Back to Journals » Journal of Pain Research » Volume 12
The challenge of developing pain medications for children: therapeutic needs and future perspectives
Authors Eerdekens M , Beuter C , Lefeber C , van den Anker J
Received 23 November 2018
Accepted for publication 27 February 2019
Published 23 May 2019 Volume 2019:12 Pages 1649—1664
DOI https://doi.org/10.2147/JPR.S195788
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Katherine Hanlon
Mariëlle Eerdekens,1 Christoph Beuter,1 Claudia Lefeber,1 John van den Anker2,3
1Grünenthal GmbH, Aachen, Germany; 2Division of Paediatric Pharmacology and Pharmacometrics, University of Basel Children’s Hospital, Basel, Switzerland; 3Division of Clinical Pharmacology, Children’s National Medical Center, Washington, DC, USA
Abstract: It is broadly accepted that children of all age groups including (preterm) neonates and young infants can perceive pain and that there is an absolute need to treat their pain safely and effectively. The approved treatment options for children, particularly (preterm) neonates and young infants, are very limited with only a few medications specifically labelled for this population. This article presents the challenges of developing pain medications for children. A short overview gives information on pain in children, including pain perception, prevalence of pain and the long-term consequences of leaving pain untreated in this vulnerable population. Current pain management practices are briefly discussed. The challenges of conducting pediatric clinical trials in general and trials involving analgesic medications in particular within the regulatory framework available to develop these medications for children are presented. Emphasis is given to the operational hurdles faced in conducting a pediatric clinical trial program. Some suggestions to overcome these hurdles are provided based on our experience during the pediatric trial program for the strong analgesic tapentadol used for the treatment of moderate to severe acute pain.
Keywords: pediatric patients, Pediatric Investigation Plan, pain relief, acute pain, tapentadol
Introduction
It is broadly accepted that children of all age groups, including (preterm) neonates and young infants,1,2 can perceive pain and that they all deserve adequate pain management irrespective of their age and development. Inadequately controlled pain is a significant cause of morbidity and even mortality in infants and children.1,3 It increases the risk of postsurgical complications and has a negative impact on quality of life, function and functional recovery.4,5 Pain has also been shown to have adverse developmental consequences in (preterm) neonates and young infants6–10 and may impact not only the short- and long-term psychomotor development of the involved infant but also will put a heavy burden on siblings and parents.
In contrast to the treatment of pain in adults, most currently used analgesics have not been systematically studied in the neonatal and pediatric population.11,12 Analgesic medication was and is still being administered to pediatric patients without prior clinical investigations of their pharmacokinetic, efficacy and safety characteristics (off-label use).13 In an attempt to improve this situation, the Best Pharmaceuticals for Children Act14 and the Pediatric Research Equity Act (PREA)14 became legally binding in the US in 2002 and 2003, respectively; this was followed in 2007 by the Pediatric Regulation15 in the European Union (EU) requiring a Pediatric Investigation Plan (PIP) for all medications in development unless a waiver has been granted.
Especially pediatric trial programs in acute pain are challenging as they have to cover extended age ranges from preterm infants to 17-year-old adolescents, thereby including a very broad weight and developmental range. In addition to ethical, formulation, dosing and a range of body maturation issues to be considered, the challenge of treating pain in the very young and preverbal children is potentiated by the difficulty in accurately measuring their pain.
In this article, we focus on the challenges encountered with pediatric trial programs for new analgesics based on experience with tapentadol. Tapentadol is an ideal candidate for development as analgesic in the pediatric population owing to its two synergistic mechanisms of action (μ-opioid receptor agonism and noradrenaline reuptake inhibition) with consequent reduced μ-load which might mitigate opioid-typical side effects,16,17 a predictable pharmacokinetic profile,18 with no metabolites contributing to the analgesic effect19 and a low drug–drug interaction potential. Tapentadol is the most recently developed and approved strong analgesic for the treatment of moderate-to-severe acute pain in adults and has been investigated across the entire pediatric age range from (preterm) neonates to adolescents.
Pain
According to the International Association for the Study of Pain (IASP), pain is defined as “An unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage”.20 The IASP does not differentiate between “adult” and “pediatric” pain; they do, however, comment, that “the inability to communicate verbally does not negate the possibility that an individual is experiencing pain and is in need of appropriate pain-relieving treatment”. The World Health Organization (WHO) states that the WHO pain ladder does not apply to pediatric patients, but a two-step approach is recommended with paracetamol or ibuprofen as a first step for mild pain and morphine as the second step for moderate-to-severe pain.21
Pain in children
Pain perception and prevalence in infants, children and adolescents
Pain transmission and reflex response in neonates, mediated by spinal cord and brainstem reflex pathways, have long been acknowledged.2 However, a true experience of pain includes emotional and affective components and requires higher-level cortical processing which was only demonstrated in the last 10–15 years.10,22–24
Owing to ongoing maturation of the central nervous system (CNS) and associated maturation of signal transmission and inhibitory pathways, pain perception in infants does, however, differ from children above the age of two and from adults.10,22–27 Importantly, it has also been shown that opioid receptors are present from early on in fetal development,28–31 and are responsive to exogenously administered morphine.32 In contrast to these early stages of development (preterm to 23 months old), no clinically significant differences in pain perception and mechanisms of analgesia are present between children (aged 2–11 years old) and adolescents (aged 12–17 years old), or between these two groups and adults.33 It is, however, acknowledged that gender may modify the pain experience, response to analgesic therapies and transition from acute to chronic pain.34–36
Acute pain in infants, children and adolescents is common and may be associated with a number of causes such as underlying disease (eg, acute painful episodes in cancer37 and sickle cell disease38), trauma (eg, fractures or burns),39–41 surgical interventions and hospital procedures.42–44 Moderate or severe pain has been reported for 33–40% of all hospitalized children.45,46 Acute pain in this population is often not sufficiently managed,44,46 leading to many pediatric patients suffering from, often avoidable, moderate-to-severe pain.
Developmental and long-term consequences of undertreated pain
Developmental and long-term consequences of acute pain during infancy and childhood vary depending on the developmental stage of the neonatal and pediatric patients, the number of acute pain experiences (eg, multiple daily interventions in preterm infants) and the severity of pain experienced (eg, major surgery, trauma). In particular, pain experiences in (preterm) infants may lead to long-term adverse outcomes in terms of physical, psychological and social well-being of the affected patient. A number of authors have described these adverse effects of early pain experience on child development.6,47–51 However, evidence of long-term consequences in terms of cognitive, motor and behavioral outcomes in this population is only slowly emerging.52–66 Poorly managed acute pain is also one of the risk factors for developing chronic pain;67–72 a higher pain intensity score after discharge from hospital was a predictor for the development of chronic pain.69 Chronic pain in children and adolescents probably has even more impact as compared to adults: in addition to high rates of functional disability, sleep disorders and depression-anxiety disorders, chronic pain may lead to poorer academic outcomes at school (frequent school absences) which may impact on occupational and social functioning later in life.71,72
Management of moderate to severe pain in children
A number of pain practice guidelines provide advice on the management of pain in children (Table 1).21,73–81 Of note, although pediatric pain management guidelines for individual EU member states, eg, Great Britain and Ireland77 and Italy,79 are available, there is still no general EU guideline on this topic. The recommended approach for postsurgical management is multidisciplinary using various analgesic options including nonpharmacological interventions and local regional techniques combined with medications having different mechanisms of action.73,74 Nonpharmacological interventions may, for example, include distraction/comforting,82–84 whereas the application of topical liposomal lidocaine85 and the transversus abdominis plane block86 are examples of local regional techniques with demonstrated efficacy.
 | Table 1 Practice guidelines for procedural and postsurgical pain in neonates and children |
For moderate-to-severe pain following major surgery, there is consensus to use opioids.74–77 Paracetamol and/or nonsteroidal anti-inflammatory drugs (NSAIDs) are recommended as part of multimodal analgesia,75–78 and addition of paracetamol has been shown to reduce the opioid requirement.87 Prolonged pain following trauma may require the use of opioids for an extended duration.75
For procedural pain in neonates such as heel lance or venepuncture, a combined pharmacological and non-pharmacological (eg, breastfeeding or sweet-tasting solution) approach is recommended.76–79 More severe pain associated with, eg, insertion of a central venous catheter can be managed with topical anesthetics in conjunction with an opioid.77–79 Severe pain associated with changing the dressing in children with burns requires a strong opioid.77 The use of both opioids and paracetamol may be associated with safety issues. The use of opioids is linked with short-term adverse events such as low blood pressure and respiratory depression and may lead to tolerance resulting in increased opioid dose and potentially in iatrogenic opioid abstinence syndrome after discontinuation.88 Paracetamol for preterm and term neonates, on the other hand, is controversially discussed in the literature, and there are reports linking paracetamol in early neonatal life to neurocognitive impairment, including attention deficit/hyperactivity disorder symptoms or autism spectrum disorders and the risk of asthma or other atopy-related diseases.88,89
Pain guidelines are mostly based on the best practice established by global experts90 given the limited availability of pediatric data from controlled clinical trials. Although pediatric regulations have been established to ensure better investigations of new drugs for the pediatric population, for older analgesics commonly used in clinical practice, additional studies are not systematically performed in this population. As a result, no label updates are possible except for further restrictions in case of safety findings obtained from real-world evidence. This results in continued use of analgesics in an off-label manner in the pediatric population. In addition, the recommendations are not supported by drug labeling in individual countries. Table 2 lists analgesics labeled for children in Germany (as a representative for the EU91) and the US.14,92 There are, however, considerable differences even amongst European countries: of note, the use of metamizole (dipyrone) is controversial. France108 has banned it altogether for risk of agranulocytosis whereas it is labeled from 3 months onwards in Germany.109 Metamizole is also banned in many other countries worldwide including the US,93 and the potential risk associated with this drug should be kept in mind in pediatric prescription.110,111 The Food and Drug Administration (FDA) issued a black-box warning on the use of codeine in children after tonsillectomy and/or adenoidectomy,94 and in contrast to Europe, morphine is not labeled for pediatric use in the US. Tramadol also carries a black-box warning in the US95 and minimum age for use is 12 years, whereas it is labeled from 1 year onwards in Germany.91 Despite considerable differences in labeling between the regions, a common thread is that very few analgesics are actually labeled for neonates and very young children, and data are limited even when approval is available. A recently published report indicated that 13–69% of prescriptions within various pediatric populations were still used off-label.13 This presents the physician, caregiver and parents with many challenges including the lack of suitable formulations which can lead to tablets marketed for adults being divided or even ground up with the inherent uncertainty around the administered dose and potential safety issues or lack of efficacy. When opioids are used off-label, equianalgesic calculations carry the danger of over- or under-dosing.
 | Table 2 Approved analgesics for use in the pediatric population in Germany and the US |
In summary, off-label use of analgesics for pediatric pain management (with the risks and concerns this entails for patients, parents and caregivers alike) is common and almost unavoidable because of a lack of labeled alternatives. It can be assumed that off-label use increases as the age of the patients decreases.
Key Messages
- Infants and children of all age-groups, including (preterm) neonates and young infants can perceive pain.
- Acute pain in infants and children is common and frequently undertreated.
- Untreated pain in infants and children may have long-term consequences in terms of adverse cognitive, motor and behavioral outcomes.
- Only very few analgesics are labelled for neonates and very young infants.
- Off-label use is common and associated with a risk of lack of efficacy and/or safety issues.
Regulatory framework for the development of medicines in children
It has long been recognized that insufficient data are available on medicines in children, particularly in neonates and infants.112 In 2004, the EU published a report concerning the high level of off-label use and concluded that harmful effects occurred and that these were under-reported.113
Measures to address this situation were initiated with the Final Pediatric Rule in the US (1997) in which the FDA made pediatric trials mandatory for all medications not yet approved. Congress subsequently passed the PREA in 2003 which made many requirements of this Rule legally binding. The International Council for Harmonisation (ICH) harmonized tripartite guideline (EU, USA and Japan) on the ‘Clinical Investigation of Medicinal Products in the Paediatric Population’ (CPMP/ICH/2711/99: ICH E11) came into force in 2001.114 An addendum to this guideline (ICH E11(R1))115 in August 2016 updated the regulatory and scientific framework. Pediatric programs have since been required by legislators in ICH countries, eg, the Pediatric Regulation was adopted in 2007 within the EU.15
The obligations of the pharmaceutical industry to carry out a PIP in the EU and the pediatric written request in the US were coupled with an incentive of a 6-month prolongation of patent protection in the EU and 6 months additional data exclusivity in the US. The long-term aim of this legal framework was to reduce off-label use and to provide evidence-based guidelines and medicines for treating children of all ages.
Challenges in pediatric trials: general
Pediatric trials pose many challenges not encountered in adult trials. The ICH E11114 provides guidance on the investigation of medicinal products in children; other more specific European Medicines Agency (EMA) guidelines are also available. The ‘Guideline on the investigation of medicinal products in the term and preterm neonate’ (EMEA/536810/2008, 2010)116 provides background information on organ development and suggestions for safety monitoring; this guideline is currently being updated. Pharmacokinetic aspects of clinical trials in pediatrics are described in the ‘Guideline on the role of pharmacokinetics in the paediatric population’ (EMEA/CHMP/EWP/147013/2004, 2006).117
Ethical considerations
An EU expert group report describes in detail the ethical considerations for pediatric trials: the interests of the child must always prevail over the interests of science.118 In most cases, it is ethically justifiable to test a new compound only in children who might receive some benefit from the medication, ie, in children suffering from the condition for which the treatment is intended.
Many aspects of the trial need to be considered. For example,
- the planned trial should not duplicate previous trials with similar objectives,
- pediatric expertise needs to be available at all sites participating in the trial,
- appropriate nonclinical data and age-appropriate formulations must be available prior to initiating clinical trials.
One particular challenge concerns informed consent which must be given by the legally designated representative of the child. If the child is able to form his/her own opinion, then he/she is also required to “assent” to participation in the clinical trial. Difficulties arise in the conduct of multi-center trials globally since the regulations concerning assent and consent are not harmonized but subject to national laws. The numerous differences between countries have been summarized in a publication by Lepola et al119
Minimum subject numbers – maximum information
Design of pediatric trials is very much a balancing act, keeping the numbers of children participating in the study to a minimum, but obtaining as much information as possible for deriving robust dosing recommendations in all age groups. Modeling and simulation play a key role throughout the program, eg, by using prior knowledge in adults to guide the selection of the first pediatric dose. Blood volumes drawn in pediatric trials are recommended not to exceed 3% of the total blood volume over a 4-week period and 1% of the total blood volume for a single draw.118 The restriction on blood volume limits the numbers of samples which can be taken from each child, particularly in neonates and young infants. Again, modeling and simulation are indispensable to design the optimal sparse sampling strategy to best characterize the concentration–time profile of the medication with the minimum numbers of patients and samples.
One key question is how many subjects are needed for achieving the objectives of the trial. Wang et al120 suggested for trials with a primary pharmacokinetic objective to prospectively “target a 95% confidence interval within 60% and 140% of the geometric mean estimates of clearance and volume of distribution for the drug in each pediatric subgroup with at least 80% power”. For efficacy trials, similar constraints on sample numbers apply: sample size must be as low as possible but still sufficient to determine efficacy with adequate power and to provide a robust safety database.
Sensitive bioanalytical methods
The bioanalytical method(s) need to work with the lowest possible plasma/serum volume. Prior to initiating pediatric trials, the available method(s) may therefore require optimization. Microsampling techniques (eg, dried blood spots) may also be considered as an alternative approach to addressing the constraints on blood volumes.121
Blood samples taken in adult pharmacokinetic trials are generally venous samples. In our experience with postsurgical trials, most investigators strongly favor arterial sampling in the very young since an arterial line was already in place.
Maturation of body functions
The rates of maturation of body functions and biochemical processes vary widely between the different age groups and can change rapidly within even a few days in the early weeks of life. Thus, linear dosing based on body weight alone can never be assumed in this vulnerable population. All aspects of drug absorption (eg, gastrointestinal activity), distribution (eg, body composition), metabolism (eg, enzyme expression and maturation) and excretion (ADME) change with age.122
Selection of age groups
The broader the age group, the more challenges are involved in designing an appropriate testing program. Age subgroups recommended in the EU are based on the maturity and developmental status of the children: eg, preterm neonates, 0–27 days, 28 days to 23 months, 2–11 years and 12–16/18 years (depending on the legal age of adulthood in the country concerned).115 However, these age subgroups may need to be modified taking into account the specific pharmacokinetic and pharmacodynamic characteristics of the compound being investigated. The challenges become greater with the lower age groups: the optimal dose for a 2-day-old baby may be different to that of a 2–3-week-old baby.123 This becomes even more challenging with preterm neonates.124,125
Although developmental changes above 2 years of age proceed slower, differences can still occur in comparison to adults. Edginton et al predicted the clearance of several drugs across the age range of birth to 18 years and showed a rapid rise in clearance following birth, which continued to rise above adult levels and then slowly returned to adult values for teenagers.126 Thus, for a number of drugs, there is a peak in clearance during childhood, which may result in lower systemic concentrations than in adults.
Key Messages
- All pediatric trials should conform to the highest ethical standards.
- Modelling and simulation play a key role in planning and supporting a pediatric program.
- Pediatric trials must be carefully designed to provide maximum information from minimum patient numbers.
- Sensitive micro-analytical techniques for pediatric pharmacokinetic trials are essential to quantify drug concentrations.
- A full understanding of body maturation on drug ADME is imperative to make reliable dose predictions in pediatric subjects.
Challenges in pediatric trials for the treatment of pain – and some ideas on how to meet these challenges
The most recent guideline on the “Clinical development of medicinal products intended for the treatment of pain” was published by the EU in 2016 (EMA/CHMP/970057/2011);127 it includes a section on special populations including the pediatric population.
For acute postsurgical pain, experience in adults shows that it is highly preferable to select a standardized pain model with comparable anesthetic procedures during the surgery.128 However, fulfilling this objective is a major challenge for pivotal pediatric trials: for recruiting sufficient patients within a reasonable time frame, the trials invariably have to be conducted globally. There is considerable variation in the standards of care for anesthesia and analgesia across countries which can make standardization a real hurdle to overcome. This is potentiated by differences in typical surgeries conducted per age groups (eg, third molar extraction often used and validated in adults and adolescents,129 tonsillectomy in younger children;128) an intra-age group standardization is possible to achieve but complete standardization across age groups is very hard to realize.
Trial centers and patient recruitment
Trial experience has shown how important the selection of providers can be for timely trial completion. In one single dose, multicenter trial in children (6 to <18 years of age) with moderate-to-severe postsurgical pain, we experienced extremely slow recruitment. A second trial with children in the same age range was conducted by a site management organization located in a hospital environment: this second trial completed considerably faster in a single center than the multicenter trial.
In response to the legal requirements for more pediatric trials, several national and international pediatric research networks have been established. These networks can assist in finding trial centers and can offer advice on protocol design.
Recruitment can be assisted by creating an atmosphere at the trial centers which helps to allay the anxiety of the children, their parents and caregivers. The patient information must be clearly and concisely written and preferably child-friendly, eg, by using pictorial representations to assist in explaining the procedures involved.
Above all, there must be a genuine commitment of the trial sponsor and the clinical research team to work at unusual times. The sponsor must be able to provide around the clock support to the trial center with frequent visits to reassure the caregivers about the compound and trial design. This is particularly the case for recruiting neonates and young infants. In contrast to trials in adults, pediatric centers often require intensive training in the use of trial protocols, case report forms, consent forms, etc.. The trial physicians also need to be available at unusual times: in our experience, most neonatal patients were recruited during the night which was only possible with committed trial physicians and nurses.
Pain assessment
In all analgesic trials, it is essential to assess the severity of pain reliably and accurately; otherwise, the validity of the trial results is jeopardized. The Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials produced a consensus statement on measures for pain trials which was subsequently endorsed by the international pediatric pain community.130
Quantification of pain in all trials testing analgesics is made more difficult by the lack of an objective biomarker.131 A number of physiological measures including changes in heart rate, oxygen saturation, skin conductance or salivary cortisol have been suggested as more objective, indirect measures to quantify pain.132 However, these measures need extensive validation and, though of high interest, have not yet reached sufficient maturity for routine monitoring in analgesic trials.
One challenge for infants and children is that no one scale fits all ages requiring a careful choice of the most appropriate scale to match the age group(s) concerned. A brief overview of some recommended scales is given in Table 3.77,130
 | Table 3 Examples of some recommended and commonly used pain assessment tools for children with postsurgical or procedural pain |
Older children are usually able to use similar scales to adults such as the visual analogue scale.136 Younger children can better communicate their pain using the revised Faces pain scale.135 Although self-reporting of pain is considered to be the “gold standard”, it is not without its challenges. Pain is a complex experience with a multitude of contributing factors such that any pain scale is an oversimplification: it is very difficult to separate the sensation of pain from anxiety or stress factors in children. Quite an advanced level of cognitive skills is required for a child to give a reliable pain assessment.77 Response biases for children in the age range 3–5 have been reported for self-reporting pain scales;137 for this age group, it may be more reliable to derive a composite picture by using both self-reporting and observational scales. For very young children (up to about 3 years of age) and neonates/infants, observational scales such as the Face, Legs, Activity, Cry, and Consolability scale are used with judgment of different aspects of the infant’s/child’s behavior.134 Once selected, it is important for these validated scales to be used in clinical trials without modification: even small changes might result in a bias and invalidate comparisons of results with other trials.77
Design of efficacy trials
Design of efficacy trials with strong analgesics, eg, for postsurgical pain relief, poses a major ethical consideration: traditional placebo-controlled trials are normally considered unethical in pediatrics. An FDA workshop considered alternative strategies for pediatric analgesic efficacy trials: a consensus report gave support to an immediate rescue design.138 The underlying concept is that constant pain relief is available for all children in the trial using the current standard of care, eg, opioid treatment administered via patient-controlled analgesia (PCA) or nurse-controlled analgesia (NCA). The infants/children entering the trial are randomly divided between the test drug and placebo groups with both groups receiving underlying PCA or NCA. The primary surrogate efficacy endpoint is defined as the opioid-sparing potential of the test in comparison to the placebo groups. This type of immediate rescue design retains scientific validity as a double-blind design and is ethically much more acceptable than a traditional placebo-controlled trial. Examples of pediatric analgesia trials adopting this approach have been reported139,140 with a review article on the opioid-sparing effects of paracetamol and NSAIDs in pediatric pain trials.141 A meta-analysis of 85 trials including opioids, NSAIDs, paracetamol and local anesthetics was published by Kossowsky et al and provides an excellent overview of this approach in practice.142 They concluded that opioid sparing is a feasible surrogate end point in pediatric analgesic trials, but commented that other end points including pain scores also need to be considered since opioid sparing alone may underestimate the analgesic efficacy of the test medication.
Maturational aspects relevant to pain trials
Specific maturational aspects relevant for testing opioids in neonates and infants include the development of the blood–brain barrier and the drug efflux protein P glycoprotein (Pgp). The blood–brain barrier helps protect the brain from the influx of potentially toxic xenobiotics: since this barrier is immature at birth, increased penetration of opioids into the brain may occur in the very young.143 Furthermore, Pgp expression is limited in neonates, increases throughout early life and reaches adult levels after about 3–6 months.144 Thus, the ability of this protein to assist in the efflux of opioids from the brain during early life may be restricted and might lead to higher concentrations.
Age-appropriate formulation(s)
Age-appropriate formulations must enable flexible and accurate dosing across the whole age range. Excipients required for the formulations need to be carefully chosen owing to potential toxicity in the young or very young.145,146 Patient acceptability, in particular palatability of oral formulations, is essential in the pediatric population. The current EMA guideline for developing age-appropriate formulations in the pediatric population suggests focusing on a minimum number of acceptable forms capable of meeting the needs of the majority of pediatric patients.147 Oral liquid formulations are generally considered acceptable for infants and children down to full-term birth and for preterms capable of swallowing and being able to accept enteral feeding.
Challenges change across the age groups
The challenges in conducting a pediatric pain program are numerous with the nature and severity of the challenges changing across age groups. Table 4 captures these changes and highlights the major issues associated within each age subgroup.
 | Table 4 Pediatric trials for pain: different challenges faced across the age groups |
The challenges are most demanding in the youngest preverbal population; however, it should be borne in mind that Table 4 is also an oversimplification. The challenges can be just as testing in older children who may be nonverbal or who may suffer from psychomotor disorders; pain assessment in these children though older can still be extremely demanding.
Key Messages
- Recruiting sufficient pediatric patients is a major challenge: pediatric networks, committed hospital staff, child-friendly environment and documentation can all help.
- The use of validated, age-appropriate pain scales is essential for reliable pain assessment.
- Immediate rescue, opioid sparing design for efficacy trials is ethically acceptable whilst maintaining scientific validity.
- Thorough understanding of body organ and system maturation is essential for deriving safe and efficacious doses across all age groups.
- Age-appropriate formulations need to be developed
Key elements of the pediatric program
Key elements of the pediatric program are discussed with reference to the strong analgesic tapentadol. Central to the pediatric plan are the clinical trials for deriving pharmacokinetic, safety and efficacy data. Prior to initiating clinical trials, consideration must be given to formulation development and a potential need for conducting non-clinical trials:
Age-appropriate formulation(s)
For tapentadol, an oral solution formulation (20 mg/mL) already marketed was considered acceptable for children with a body weight >20 kg. For children <20 kg, an additional dose strength of 4 mg/mL was formulated.
For seriously ill children and clinically unstable term or preterm babies, a parenteral formulation is generally required. The concentration of the solution for parenteral application needs to be carefully chosen: neonates may only accept very low volumes to prevent volume overload with concurrent fluid nutrition.147 A 1 mg/mL solution for injection of tapentadol was formulated in preparation for testing in preterm neonates and young infants up to 2 years of age.
Nonclinical testing
Prior to the initiation of the pediatric program, a comprehensive preclinical safety package had already been completed for tapentadol to support the adult program. This package included one study in young animals which was considered sufficient to support clinical trials of tapentadol in adolescents (12 to <18 year olds). For supporting the administration of tapentadol to children <12 years of age, one additional study in juvenile animals was initiated. For compounds developed specifically for the pediatric population, both EMA and FDA guidelines discuss conditions in which toxicity testing in juvenile animals can be helpful for predicting toxicity in pediatric patients.148,149 In either case, the design of nonclinical trials is based on a complex array of factors including prior knowledge of the compound in question, pharmacological class of compound and age range of children involved.150
Clinical trials
Tapentadol’s µ-opioid agonism and noradrenaline reuptake inhibitory activity in the treatment of pain are well understood in adults. Despite an age-dependent variation in the antinociceptive potency of µ-opioid agonism after birth, its analgesic activity has been well established also in newborns.30,32,33 Although the descending noradrenergic system does not seem to fully function as a pain inhibitory system at birth, the spinal elements necessary for the functioning of the noradrenaline reuptake inhibition mechanism are developed at birth and can be utilized by tapentadol.151–153 Therefore a similar exposure–response relationship for tapentadol in adults and children was initially assumed. A range of systemic exposure (maximum plasma concentration, area under the concentration–time curve) known to be safe and efficacious in adults was targeted for the adolescent and lower age groups. As data were derived moving down the age range from children less than 18 years of age to preterm neonates, this assumption was tested and if necessary, could be modified.
The clinical pediatric program for tapentadol based on prior knowledge in adults is summarized in Table 5. As each age sub-group was completed in the single-dose trials, an assessment of pharmacokinetics, safety and exploratory analgesic efficacy data was conducted by an internal review panel composed of experts from the relevant departments. If the clinical and safety data were acceptable and tapentadol serum concentrations within the targeted range, then dosing could proceed to the next lower age group. For trials in children under 2 years of age and for the multiple efficacy dose trial, an external, independent Data Monitoring Committee was additionally set up to oversee the patients’ safety.
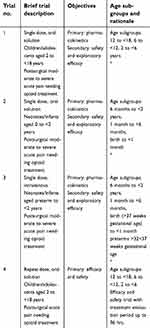 | Table 5 Pediatric clinical program for the strong analgesic tapentadol in treating acute pain |
All the pediatric clinical trials listed in Table 5 were conducted in patients with acute pain. Although the single-dose trials focused on the assessment of pharmacokinetics, some exploratory efficacy data could also be collected. More extensive efficacy and safety data were subsequently collected in a multiple dose trial in children experiencing moderate-to-severe postsurgical pain (trial 4 in Table 5). This double-blind, placebo-controlled trial utilized the opioid-sparing effect trial design discussed above in order to retain scientific validity while granting as much operational flexibility as possible to the involved investigators.
Drugs in development for the treatment of pain in children
In 2006, the EMA published a report assessing the pediatric needs and requirements for analgesic medications.154 This report highlighted large gaps in knowledge concerning pharmacokinetics, safety and efficacy in children of medications approved for adult use and a lack of age-appropriate formulations. In particular, only very few on-label medications are available for the treatment of moderate-to-severe pain in children under 2 years of age. An appraisal of the EMA website indicates that only 12 PIPs in total for pain medications are currently approved. Table 6 shows a list of analgesic medications with approved PIPs on the EMA website as of September 2018.91
 | Table 6 Analgesics under investigation in the pediatric population as agreed in a Pediatric Investigation Plan |
Only one novel analgesic medication (tapentadol) is currently in development across the entire age range of birth to <18 years of age and has been recently approved for acute pain in children aged 2 years and above.155,156 The development of tapentadol in the pediatric population is still ongoing to also include patients below 2 years of age. Owing to the two synergistic mechanisms of action of tapentadol (µ-opioid receptor agonism and noradrenaline reuptake inhibition) with consequent reduced µ-load which might mitigate opioid-typical side effects,16,17 a predictable pharmacokinetic profile18 with no metabolites contributing to the analgesic effect19 and a low drug–drug interaction potential, tapentadol is an ideal candidate for development in the pediatric population.
Tapentadol was the first analgesic to go through the formal EU PIP process and as such had a forerunner role. Since the tapentadol pediatric program was conducted with input from both the EMA and FDA, some aspects of the trials had variations included to fulfill the requirements of both authorities. Details of these variations and results of all the pediatric clinical trials in the acute pediatric program for tapentadol will be reported in a series of publications which will be published as a thematic series in this journal.
Development of medicines for the pediatric population – a wider perspective
The EMA and its Paediatric Committee produced a report for the EU in 2017 describing the experiences gained during the 10-year period of the pediatric regulation being in place.157 A comparison before and after the introduction of the regulation showed a positive impact with more than 260 medicines and indications authorized for use in children during this time period. It was acknowledged, however, that approval did not always translate directly into availability of the medication on the market for children.
Abbreviation list
ADME, absorption, distribution, metabolism, and excretion; BPCA, Best Pharmaceuticals for Children Act; CNS, central nervous system; EMA, European Medicines Agency; EU, European Union; FDA, Food and Drug Administration; FLACC, Face, Legs, Activity, Cry, and Consolability; FPS-R, revised Faces pain scale; IASP, International Association for the Study of Pain; ICH, International Council for Harmonisation; NCA, nurse controlled analgesia; NSAID, nonsteroidal anti-inflammatory drug; PCA, patient-controlled analgesia; Pgp, P glycoprotein; PIP, Pediatric Investigation Plan; PREA, Pediatric Research Equity Act; VAS, Visual Analogue Scale; WHO, World Health Organization.
Acknowledgments
Writing assistance was provided by Martin Brett, Elke Grosselindemann and Birgit Brett and was funded by Grünenthal GmbH.
Disclosure
Mariëlle Eerdekens, Christoph Beuter, and Claudia Lefeber are employees of Grünenthal GmbH. They report personal fees from Grünenthal GmbH, outside the submitted work. John van den Anker is a paid consultant for Grünenthal. The authors report no other conflicts of interest in this work.
References
1. Anand KJ, Hickey PR. Pain and its effects in the human neonate and fetus. N Engl J Med. 1987;317(21):1321–1329. doi:10.1056/NEJM198711193172105
2. Fitzgerald M. The development of nociceptive circuits. Nat Rev Neurosci. 2005;6(7):507–520. doi:10.1038/nrn1701
3. Anand KJ, Hickey PR. Halothane-morphine compared with high-dose sufentanil for anesthesia and postoperative analgesia in neonatal cardiac surgery. N Engl J Med. 1992;326(1):1–9. doi:10.1056/NEJM199201023260101
4. Fortier MA, Chou J, Maurer EL, Kain ZN. Acute to chronic postoperative pain in children: preliminary findings. J Pediatr Surg. 2011;46(9):1700–1705. doi:10.1016/j.jpedsurg.2011.03.074
5. Chidambaran V, Ding L, Moore DL, et al. Predicting the pain continuum after adolescent idiopathic scoliosis surgery: a prospective cohort study. Eur J Pain. 2017;21(7):1252–1265. doi:10.1002/ejp.1025
6. Ranger M, Grunau RE. Early repetitive pain in preterm infants in relation to the developing brain. Pain Manag. 2014;4(1):57–67. doi:10.2217/pmt.13.61
7. Beggs S. Long-term consequences of neonatal injury. Can J Psychiatry. 2015;60(4):176–180. doi:10.1177/070674371506000404
8. Cunliffe M, Roberts SA. Pain management in children. Curr Anaesth Crit Care. 2004;15(4–5):272–283. doi:10.1016/j.cacc.2004.08.014
9. Vinall J, Grunau RE. Impact of repeated procedural pain-related stress in infants born very preterm. Pediatr Res. 2014;75(5):584–587. doi:10.1038/pr.2014.16
10. Fitzgerald M. What do we really know about newborn infant pain? Exp Physiol. 2015;100(12):1451–1457. doi:10.1113/EP085134
11. Wharton GT, Murphy MD, Avant D, et al. Impact of pediatric exclusivity on drug labeling and demonstrations of efficacy. Pediatrics. 2014;134(2):e512–e518. doi:10.1542/peds.2013-2987
12. The 2017 Commission Report on the Paediatric Regulation in the EU. State of paediatric medicines in the EU. 10 years of the EU Paediatric Regulation. Available from:
13. Weda M, Hoebert J, Vervloet M, et al. Study on off-label use of medicinal products in the European Union. European Union; 2017. Available from:
14.
15.
16. Tzschentke TM, Christoph T, Kögel BY. The mu-opioid receptor agonist/noradrenaline reuptake inhibition (MOR-NRI) concept in analgesia: the case of tapentadol. CNS Drugs. 2014;28:319–329. doi:10.1007/s40263-014-0151-9
17. Raffa RB, Elling C, Tzschentke TM. Does ‘strong analgesic’ equal ‘strong opioid’? Tapentadol and the concept of ‘µ-load'. Adv Ther. 2018;35(10):1471–1484.. doi:10.1007/s12325-018-0778-x
18. Göhler K, Brett M, Smit JW, Rengelshausen J, Terlinden R. Comparative pharmacokinetics and bioavailability of tapentadol following oral administration of immediate- and prolonged-release formulations. Int J Clin Pharmacol Ther. 2013;51(4):338–348. doi:10.5414/CP201722
19. Terlinden R, Kogel BY, Englberger W, Tzschentke TM. In vitro and in vivo characterization of tapentadol metabolites. Methods Find Exp Clin Pharmacol. 2010;32:31–38. doi:10.1358/mf.2010.32.1.1434165
20.
21.
22. Slater R, Cantarella A, Gallella S, et al. Cortical pain responses in human infants. J Neurosci. 2006;26(14):3662–3666. doi:10.1523/JNEUROSCI.0348-06.2006
23. Slater R, Worley A, Fabrizi L, et al. Evoked potentials generated by noxious stimulation in the human infant brain. Eur J Pain. 2010;14(3):321–326. doi:10.1016/j.ejpain.2009.05.005
24. Verriotis M, Fabrizi L, Lee A, Ledwidge S, Meek J, Fitzgerald M. Cortical activity evoked by inoculation needle prick in infants up to one-year old. Pain. 2015;156(2):222–230. doi:10.1097/01.j.pain.0000460302.56325.0c
25. Fitzgerald M, Walker SM. Infant pain management: a developmental neurobiological approach. Nat Clin Pract Neurol. 2009;5(1):35–50. doi:10.1038/ncpneuro0984
26. Barkovich AJ. Concepts of myelin and myelination in neuroradiology. Am J Neuroradiol. 2000;21(6):1099–1109.
27. Bremner LR, Fitzgerald M. Postnatal tuning of cutaneous inhibitory receptive fields in the rat. J Physiol. 2008;586(6):1529–1537. doi:10.1113/jphysiol.2007.145672
28. Attali B, Saya D, Vogel Z. Pre- and postnatal development of opiate receptor subtypes in rat spinal cord. Brain Res Dev Brain Res. 1990;53(1):97–102.
29. Georges F, Normand E, Bloch B, Le Moine C. Opioid receptor gene expression in the rat brain during ontogeny, with special reference to the mesostriatal system: an in situ hybridization study. Brain Res Dev Brain Res. 1998;109(2):187–199.
30. Nandi R, Fitzgerald M. Opioid analgesia in the newborn. Eur J Pain. 2005;9(2):105–108. doi:10.1016/j.ejpain.2004.05.005
31. Rahman W, Dashwood MR, Fitzgerald M, Aynsley-Green A, Dickenson AH. Postnatal development of multiple opioid receptors in the spinal cord and development of spinal morphine analgesia. Brain Res Dev Brain Res. 1998;108(1–2):239–254.
32. Bouwmeester NJ, Hop WC, van Dijk M, Anand KJ, van den Anker JN, Tibboel D. Postoperative pain in the neonate: age-related differences in morphine requirements and metabolism. Intensive Care Med. 2003;29(11):2009–2015. doi:10.1007/s00134-003-1899-4
33. Walker PC, Wagner DS. Treatment of pain in pediatric patients. J Pharm Pract. 2003;16(4):261–275. doi:10.1177/0897190003258505
34. Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, Riley JL
35. Musey PI
36. Holley AL, Wilson AC, Palermo TM. Predictors of the transition from acute to persistent musculoskeletal pain in children and adolescents: a prospective study. Pain. 2017;158(5):794–801. doi:10.1097/j.pain.0000000000000817
37.
38.
39.
40.
41.
42. Stevens BJ, Zempsky WT. Prevalence and distribution of pain in children. In: McGrath PJ, Stevens BJ, Walker SM, Zempsky WT, editors. Oxford Textbook of Paediatric Pain. Oxford:Oxford University Press; 2014:12–19.
43. Baarslag MA, Jhingoer S, Ista E, Allegaert K, Tibboel D, van Dijk M. How often do we perform painful and stressful procedures in the paediatric intensive care unit? A prospective observational study. Aust Crit Care. 2019;32(1):4–10. pii:S1036-7314(17)30500-3.
44. Stevens BJ, Abbott LK, Yamada J, et al. Epidemiology and management of painful procedures in children in Canadian hospitals. CMAJ. 2011;183(7):E403–E410. doi:10.1503/cmaj.101341
45. Stevens BJ, Harrison D, Rashotte J, et al. Pain assessment and intensity in hospitalized children in Canada. J Pain. 2012;13(9):857–865. doi:10.1016/j.jpain.2012.05.010
46. Kozlowski LJ, Kost-Byerly S, Colantuoni E, et al. Pain prevalence, intensity, assessment and management in a hospitalized pediatric population. Pain Manag Nurs. 2014;15(1):22–35. doi:10.1016/j.pmn.2012.04.003
47. Brummelte S, Grunau RE, Chau V, et al. Procedural pain and brain development in premature newborns. Ann Neurol. 2012;71(3):385–396. doi:10.1002/ana.22267
48. Zwicker JG, Grunau RE, Adams E, et al. Score for neonatal acute physiology-II and neonatal pain predict corticospinal tract development in premature newborns. Pediatr Neurol. 2013;48(2):123–129.e1. doi:10.1016/j.pediatrneurol.2012.10.016
49. Smith GC, Gutovich J, Smyser C, et al. Neonatal intensive care unit stress is associated with brain development in preterm infants. Ann Neurol. 2011;70(4):541–549. doi:10.1002/ana.22545
50. Grunau RE, Holsti L, Haley DW, et al. Neonatal procedural pain exposure predicts lower cortisol and behavioral reactivity in preterm infants in the NICU. Pain. 2005;113(3):293–300. doi:10.1016/j.pain.2004.10.020
51. Vinall J, Miller SP, Chau V, Brummelte S, Synnes AR, Grunau RE. Neonatal pain in relation to postnatal growth in infants born very preterm. Pain. 2012;153(7):1374–1381. doi:10.1016/j.pain.2012.02.007
52. Ranger M, Chau CM, Garg A, et al. Neonatal pain-related stress predicts cortical thickness at age 7 years in children born very preterm. PLoS ONE. 2013;8(10):e76702. doi:10.1371/journal.pone.0076702
53. Grunau RE, Whitfield MF, Petrie-Thomas J, et al. Neonatal pain, parenting stress and interaction, in relation to cognitive and motor development at 8 and 18 months in preterm infants. Pain. 2009;143(1–2):138–146. doi:10.1016/j.pain.2009.02.014
54. Ranger M, Zwicker JG, Chau CM, et al. Neonatal pain and infection relate to smaller cerebellum in very preterm children at school age. J Pediatr. 2015;167(2):292–298.e1. doi:10.1016/j.jpeds.2015.04.055
55. Doesburg SM, Chau CM, Cheung TP, et al. Neonatal pain-related stress, functional cortical activity and visual-perceptual abilities in school-age children born at extremely low gestational age. Pain. 2013;154(10):1946–1952. doi:10.1016/j.pain.2013.04.009
56. Duerden EG, Grunau RE, Guo T, et al. Early procedural pain is associated with regionally-specific alterations in thalamic development in preterm neonates. J Neurosci. 2018;38(4):878–886. doi:10.1523/JNEUROSCI.0867-17.2017
57. Vinall J, Miller SP, Bjornson BH, et al. Invasive procedures in preterm children: brain and cognitive development at school age. Pediatrics. 2014;133(3):412–421. doi:10.1542/peds.2013-1863
58. Grunau RE, Weinberg J, Whitfield MF. Neonatal procedural pain and preterm infant cortisol response to novelty at 8 months. Pediatrics. 2004;114(1):e77–e84.
59. Brummelte S, Chau CM, Cepeda IL, et al. Cortisol levels in former preterm children at school age are predicted by neonatal procedural pain-related stress. Psychoneuroendocrinology. 2015;51:151–163. doi:10.1016/j.psyneuen.2014.09.018
60. Vinall J, Miller SP, Synnes AR, Grunau RE. Parent behaviors moderate the relationship between neonatal pain and internalizing behaviors at 18 months corrected age in children born very prematurely. Pain. 2013;154:1831–1839.
61. Ranger M, Synnes AR, Vinall J, Grunau RE. Internalizing behaviours in school-age children born very preterm are predicted by neonatal pain and morphine exposure. Eur J Pain. 2014;18(6):844–852. doi:10.1002/j.1532-2149.2013.00431.x
62. Walker SM, Franck LS, Fitzgerald M, Myles J, Stocks J, Marlow N. Long-term impact of neonatal intensive care and surgery on somatosensory perception in children born extremely preterm. Pain. 2009;141(1–2):79–87. doi:10.1016/j.pain.2008.10.012
63. Hermann C, Hohmeister J, Demirakça S, Zohsel K, Flor H. Long-term alteration of pain sensitivity in school-aged children with early pain experiences. Pain. 2006;125(3):278–285. doi:10.1016/j.pain.2006.08.026
64. Taddio A, Goldbach M, Ipp M, Stevens B, Koren G. Effect of neonatal circumcision on pain responses during vaccination in boys. Lancet. 1995;345:291–292.
65. Kassab M, Hamadneh S, Nuseir K, ALmomani B, Hamadneh J. Factors associated with infant pain severity undergoing immunization injections. J Pediatr Nurs. 2018;42:e85–e90. doi:10.1016/j.pedn.2018.04.002
66. Taddio A, Shah V, Atenafu E, Katz J. Influence of repeated painful procedures and sucrose analgesia on the development of hyperalgesia in newborn infants. Pain. 2009;144(1–2):43–48. doi:10.1016/j.pain.2009.02.012
67. Kristensen AD, Pedersen TA, Hjortdal VE, Jensen TS, Nikolajsen L. Chronic pain in adults after thoracotomy in childhood or youth. Br J Anaesth. 2010;104(1):75–79. doi:10.1093/bja/aep317
68. Williams G, Howard RF, Liossi C. Persistent postsurgical pain in children and young people: prediction, prevention, and management. Pain Rep. 2017;2(5):e616. doi:10.1097/PR9.0000000000000616
69. Pagé MG, Stinson J, Campbell F, Isaac L, Katz J. Identification of pain-related psychological risk factors for the development and maintenance of pediatric chronic postsurgical pain. J Pain Res. 2013;6:167–180. doi:10.2147/JPR.S40846
70. Aasvang EK, Kehlet H. Chronic pain after childhood groin hernia repair. J Pediatr Surg. 2007;42(8):1403–1408. doi:10.1016/j.jpedsurg.2007.03.042
71. Jones K, Nordstokke D, Wilcox G, Schroeder M, Noel M. The ‘work of childhood’: understanding school functioning in youth with chronic pain. Pain Manag. 2018;8(2):139–153. doi:10.2217/pmt-2017-0048
72. Cáceres-Matos R, Gil-García E, Barrientos-Trigo S, Molina E, Porcel-Gálvez AM. Consequences of chronic pain in childhood and adolescence. [Article in Spanish]. Gac Sanit. Epub 2018 Feb 13;pii:S0213-9111(18)30004-9.
73.
74. Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain. 2016;17(2):131–157. doi:10.1016/j.jpain.2015.12.008
75.
76. Batton DG, Barrington KJ, Wallman C;
77.
78. Anand KJ. International Evidence-Based Group for Neonatal Pain. Consensus statement for the prevention and management of pain in the newborn. Arch Pediatr Adolesc Med. 2001;155(2):173–180.
79. Lago P, Garetti E, Merazzi D, et al. Guidelines for procedural pain in the newborn. Acta Paediatr. 2009;98(6):932–939.
80. Taddio A, Appleton M, Bortolussi R, et al. Reducing the pain of childhood vaccination: an evidence-based clinical practice guideline. CMAJ. 2010;182(18):E843–E855. doi:10.1503/cmaj.092053
81. Spence K, Henderson-Smart D, New K, Evans C, Whitelaw J, Woolnough R. Evidenced based clinical practice guideline for management of newborn pain. J Paediatr Child Health. 2010;46(4):184–192. doi:10.1111/j.1440-1754.2009.01659.x
82. Pillai Riddell RR, Racine NM, Gennis HG, et al. Non-pharmacological management of infant and young child procedural pain. Cochrane Database Syst Rev. 2015;(12):CD006275. Available from: https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD006275.pub3/full. Accessed April 02, 2019.
83. Johnston C, Campbell-Yeo M, Disher T, et al. Skin-to-skin care for procedural pain in neonates. Cochrane Database Syst Rev. 2017;2:CD008435.
84. Riddell RP, O’Neill MC, Campbell L, Taddio A, Greenberg S, Garfield H. Featured article: the ABCDs of pain management: a double-blind randomized controlled trial examining the impact of a brief educational video on infants’ and toddlers’ pain scores and parent soothing behavior. J Pediatr Psychol. 2018;43(3):224–233. doi:10.1093/jpepsy/jsx122
85. Taddio A, Riddell RP, Ipp M, et al. Relative effectiveness of additive pain interventions during vaccination in infants. CMAJ. 2017;189(6):E227–E234. doi:10.1503/cmaj.171389
86. Boric K, Dosenovic S, Jelicic Kadic A, et al. Interventions for postoperative pain in children: an overview of systematic reviews. Paediatr Anaesth. 2017;27(9):893–904. doi:10.1111/pan.13203
87. Ceelie I, de Wildt SN, van Dijk M, et al. Effect of intravenous paracetamol on postoperative morphine requirements in neonates and infants undergoing major noncardiac surgery: a randomized controlled trial. JAMA. 2013;309(2):149–154. doi:10.1001/jama.2012.148050
88. van den Anker JN, Allegaert K. Treating pain in preterm infants: moving from opioids to acetaminophen. J Pediatr. 2016;168:13–15. doi:10.1016/j.jpeds.2015.09.061
89. van den Anker JN, Allegaert K. Acetaminophen in the neonatal intensive care unit: shotgun approach or silver bullet. J Pediatr. 2018;198:10–11. doi:10.1016/j.jpeds.2018.02.046
90. Grégoire MC, Finley GA. Drugs for chronic pain in children: a commentary on clinical practice and the absence of evidence. Pain Res Manag. 2013;18(1):47–50.
91. Beuter C, Eerdekens M. Analgesia in children: is there still a need for novel approved medications?
92.
93. Kötter T, Da Costa BR, Fässler M, et al. Metamizole-associated adverse events: a systematic review and meta-analysis. PLoS One. 2015;10(4):e0122918. doi:10.1371/journal.pone.0122918
94.
95.
96.
97.
98.
99.
100.
101.
102.
103.
104.
105.
106.
107.
108.
109.
110. de Leeuw TG, Dirckx M, Gonzalez Candel A, Scoones GP, Huygen FJPM, de Wildt SN. The use of dipyrone (metamizol) as an analgesic in children: what is the evidence? A review. Paediatr Anaesth. 2017;27(12):1193–1201. doi:10.1111/pan.13257
111. Ziesenitz VC, Erb TO, Trachsel D, van den Anker JN. Safety of dipyrone (metamizole) in children - What’s the risk of agranulocytosis? Paediatr Anaesth. 2018;28(2):186–187. doi:10.1111/pan.13312
112.
113.
114.
115.
116.
117.
118.
119. Lepola P, Needham A, Mendum J, Sallabank P, Neubauer D, de Wildt S. Informed consent for paediatric clinical trials in Europe. Arch Dis Child. 2016;101(11):1017–1025. doi:10.1136/archdischild-2015-310001
120. Wang Y, Jadhav PR, Lala M, Gobburu JV. Clarification on precision criteria to derive sample size when designing pediatric pharmacokinetic studies. J Clin Pharmacol. 2012;52(10):1601–1606. doi:10.1177/0091270011422812
121. Pandya H, Spooner N, Mulla H. Dried blood spots, pharmacokinetic studies and better medicines for children. Bioanalysis. 2011;3(7):779–786. doi:10.4155/bio.11.19
122. van den Anker J, Reed MD, Allegaert K, Kearns GL. Developmental changes in pharmacokinetics and pharmacodynamics. J Clin Pharmacol. 2018;58(Suppl 10):S10–S25. doi:10.1002/jcph.1284
123. Anand KJ, Aranda JV, Berde CB, et al. Analgesia and anesthesia for neonates: study design and ethical issues. Clin Ther. 2005;27(6):814–843. doi:10.1016/j.clinthera.2005.06.021
124. Johnston CC, Stevens B, Craig KD, Grunau RV. Developmental changes in pain expression in premature, full-term, two- and four-month-old infants. Pain. 1993;52(2):201–208.
125. Craig KD, Whitfield MF, Grunau RV, Linton J, Hadjistavropoulos HD. Pain in the preterm neonate: behavioural and physiological indices. Pain. 1993;52(3):287–299.
126. Edginton AN, Schmitt W, Voith B, Willmann S. A mechanistic approach for the scaling of clearance in children. Clin Pharmacokinet. 2006;45(7):683–704. doi:10.2165/00003088-200645070-00004
127.
128. Walco GA, Kopecky EA, Weisman SJ, et al. Clinical trial designs and models for analgesic medications for acute pain in neonates, infants, toddlers, children, and adolescents: ACTTION recommendations. Pain. 2018;159(2):193–205. doi:10.1097/j.pain.0000000000001104
129. Singla NK, Desjardins PJ, Chang PD. A comparison of the clinical and experimental characteristics of four acute surgical pain models: dental extraction, bunionectomy, joint replacement, and soft tissue surgery. Pain. 2014;155(3):441–456. doi:10.1016/j.pain.2013.09.002
130. McGrath PJ, Walco GA, Turk DC, et al. Core outcome domains and measures for pediatric acute and chronic/recurrent pain clinical trials: pedIMMPACT recommendations. J Pain. 2008;9(9):771–783. doi:10.1016/j.jpain.2008.04.007
131. Baarslag MA, Allegaert K, Van Den Anker JN, et al. Paracetamol and morphine for infant and neonatal pain; still a long way to go? Expert Rev Clin Pharmacol. 2017;10(1):111–126. doi:10.1080/17512433.2017.1254040
132. Arias MC, Guinsburg R. Differences between uni-and multidimensional scales for assessing pain in term newborn infants at the bedside. Clinics (Sao Paulo). 2012;67(10):1165–1170.
133. Stevens B, Johnston C, Petryshen P, Taddio A. Premature infant profile development and initial validation. Clin J Pain. 1996;12:13–22.
134. Merkel SI, Voepel-Lewis T, Shayevitz JR, Malviya S. The FLACC: a behavioral scale for scoring postoperative pain in young children. Pediatr Nurs. 1997;23(3):293–297.
135. Hicks CL, von Baeyer CL, Spafford PA, van Korlaar I, Goodenough B. The faces pain scale-revised: toward a common metric in pediatric pain measurement. Pain. 2001;93(2):173–183.
136. Scott P, Ansell B, Huskisson E. Measurement of pain in juvenile chronic polyarthritis. Ann Rheum Dis. 1977;36:186–187.
137. von Baeyer CL, Forsyth SJ, Stanford EA, Watson M, Chambers CT. Response biases in preschool children’s ratings of pain in hypothetical situations. Eur J Pain. 2009;13(2):209–213. doi:10.1016/j.ejpain.2008.03.017
138. Berde CB, Walco GA, Krane EJ, et al. Pediatric analgesic clinical trial designs, measures, and extrapolation: report of an FDA scientific workshop. Pediatrics. 2012;129(2):354–364. doi:10.1542/peds.2010-3591
139. Rusy LM, Hainsworth KR, Nelson TJ, et al. Gabapentin use in pediatric spinal fusion patients: a randomized, double-blind, controlled trial. Anesth Analg. 2010;110(5):1393–1398. doi:10.1213/ANE.0b013e3181d41dc2
140. Rugyte D, Kokki H. Intravenous ketoprofen as an adjunct to patient-controlled analgesia morphine in adolescents with thoracic surgery: a placebo controlled double-blinded study. Eur J Pain. 2007;11(6):694–699. doi:10.1016/j.ejpain.2006.11.001
141. Wong I, St John-Green C, Walker SM. Opioid-sparing effects of perioperative paracetamol and nonsteroidal anti-inflammatory drugs (NSAIDs) in children. Pediatr Anesth. 2013;23:475–495. doi:10.1111/pan.12163
142. Kossowsky J, Donado C, Berde CB. Immediate rescue designs in pediatric analgesic trials: a systematic review and meta analysis. Anesthesiology. 2015;122(1):150–171. doi:10.1097/ALN.0000000000000445
143. Lam J, Koren G. P-glycoprotein in the developing human brain: a review of the effects of ontogeny on the safety of opioids in neonates. Ther Drug Monit. 2014;36(6):699–705. doi:10.1097/FTD.0000000000000087
144. Lam J, Baello S, Iqbal M, et al. The ontogeny of P-glycoprotein in the developing human blood brain barrier: implication for opioid toxicity in neonates. Pediatr Res. 2015;78(4):417–421. doi:10.1038/pr.2015.119
145.
146. Ivanovska V, Rademaker CMA, Dijk L, Mantel-Teeuwisse AK. Pediatric drug formulations: a review of challenges and progress. Pediatrics. 2014;134:361–372. doi:10.1542/peds.2013-3225
147.
148.
149.
150. Barrow PC, Schmitt G. Juvenile nonclinical safety studies in support of pediatric drug development. In: Gautier JC, editor. Drug Safety Evaluation. Methods and Protocols. Humana Press, Springer; 2017:25–67.
151. Walker SM, Fitzgerald M. Characterization of spinal alpha-adrenergic modulation of nociceptive transmission and hyperalgesia throughout postnatal development in rats. Br J Pharmacol. 2007;151(8):1334–1342. doi:10.1038/sj.bjp.0707290
152. Hathway GJ, Koch S, Low L, Fitzgerald M. The changing balance of brainstem-spinal cord modulation of pain processing over the first weeks of rat postnatal life. J Physiol. 2009;587(Pt 12):2927–2935. doi:10.1113/jphysiol.2008.168013
153. Schwaller F, Kanellopoulos AH, Fitzgerald M. The developmental emergence of differential brainstem serotonergic control of the sensory spinal cord. Sci Rep. 2017;7(1):2215. doi:10.1038/s41598-017-02509-2
154.
155.
156.
157.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
