest real-world study population of US patients with subspecialist-confirmed severe asthma since the TENOR study. Future analyses and long-term follow-up will greatly improve our understanding of severe asthma in the United States and potentially enable improvements in the standard of care. The CHRONICLE study cohort is particularly important given the decentralized nature of severe asthma management by US subspecialists. With the potential to integrate data from CHRONICLE and ISAR,15,16 US subspecialist patients with severe asthma will be better represented in a large global sample of patients with severe asthma. Potential benefits of pooling data across multiple national registries include enhanced accuracy of epidemiologic estimates, improved recognition of rare events, and greater ability to examine possible drug-demographic, drug-disease, and drug–drug interactions in various subpopulations.16
Despite the strengths of the CHRONICLE study, there are several potential limitations. First, patient recall bias and missing data from medical records could limit the utility of the results. To address this issue, study sites routinely request supplementary information regarding each patient’s health care utilization from the patient’s primary care physician; additionally, acquisition of pharmacy claims information is planned. However, information regarding actual treatment costs from patient, insurer, and societal perspectives are not collected, as providers and patients have limited access to accurate payment data. The study collects some information about financial decisions (eg, missed medication doses due to cost, declination of biologic therapy due to cost, etc.), but a complete assessment of financial decision-making is beyond the scope of the study. Another limitation is that laboratory testing, pulmonary function testing, and other procedures are not mandated but are performed according to standard clinical practice. As a result, not all patients will contribute data to all outcomes of interest, and those tested represent a selected sample. However, the real-world frequency of testing and procedures can be ascertained, and the available data will reflect real-world care. Despite efforts to involve a diverse mix of study sites, the site selection procedure was not random, and the precise generalizability of the study results is unknown. To address this uncertainty, the characteristics of the study cohort will continue to be compared with other cohorts described in the literature or other sources. Finally, the study does not include patients with severe asthma who were not treated by subspecialists, and children and adolescents with severe asthma are not enrolled. These were purposeful decisions made to align with ISAR and to study adults with subspecialist-confirmed severe asthma.
Conclusions
There is an urgent need for improved management and treatment approaches for adults with severe asthma. The CHRONICLE study is a longitudinal, prospective, observational study that provides high-quality data on patient characteristics, treatment patterns, and health outcomes among a large, geographically diverse cohort of adults with severe asthma treated by US subspecialists. The study will provide a rich source of real-world data in the years ahead to expand our understanding of severe asthma and potentially improve the standard of care in the United States and globally.
Abbreviations
ACT, Asthma Control Test; AI, allergist/immunologist; AMA, American Medical Association; AQLQ, Asthma Quality of Life Questionnaire; ATAQ, Asthma Therapy Assessment Questionnaire; ATS, American Thoracic Society; BAL, bronchoalveolar lavage; BMI, body mass index; CBC, complete blood count; CDC, Centers for Disease Control and Prevention; ERS, European Respiratory Society; FDA, US Food and Drug Administration; FeNO, fractional exhaled nitric oxide; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; GETE, Global Evaluation of Treatment Effectiveness; GINA, Global Initiative for Asthma; HCP, health care provider; HCRU, health care resource utilization; HMO, health maintenance organization; ICS, inhaled corticosteroids; ICU, intensive care unit; IgE, immunoglobulin E; IL, interleukin; ISAR, International Severe Asthma Registry; LABA, long-acting beta-agonist; LLN, lower limit of normal; mSCS, maintenance systemic corticosteroids; NAEPP, National Asthma Education and Prevention Program; NAS, National Asthma Survey of Physicians; NCT, National Clinical Trial; NR, not reported; P, pulmonologist; PCP, primary care physician; PFT, pulmonary function test; ppb, parts per billion; PRO, patient-reported outcome; RCT, randomized controlled trial; SD, standard deviation; SGRQ, St. George’s Respiratory Questionnaire; TBD, to be determined; TENOR, The Epidemiology and Natural History of Asthma: Outcomes and Treatment Regimens; VA, Veterans Affairs; WPAI-Asthma, Work Productivity and Activity Impairment Asthma questionnaire.
Data Sharing Statement
CHRONICLE is an ongoing study; individual de-identified participant data cannot be shared until the study concludes. The full study protocol is available upon request of the corresponding author. Individuals who were or were not involved in the study may submit publication proposals to the study’s Publication Steering Committee by contacting the corresponding author.
Ethics Approval and Informed Consent
The CHRONICLE study protocol received central institutional review board (Advarra, Columbia, MD) approval on November 3, 2017, and was registered on ClinicalTrials.gov on December 14, 2017 (NCT03373045).
Acknowledgments
Portions of this manuscript have previously been presented at the following conferences: The American Academy of Allergy, Asthma, and Immunology (AAAAI) 2019 meeting as a poster presentation with interim findings, and the abstract was published in The Journal of Allergy and Clinical Immunology (DOI: https://doi.org/10.1016/j.jaci.2018.12.971); the American Thoracic Society (ATS) 2019 meeting in 3 separate poster presentations with interim findings, and the abstracts were published in the American Journal of Respiratory and Critical Care Medicine (https://doi.org/10.1164/ajrccm-conference.2019.199.1_MeetingAbstracts.A2679; https://doi.org/10.1164/ajrccm-conference.2019.199.1_MeetingAbstracts.A2678; https://doi.org/10.1164/ajrccm-conference.2019.199.1_MeetingAbstracts.A2681); the 2019 Eastern Allergy Conference as a poster presentation with interim findings, and the abstract was printed in the meeting program; the 2019 Eastern Pulmonary Conference as a poster presentation with interim findings, and the abstract was printed in the meeting program; the 2019 American College of Allergy, Asthma & Immunology meeting as a poster presentation with interim findings, and the abstract was published in the Annals of Allergy, Asthma & Immunology (DOI: https://doi.org/10.1016/j.anai.2019.08.271); the 2019 American College of Chest Physicians annual meeting as an oral presentation with interim findings, and the presentation’s abstract was published in Chest (DOI: https://doi.org/10.1016/j.chest.2019.08.272). The authors gratefully acknowledge Nabeel Farooqui and James Zangrilli for their early input into study design, as well as Ramona Bosley for her input into the electronic data capture system that was used for study data collection, and Laura Belton for her assistance with analysis of the initial patient cohort. Medical writing support was provided by Katie Gersh, PhD, of MedErgy (Yardley, PA, USA), which was in accordance with Good Publication Practice (GPP3) guidelines and funded by AstraZeneca (Wilmington, DE, USA).
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. CSA, FT, and TNT contributed to funding acquisition.
Disclosure
Christopher S. Ambrose, Frank Trudo, and Trung N. Tran are employees of AstraZeneca. Bradley E. Chipps is an advisor for, received consultancy fees from, and is on the speakers’ bureau for AstraZeneca, Boehringer Ingelheim, Genentech, GlaxoSmithKline, Novartis, Regeneron, and Sanofi Genzyme. Wendy C. Moore is on advisory boards for and reports grant and personal fees from AstraZeneca, Sanofi Regeneron, Genentech, and GlaxoSmithKline. She is also a member of the writing and steering committee for the CHRONICLE study as well as a PI of the clinical trial at Wake Forest. Weily Soong is a consultant for and reports grants from AstraZeneca, Genentech, GlaxoSmithKline, Mandala, Novartis, Regeneron, Sanofi, and Teva; and is a speaker for AstraZeneca, GlaxoSmithKline, Regeneron, Sanofi, and Optinose. Jennifer Trevor is a consultant for AstraZeneca; and is on advisory boards for AstraZeneca, GlaxoSmithKline, and Boehringer Ingelheim. Dennis K. Ledford reports research support (paid to University) from Genentech/Roche, Novartis, and AstraZeneca. He reports royalties from Wolters Kluwer Health and Taylor and Francis. He is an Editor for the American Academy of Allergy, Asthma & Immunology (AAAAI). He is also a consultant/speaker for and reports personal fees from ALK, AstraZeneca, Boehringer Ingelheim, Genentech/Roche, GlaxoSmithKline, Meda, Novartis, Sanofi/Regeneron, and Teva, and fees received for legal opinions on drug allergy, metal allergy, radiocontrast reaction, and asthma death. Warner W. Carr is a speaker for AstraZeneca, Teva, Boehringer Ingelheim, Regeneron, and Sanofi; and a consultant for AstraZeneca, Teva, Boehringer Ingelheim, Regeneron, Sanofi, Circassia, CSL Behring, Genentech, GlaxoSmithKline, Horizon Pharma, Kaleo, Mylan, Pfizer, Shire, Meda, Baxalta, Novartis, Greer Laboratories, Alcon Laboratories, Valeant Pharmaceuticals, Grifols, Optinose, and Aerocrine. Njira Lugogo is on advisory boards for AstraZeneca, Sanofi, Genentech, Novartis, Teva, and GlaxoSmithKline. She also reports clinical trial funding to her institution from AstraZeneca, GlaxoSmithKline, and Sanofi. Reynold A. Panettieri Jr is on advisory boards for and reports grant support/personal fees from AstraZeneca, Sanofi, Genentech, Regeneron, MedImmune, RIFM, Equillium, Theravance, Avillion, OncoArendi, Meresa, NIH, and Novartis. The authors report no other conflicts of interest in this work.
References
1. Martinez FD, Vercelli D. Asthma. Lancet. 2013;382(9901):1360–1372. doi:10.1016/S0140-6736(13)61536-6
2. Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43(2):343–373. doi:10.1183/09031936.00202013
3. Global Initiative for Asthma. Global strategy for asthma management and prevention. Available from: http://www.ginasthma.org.
4. Luskin AT, Chipps BE, Rasouliyan L, Miller DP, Haselkorn T, Dorenbaum A. Impact of asthma exacerbations and asthma triggers on asthma-related quality of life in patients with severe or difficult-to-treat asthma. J Allergy Clin Immunol Pract. 2014;2(5):
5. Lee LK, Obi E, Paknis B, Kavati A, Chipps B. Asthma control and disease burden in patients with asthma and allergic comorbidities. J Asthma. 2018;55(2):208–219. doi:10.1080/02770903.2017.1316394
6. Chipps BE, Zeiger RS, Dorenbaum A, et al. Assessment of asthma control and asthma exacerbations in the epidemiology and natural history of asthma: outcomes and treatment regimens (TENOR) observational cohort. Curr Respir Care Rep. 2012;1(4):259–269. doi:10.1007/s13665-012-0025-x
7. Godard P, Chanez P, Siraudin L, Nicoloyannis N, Duru G. Costs of asthma are correlated with severity: a 1-yr prospective study. Eur Respir J. 2002;19(1):61–67. doi:10.1183/09031936.02.00232001
8. Peters SP, Ferguson G, Deniz Y, Reisner C. Uncontrolled asthma: a review of the prevalence, disease burden and options for treatment. Respir Med. 2006;100(7):1139–1151. doi:10.1016/j.rmed.2006.03.031
9. Trevor JL, Chipps BE. Severe asthma in primary care: identification and management. Am J Med. 2018;131(5):484–491. doi:10.1016/j.amjmed.2017.12.034
10. Nunes C, Pereira AM, Morais-Almeida M. Asthma costs and social impact. Asthma Res Pract. 2017;3:1. doi:10.1186/s40733-016-0029-3
11. Brown T, Jones T, Gove K, et al. Randomised controlled trials in severe asthma: selection by phenotype or stereotype. Eur Respir J. 2018;52(6):1801444. doi:10.1183/13993003.01444-2018
12. Siddiqui S, Denlinger LC, Fowler SJ, et al. Unmet needs in severe asthma subtyping and precision medicine trials. Bridging clinical and patient perspectives. Am J Respir Crit Care Med. 2019;199(7):823–829. doi:10.1164/rccm.201809-1817PP
13. Chipps BE, Haselkorn T, Paknis B, et al. More than a decade follow-up in patients with severe or difficult-to-treat asthma: the epidemiology and natural history of asthma: outcomes and treatment regimens (TENOR) II. J Allergy Clin Immunol. 2018;141(5):1590–1597.e1599. doi:10.1016/j.jaci.2017.07.014
14. Chipps BE, Zeiger RS, Borish L, et al. Key findings and clinical implications from the epidemiology and natural history of asthma: outcomes and treatment regimens (TENOR) study. J Allergy Clin Immunol. 2012;130(2):332–342.e310. doi:10.1016/j.jaci.2012.04.014
15. International Severe Asthma Registry (ISAR). Website. Available from: http://isaregistries.org.
16. Bulathsinhala L, Eleangovan N, Heaney LG, et al. Development of the International Severe Asthma Registry (ISAR): a modified Delphi study. J Allergy Clin Immunol Pract. 2019;7(2):578–588. doi:10.1016/j.jaip.2018.08.016
17. Hetherington KJ, Heaney LG. Drug therapies in severe asthma – the era of stratified medicine. Clin Med. 2015;15(5):452–456. doi:10.7861/clinmedicine.15-5-452
18. Lommatzsch M, Virchow JC. Severe asthma: definition, diagnosis and treatment. Dtsch Arztebl Int. 2014;111(50):847–855. doi:10.3238/arztebl.2014.0847
19. Aaron SD, Boulet LP, Reddel HK, Gershon AS. Underdiagnosis and overdiagnosis of asthma. Am J Respir Crit Care Med. 2018;198(8):1012–1020. doi:10.1164/rccm.201804-0682CI
20. Dolan CM, Fraher KE, Bleecker ER, et al. Design and baseline characteristics of the epidemiology and natural history of asthma: outcomes and treatment regimens (TENOR) study: a large cohort of patients with severe or difficult-to-treat asthma. Ann Allergy Asthma Immunol. 2004;92(1):32–39. doi:10.1016/S1081-1206(10)61707-3
21. Battisti WP, Wager E, Baltzer L, et al. Good publication practice for communicating company-sponsored medical research: GPP3. Ann Intern Med. 2015;163(6):461–464. doi:10.7326/M15-0288
22. International Committee of Medical Journal Editors. Recommendations for the conduct, reporting, editing and publication of scholarly work in medical journals. Available from: http://www.ICMJE.org.
23. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61(4):344–349. doi:10.1016/j.jclinepi.2007.11.008
24. Cloutier MM, Salo PM, Akinbami LJ, et al. Clinician agreement, self-efficacy, and adherence with the guidelines for the diagnosis and management of asthma. J Allergy Clin Immunol Pract. 2018;6(3):886–894.e884. doi:10.1016/j.jaip.2018.01.018
25. American Medical Association. Physician Characteristics and Distribution in the US. American Medical Association; 2015.
26. Price DB, Trudo F, Voorham J, et al. Adverse outcomes from initiation of systemic corticosteroids for asthma: long-term observational study. J Asthma Allergy. 2018;11:193–204. doi:10.2147/JAA.S176026
27. Rice JB, White AG, Scarpati LM, Wan G, Nelson WW. Long-term systemic corticosteroid exposure: a systematic literature review. Clin Ther. 2017;39(11):2216–2229. doi:10.1016/j.clinthera.2017.09.011
28. Canonica GW, Colombo GL, Bruno GM, et al. Shadow cost of oral corticosteroids-related adverse events: a pharmacoeconomic evaluation applied to real-life data from the Severe Asthma Network in Italy (SANI) registry. World Allergy Organ J. 2019;12(1):100007. doi:10.1016/j.waojou.2018.12.001
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
