Back to Journals » Infection and Drug Resistance » Volume 15
The Antimicrobial Efficacy Against Selective Oral Microbes, Antioxidant Activity and Preliminary Phytochemical Screening of Zingiber officinale
Authors Ahmed N , Karobari MI , Yousaf A, Mohamed RN, Arshad S, Basheer SN, Peeran SW, Noorani TY, Assiry AA, Alharbi AS, Yean CY
Received 1 March 2022
Accepted for publication 17 May 2022
Published 31 May 2022 Volume 2022:15 Pages 2773—2785
DOI https://doi.org/10.2147/IDR.S364175
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Naveed Ahmed,1 Mohmed Isaqali Karobari,2– 4 Anam Yousaf,5 Roshan Noor Mohamed,6 Sohaib Arshad,7 Syed Nahid Basheer,8 Syed Wali Peeran,9 Tahir Yusuf Noorani,4 Ali A Assiry,10 Abdulaziz S Alharbi,11 Chan Yean Yean1
1Department of Medical Microbiology and Parasitology, School of Medical Sciences, Universiti Sains Malaysia, Kubang Kerian, 16150, Malaysia; 2Department of Restorative Dentistry & Endodontics, Faculty of Dentistry, University of Puthisastra, Phnom Penh, 12211, Cambodia; 3Center for Transdisciplinary Research (CFTR), Saveetha Dental College & Hospitals, Saveetha Institute of Medical and Technical Sciences University, Chennai, Tamil Nadu, 600077, India; 4Conservative Dentistry Unit, School of Dental Sciences, Universiti Sains Malaysia, Health Campus, Kota Bharu, Kelantan, 16150, Malaysia; 5Department of Pathology Laboratory, Pakistan Kidney and Liver Institute & Research Center, Lahore, Pakistan; 6Department of Pediatric Dentistry, Faculty of Dentistry, Taif University, Taif, 21944, Saudi Arabia; 7Periodontics Unit, School of Dental Sciences, Health Campus, Universiti Sains Malaysia, Kubang Kerian, Kelantan, 16150, Malaysia; 8Division of Operative Dentistry, Department of Restorative Dental Sciences, College of Dentistry, Jazan University, Jazan, Saudi Arabia; 9Department of Periodontics, Armed Forces Hospital Jizan, Jizan, Kingdom of Saudi Arabia; 10Preventive Dental Science Department, Faculty of Dentistry, Najran University, Najran, Kingdom of Saudi Arabia; 11Saudi Board of Pediatric Dentistry (SB-PD), King Fahad Military Medical Complex – KFMMC, Dammam, Saudi Arabia
Correspondence: Mohmed Isaqali Karobari, Department of Restorative Dentistry & Endodontics, Faculty of Dentistry, University of Puthisastra, Phnom Penh, 12211, Cambodia, Email [email protected] Chan Yean Yean, Department of Medical Microbiology and Parasitology, School of Medical Sciences, Universiti Sains Malaysia, Kubang Kerian, 16150, Malaysia, Email [email protected]
Abstract:
Introduction: Ginger (Zingiber officinale) has been one of the most commonly consumed herbal medicines for a long time to treat several common diseases. Antibacterial activity, antioxidant properties and many bioactive compounds in ginger have been identified previously, which could be used as an alternative method to treat many infectious diseases.
Methods: The current study evaluates ginger’s biochemical profile using qualitative and quantitative analysis and its bioactive potentials using antioxidant and antimicrobial assays against Streptococcus mutans and selective oral microbes. HPLC analysis was performed for the quantitative analysis. DPPH and disc diffusion assays were used for antioxidant and antimicrobial activities. The antimicrobial activity was checked against Streptococcus mutans, Enterococcus faecalis, Staphylococcus spp., and Lactobacillus spp. All solvents were removed by rotary evaporation before testing the dried extracts.
Results: The observed IC50 value showed that distilled water extract exhibited the highest antioxidant activity (43.9), followed by ethanol extract (52.4), and the lowest activity was observed in n-butanol extract (91.2) and n-hexane (90.6). Different plant extracts have shown significant antibacterial activity (p = 0.001) against each bacterium. The highest antibacterial activity against tested bacteria was observed in n-hexane, chloroform and ethanol extracts. In comparison, the ethyl acetate, n-butanol and water extracts showed low antibacterial activity.
Conclusion: This study emphasizes that Zingiber officinale (Z. officinale) against Gram-positive bacteria is an effective antimicrobial herb. Furthermore, it can be used as a potential natural source of antioxidants. Further studies on the toxicity analysis of ginger are recommended.
Keywords: Streptococcus mutans, ginger, dental, oral microbes, herbal medicine, medicinal plants, healthy food
Introduction
Ginger (Zingiber officinale) is a flowering plant that originated and is commonly grown in India, China, Southeast Asia, the West Indies, Mexico, and many other regions throughout the world.1 Ginger is among the healthiest and most delicious foods. The rhizome (underground part of the stem) is commonly used as a spice. Apart from its use as spices, it is one of the most utilized herbs in traditional medicine. It belongs to the Zingiberaceae family and is closely related to turmeric, cardamom, and galangal. Ginger is used as a spice and flavouring agent worldwide, and it is well-identified for a variety of health benefits, including pharmacological effects, antioxidant, antibacterial, anti-inflammatory, anti-nociceptive, anti-mutagenic, and hepatoprotective.2–4
Phenolic and terpene molecules are among the main active ingredients of ginger. The phenolic constituents of ginger include paradols, shogaols, and gingerols. The most abundant phenolic compounds in fresh ginger are gingerols, such as 6-gingerol, 8-gingerol, and 10-gingerol.5 Through heat treatment or long-term preservation, the gingerols could be converted to matching shogaols. After the hydrogenation, these shogaols could be converted to paradols.6 Quercetin, gingerenone-A, zingerone, and 6-dehydrogingerdione are the phenolic compound also found in ginger. Furthermore, ginger contains various terpene components, including α-curcumene, α-farnesene, β-bisabolene, β-sesquiphellandrene, and zingiberene, which are the significant ingredients of ginger essential oils.7 In addition to these, ginger contains lipids, polysaccharides, raw fibres and organic acids.8
The oxidation of biological molecules has been associated with many pathological events such as Parkinson’s disease carcinogenesis, atherogenesis and ageing.9 Oxidative stress occurs when the natural antioxidant capacity of cells is reduced or when the number of reactive oxygen species in organisms increases.10 It is generally known that free radicals are linked to cell degeneration, particularly in the brain. Consumption of foods high in antioxidants and phytochemicals, on the other hand, may aid in the prevention of degenerative illnesses induced by oxidative stress by increasing the body’s antioxidant state.7,11 The ginger bioactive, such as zingiberene, zingerone, shogaols, and gingerols, are responsible for its antioxidant activity.8
Phytotherapy has emerged as an acceptable choice for preventing and treating many bacterial infections.12 The rhizome (root) of ginger is widely used in medicine to treat a variety of diseases, including diabetes, gastrointestinal ulcers, vomiting, nausea, diarrhoea, fever, rheumatoid arthritis, arterial problems, xerostomia, migraine headaches, different type of cancer, sore throat, and some respiratory diseases.13
Many gram-positive bacteria of the oral microbiome are involved in oral infections.1,14 Caries are most frequently associated with Streptococcus mutans (S. mutans) and Lactobacillus species.15 S. mutans is a facultatively anaerobic and Gram-positive bacterium commonly found in the human oral cavity and is one of the leading causes of dental caries. Acidogenicity, adhesion, and acid tolerance are the most important virulence factors linked to cariogenicity.16,17 Each of these features interacts with others to change the ecology of dental plaque. The synthesis of mutacins (bacteriocins) by S. mutans is considered a key element in the colonization and establishment of dental biofilms.18 The antibacterial activity of ginger essential oils (ethanolic extract) against various bacteria, including E. coli and S. typhi, has been proven previously.17
The World Health Organization (WHO) believes that 80% of the world’s population still uses traditional medicine, which mainly includes the usage of plant-based medications.9 In Indian traditional medicine, Zingiber officinale is one of the most used herbs. Specific elements in this plant can also serve as antioxidants, which a substances that can stop or prolong the oxidation processes in the human body.19 Keeping in mind the biochemical/phenolic components, antioxidant and antibacterial properties of ginger, and to motivate the usage of ginger at the household level, the current study was conducted to prove its biochemical profile, antioxidant activity and antimicrobial efficacy against particular oral microbes.
Materials and Methods
Ethical Clearance
Ethical approval was obtained from the Institutional review board of Saveetha University, Chennai, Tamil Nadu, India, with ethical clearance code: IHEC-SDC-FACULTY/21/ENDO/199.
Extraction and Fractionation
The fresh ginger plant was collected from a local market in Chennai, India. The plant was identified taxonomically at the Department of Pharmacology, Saveetha Dental College (SDC), Saveetha Institute of Medical and Technical Science (SIMTS), and Saveetha University (SU) Chennai, India. After collection, the rhizomes wash thoroughly with distilled water, shade dried, and identified by the Herbarium, where a voucher (HA#270916) specimen of plant species was deposited for further reference. Ten kilograms (10 KG) of Ginger (Z. officinale) was cleaned with sterilized distilled water to eliminate the unwanted contaminants and dust. Then it was dried at room temperature (25℃) for two weeks. Post-drying grinding was done using a grinder (Zhengzhou Tamok Machinery Co., Ltd. Henan, China) until a fine powder was obtained, spread on a cloth to dry under shade for 30 days. After drying, a total of 400 grams of powder was obtained, from which 50 grams were weighed separately and proceeded further by mixing in 150 mL of each polar solvents, including 95% ethanol, HPLC grade chloroform, and ethyl acetate n-hexane, n-butanol, and distilled water. The mixture was shaken with hands daily and kept for 15 days. After 15 days, each mixture was filtered via Whatman filter paper, and the filtrates were transferred into new glass bottles. The filtrates were then evaporated using a rotary evaporator (Westlab, Mitchell Park, Australia), after which a semi-solid form of the extract was obtained.20,21 The weight of each crude extract left behind was 3 to 4 grams. The dried extracts were transferred to separated glass tubes and stored at 2 to 8℃.
Preliminary Phytochemical Screening (Qualitative Analysis)
The preliminary phytochemical analysis was performed to check the presence of alkaloids, tannins, glycosides, flavonoids, terpenoids, and quinones. Alkaloid’s determination was done using Wagner’s test.22 To check the presence of saponins, 5 mg of extract (powder) was weighed and added to 6 test tubes separately, and then 20 mL of 95% ethanol was added to each test tube. The tubes were placed on a shaking incubator (Merck, New Jersey, United States) for 15 min. The appearance of soap indicated the presence of saponin in the tested sample.23 To check the presence of tannin, 6 sample tubes were loaded with 5 mg extract (powder) and 5 mL of 95% ethanol separately, and each extract was encumbered with a few drops of 5% ferric chloride. Blue or dark colour precipitation indicated that tannins were present in the tested sample.23
The flavonoids were determined using the extract with 6 different solvents separately, and 3 mL of sodium hydroxide was added to each test tube. The mixture was stirred gently to produce the colour, and the disappearance of the colour indicated the presence of flavonoids.22 For terpenoids, extracts obtained using different solvents were dissolved in 2 mL of chloroform (pure), and then 2 mL of concentrated H2SO4 (5 M) was added. The formation of the red-brown precipitate indicated the presence of the terpenoids.24 For glycosides, a few drops of 2% ferric chloride, 2 mL of 95% glacial acetic acid, and 1 mL H2SO4 were added in 6 test tubes containing 5 mg extract and 5 ml of 6 different solvents, respectively. Glycoside presence was confirmed by forming the brown or violet coloured ring just at the interface of the two liquids.25 For Quinone, a few drops of concentrated H2SO4 were added in 6 different extracts separately. The formation of red precipitates confirmed the presence of quinones.26
Total Phenolic Contents Determination (TPC)
Folin-Ciocalteu (FC) assessment was used for the TPC evaluation.27,28 The prepared individual extracts (1 g) were placed in separate test tubes, thoroughly mixed with 5 mL of FC reagent, and then kept for 5 minutes at room temperature. After 5 minutes, 4 mL of sodium carbonate (Na2CO3) was added to each of the tubes and allowed to react at room temperature for 2 hours. After 2 hours, the absorbance was determined by UV-VIS Spectrophotometry at 760 nm absorbance.
Antioxidant Assay
Free Radical Scavenging Activity by DPPH
The antioxidant activity was determined using the free radical scavenging activity of the 2,2-Diphenyl-1 picrylhydrazyl (DPPH).29 At first, the DPPH solution (0.1 mM) was dissolved in 82% of solvent (ethanol). Then, 100 mg of each plant extract was mixed into 1 ml of 10% DMSO as a stock solution, and 7 different concentrations (0.0125, 0.025, 0.05, 0.1, 0.5 and 1.0 mg/ml) were prepared from it. Two hundred (200) µL of this mixture proceeded for the absorbance using a UV double beam spectrophotometer at a wavelength of 517 nm in triplicates at an interval of 20, 40, and 60 min. In this assay, Ascorbic acid was used as a standard. A blank test was also prepared, having only water instead of the sample. The results were expressed by IC50 values (mg/mL). The IC50 values are inversely proportional to the antioxidant activity, ie, the lowest IC50 value will show higher antioxidant activity.30 To measure the percentage of radical scavenging activity, the following formula was used:
% Radical scavenging activity = A0 - A1/A0 × 100
Where A0=The absorbance of blank and A1= The absorbance of the sample.
Antibacterial Activity
The oral swabs were collected from the infected patients and inoculated on blood agar plates. After the inoculation of samples, the agar plates were incubated for 24–48 hours at 37℃. Then the agar plates were checked for the presence of bacterial growth. The bacterial species were identified using standard identification protocols (colonial characteristics, microscopic and biochemical identification).31,32
After the colonies of Streptococcus mutans, Enterococcus faecalis, Staphylococcus spp., and Lactobacillus spp. were identified, these were grown in 5 ml Luria-Bertani (LB) broth overnight and incubated in a shaking incubator at 37℃. Hundred (100) µL from the overnight bacterial culture suspension were transferred onto the blood agar plates and spread (lawn). At first, the direct sensitivity of Ciprofloxacin (CIP, 5 µg), Chloramphenicol (C, 30 µg), Tetracycline (TE, 30 µg), and Vancomycin (VA, 30 µg) was tested without any ginger extract. To check the sensitivity using ginger extracts, the filter paper discs were soaked in each of the prepared extracts to absorb the adequate quantity of these extracts and were then placed on the second inoculated agar plate. After placing discs on the agar plates, these were incubated for 24 hrs at 37℃.33 The filter paper discs containing 1% Dimethyl sulfoxide (DMSO) were used as a negative control. After incubation, the zone of inhibitions around filter paper discs was measured. A zone of inhibition indicated the activity as sensitive or resistant. For CIP and C, a zone diameter of ≥ 21 mm was considered sensitive, and < 21 was considered resistant. Similarly, for TE and VA, sensitive zones were ≥ 23 and ≥ 17 mm, respectively. The zone of inhibitions was followed by the Clinical Laboratory Standards Institute’s guidelines (2017) for antibacterial testing.34 The zone of inhibitions after direct sensitivity and sensitivity testing after the ginger extracts were compared statistically.
Hemolytic Activity
A blood sample (5 ml) in EDTA containing vial was collected from a healthy individual. The sample was centrifuged for 3 min at 1500 rpm to obtain the concentrated cells. After centrifugation, the supernatant (Plasma) was discarded and used the sterile phosphate buffer saline solution (pH 7.2 ± 0.2); the pellet was washed 3 times by centrifugation for 5 min at 1500 rpm. After the washing, the cells were resuspended in normal saline to be used for further testing. In each plant extract (0.5 ml), 0.5 ml of the cell suspension was mixed and incubated at 37℃ for 30 min. After the incubation period, the mixture was centrifuged for 10 min at 1500 rpm. Finally, the free haemoglobin (Hb) in the supernatants was measured using UV-Vis Spectrophotometer at the wavelength of 540 nm.35 Triton X-100 (0.1%) was used as a standard lysis buffer with the 99% lysis of red blood cells.
HPLC Analysis
Extracts were analyzed for qualitative phytochemical analysis using HPLC analysis. Total phenolic contents were estimated using the protocol defined in a previous study.36 Different standard fractions of ginger contain various phenolic compounds, ie, sinapic acid, Quercetin, cinnamic acid, chlorogenic acid, p-coumaric acid, gallic acid, vanillic acid, meta coumaric acid, syringic acid, caffeic acid, and benzoic acid were tested. 5 mg extract was dissolved in 1 mL of 95% ethanol and filtered through a 0.2 µm syringe filter. The filtrate is then added to the HPLC vials, screwed with a cap, and placed in the HPLC machine (Thermo Fisher Scientific, USA). HPLC analysis was performed in the HPLC LC-10A series equipped with liquid pump LS-10AS, C-18 column (CLC-ODS 25 cm×4.6 mm 5 μm), and a fluorescence UV-visible detector (280 nm). The column temperature was maintained at 25℃. A mobile phase containing ethanol and water with a ratio of 95:1 was used with a flow rate of 1 mL per minute. The compounds in HPLC were resolved using isocratic elution techniques. CSW32 Chromatography Station (DataApex, Prague, The Czech Republic) software was used to acquire, process and evaluate data. The retention time, area (%) and concentration (ppm) were measured using the ISTD2 method.
Identification of phenolic and flavanols was carried out by comparing their retention times with those of reference standards (Sigma Chemicals Co., St Louis, MO, USA). Quantitative determination was carried out using calibration curves of the standards. The HPLC analysis separated the phenolic and flavonoid (standard) compounds with retention time (RT) of peak noted. The retention time was used to qualitatively pick a peak from the analyte with equal or similar retention time to the standard time. The HPLC analytical instrument was able to detect the presence of phenolic and flavonoid compounds that lie within the run time of the developed method or exhibited properties similar to the standards and thus eluted at the same retention time or close.
Statistical Analysis
The statistical analyses were performed using SPSS (Statistical Package for the Social Sciences) software version 26. Means and standard deviations (SD) were used to summarise continuous data. A paired samples t-Test was run to check the significance between biochemical tests v/s different extracts, DPPH scavenging activity at different concentrations, and tested bacteria v/s antibiotic susceptibility patterns. In the statistical analysis, p < 0.05 was considered significant.
Results
Screening of Phytochemicals
The predominant phytoconstituents of the ethanolic extract contain alkaloids, flavonoids, saponin, and terpenoids, whereas n-hexane extracts contain alkaloids and tannins, saponin and flavonoids. Alkaloids were present in all fractions. The phytochemical reactions against each extract have been shown in Table 1.
 |
Table 1 Phytochemical Analysis of Various Extracts of Ginger |
The Phenolic Contents
The total phenolic contents (TPC) present in ginger extracts showed varied content, as shown in Table 2. Water extract has shown higher TPC (75.66 mg/g of GAE +/-) while the chloroform extract showed comparatively low TPC (15.66 mg/g of GAE). The results showed significantly higher TPC in the aqueous extracts compared to the chloroform extracts.
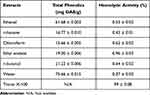 |
Table 2 Total Phenolic Contents and Hemolytic Activity of Ginger Extracts |
Free Radical Scavenging Activity by Using DPPH
Overall, the highest antioxidant activity was found in distilled water and ethanol extracts at different tested concentrations. The lowest concentration (0.025 mg/ml) has shown the highest antioxidant activity as compared to the higher concentration (0.05, 0.01, 0.25, 0.5 and 1 mg/ml). At the concentration of 0.025 mg/ml, the highest antioxidant activity was noted in ethyl acetate (4.69 mg/ml) followed by distilled water (7.06 mg/ml), ethanol (10.04 mg/ml), chloroform (10.3 mg/ml), n-butanol (10.5 mg/ml), and n-hexane (16.2 mg/ml) extracts. All tested extracts have shown the least antioxidant activity at a concentration of 1.0.
The Ascorbic acid has shown the highest antioxidant activity at all tested concentrations, proving the test’s accuracy. Compared with the standard (Ascorbic acid), all other extracts have shown a significant difference (p < 0.05). The scavenging activity of each extract is shown in Figure 1.
 |
Figure 1 Graphical representation of DPPH radical scavenging activity in ginger extracts. Values are expressed as mean ± standard deviation. Ascorbic acid was used as a standard. |
Antibacterial Activity
Among the tested bacterial isolates, Streptococcus mutans showed sensitivity against all tested antibiotics, while Enterococcus faecalis showed resistance against CIP in n-butanol, ethyl acetate, and water extracts. Staphylococcus spp. Showed resistance against C in Ethanol extracts and CIP in water extracts. Lactobacillus spp. Showed resistance against CIP in n-hexane and water extracts while resistance against C in ethanol extracts. Chloroform and ethyl acetate extracts showed the highest antibacterial activity against Lactobacillus spp. and the least antibacterial activity against n-hexane extracts. None of the tested bacterial isolates was resistant to TE and VA among all extracts. No inhibition zone for antibiotics was found while checking the activity of the tested organism in extracts containing 1% DMSO. A significant correlation (p = 0.001) was observed between the tested bacteria and their antibiotic patterns. The antibacterial activities of tested bacteria against different antibiotics (with the treatment of different extracts) have been shown in Figure 2.
 |
Figure 2 Antibacterial activity of different extracts against different bacteria. |
Hemolytic Activity
Ginger showed potent hemolytic activity against human red blood cells, as summarized in Table 2. Hemolysis is the breakdown of heme protein present in red blood cells. Among all tested extracts, the ethyl acetate extract showed higher hemolytic activity (0.96%), while the water extract showed low hemolytic activity (0.37%). The hemolytic activity of all ginger extracts has shown that the ginger is non-toxic to human erythrocytes.
Identification of Phenolic Acid in Different Sub-Fraction of Ginger by HPLC
Ethanol fraction showed the highest concentrations of cinnamic acid. Syringic acid in water and n-hexane, P-coumaric acid in n-butanol, Ferulic acid in Ethyl acetate, and caffeic acid in chloroform extracts were found in the highest concentration. Quercetin was found unanimously in all the fractions with varying concentrations. A high concentration of Quercetin was observed in the fraction of water and ethanol because of their polarity, while chloroform showed a comparatively low concentration of Quercetin. The retention time, area, and concentration of each phenolic and flavonoid component have been shown in Table 3. Figures S1‒S6 show a representative chromatogram of phenolic and flavonoid for Z. officinale extracts.
 |
Table 3 HPLC Analysis of Ginger Extracts |
Discussion
The most prevalent disorders in dentistry are dental caries and periodontal diseases. On the other hand, dental caries is the most common infectious disease of bacterial origin, with S. mutans as the primary causative organism.9,32 The rise in antibiotic-resistant strains and concerns about antibiotic usage has prompted the need for new novel antimicrobial agents or parallel ways of treatment.37 The current study was designed to perform a preliminary phytochemical screening of ginger using qualitative and quantitative analysis of different extracts and evaluate its bioactive potentials using enzymatic, antioxidant, and antimicrobial assays. HPLC analysis was performed for the quantitative analysis, and DPPH and disc diffusion assays were used for antioxidant and antimicrobial activities, respectively. The antibacterial impact of different ginger extracts concentrations in-vitro was tested against S. mutans, E. faecalis, Staphylococcus spp., and Lactobacillus spp.
In the latest previous in-vivo and in-vitro lab-based studies, the antioxidant properties of ginger and its components have been investigated, which showed significant antioxidant properties.8,22,37 Ginger extract has been proven to have antioxidant properties in tested animals.38,39 The current study showed the highest antioxidant activity in distilled water and ethanol extracts. Ascorbic acid has proceeded as a control, showing the highest antioxidant activities at 0.25, 0.5, and 1.0 mg/ml concentrations. However, the highest antioxidant activity was observed in methanol extracts.40 The differences in IC50 values of the tested extracts were most likely due to the differences in the structure and nature of the solvents.
Ginger (Zingiber officinale) was chosen for this study because it is a medicinal herb that has been used worldwide since antiquity. In ancient Sanskrit, Chinese history, surviving Arabic, Persian, Roman, and Greek sources, ginger was mentioned as a medicinal and a spice.5 The principal rationale for utilizing ginger was its direct antimicrobial action, suggesting that it can be used to treat various bacterial infections.5,37 Mohammed et al41 investigated the antibacterial activity of ginger extracts using n-hexane, ethyl acetate, ethanolic Soxhlet, and water as solvents and reported the significant antibacterial activity of ginger extracts. In comparison, Naseer et al42 showed no significant differences in the antibacterial effects of ginger’s water and ethanol extracts on S. aureus and S. pyogenes.
Ciprofloxacin, tetracycline, chloramphenicol, and vancomycin drugs were used in the current study. Results of the current study found that the ethanol extracts (containing antibiotics) have shown the highest antibacterial activity against Lactobacillus spp. Ethanol and water extracts against S. mutans, n-hexane against E. faecalis, and chloroform against Staphylococcus spp. The VA and TE did not show resistance to tested bacteria. Antibiotics containing Ethyl acetate, n-butanol, and water extracts have shown no antibacterial activity of E. faecalis against CIP. These results contradict the previous studies that reported ginger as an effective antibacterial agent against Gram-positive bacteria.43–45 Based on a study conducted by Wang et al ginger has significant inhibitory zones against S. aureus and E. coli.46 Another study also reported these antibacterial properties, which stated that ginger has direct antibacterial activity and thus can be used to treat various bacterial infections.9
Ginger has proven the cytotoxic potential in the current study because of saponins, steroids, alkaloids, and anthraquinones. These results are similar to the previous studies,40,41 which reported that ginger has solid cytotoxic potential. As compared to other herbal products, ginger has secondary side effects. If taken in powder form, it can cause acid reflux.27 The intense effect of Ginger is Gallstones formation and may affect blood pressure with abnormal cardiac rhythm.6 According to the chemical analysis of ginger, it has been observed that it contains almost 400 different compounds.40,47 Raffinose and gingerol, two chemicals found in ginger, have been proven to relieve inflammation and toothaches temporarily. Ginger’s components may also assist in decreasing the germs in the mouth that cause cavities and gum disease, making it a valuable addition to any oral health routine. In addition, it may also be used to whiten teeth when combined with salt.48,49 A recent study has also proven that the presence of 6-shogaol and 6-gingerol in extracts was the most critical factor in ginger cytotoxicity, with a substantial cytotoxic impact in the rhizome contrasted to a non-cytotoxic effect in the callus extracts.50
HPLC is the most reliable analysis method for quantitatively analyzing ginger components, gingerols, and shogaols.8 The HPLC method can quantify and identify phenolic chemicals in plants more efficiently.36 The current study showed that different fractions of ginger contain various phenolic compounds, ie, sinapic acid, chlorogenic acid, Quercetin, gallic acid, cinnamic acid, p-coumaric acid, meta coumaric acid, vanillic acid, caffeic acid, benzoic acid, and syringic acid.
Results of the current study clearly showed that the amount of phenolic and flavonoids compounds varied considerably from one extract to another. Our results showed resemblance with a previous study in which compounds’ variations among different extracts have been noted.37 The highest concentrations of cinnamic acid were found in the Ethanol fraction. Similarly, Syringic acid in water and n-hexane, Para-coumaric acid in n-butanol, Ferulic acid in Ethyl acetate, and caffeic acid in chloroform extracts were found in the highest concentration. Our results are consistent with Yousfi et al which also showed that the ethanolic extracts have the highest polyphenols content while ethyl acetate extract showed the lowest amount of polyphenols.37 Previously, high phenolic content was observed in the methanolic extract.8 Similar results were also reported by Ezez and Tefera (2021), in which the methanol extract showed the maximum phenolic content, and the most negligible phenolic content was found in acetone extract.40
The best-researched flavonoid, Quercetin, has broad-spectrum biological activity, including antibacterial properties.51 Quercetin affects quorum sensing and functions as an anti-biofilm agent against Staphylococcus spp. Quercetin is also a known anticancer and antioxidant compound.49,52 In the present study, Quercetin was found abundantly in all fractions with different concentrations with maximum concentration in the fraction of water and ethanol because of their polarity, while chloroform showed a minor concentration. The main constituents in the current study were the presence of quinones, alkaloids, saponins, flavonoids, glycosides, tannins, and terpenoids. The predominant phytoconstituents of the methanolic extract contained alkaloids, flavonoids, saponin, and terpenoids, whereas, in n-hexane extract, alkaloids, tannins, saponin, and flavonoids were found prominent. Similar results have been found in a study conducted by Egbuna et al13 which reveals the presence of tannins, alkaloids, saponins, flavonoids, phenols, steroids, terpenoids and carboxylic acids in the Ginger ethanolic extracts. Phytochemicals are observed to differ from one another based on their chemical composition, and because of the differences in their chemical makeup, they would have distinct metabolic responses.
Ginger has been used as an antipyretic agent and anti-inflammatory in Asian and Ayurvedic medicine for thousands of years to treat toothache, nausea, indigestion, stomach ache, flatulent intestinal colic, insomnia, urinary tract and respiratory infections and diabetes, rheumatism, nervous diseases, infertility, and to strengthen the memory.27 In the United States, the Physicians employed ginger to treat nausea and intestinal ailments around the turn of the 18th century.42 Also, ginger is used to cure colds, headaches, nausea, and other gastrointestinal issues in China and Japan.46 Ginger’s aromatic carminative and absorbent properties are responsible for its efficacy.42 Ginger is thought to increase the muscular tone and peristalsis in the stomach via gastrointestinal actions. Even though the exact mechanism of action is still not precise.22 Recent research studies on experimental animals have reported ginger’s exclusive gastric stimulation, increased gastric emptying mediated by cholinergic action in the lower intestine, and antispasmodic characteristics mediated by calcium antagonist activity.10,35,43 Avoiding the CNS adverse effects, ginger works directly on the digestive system. The main components involved in the anti-nauseant actions of ginger are 6-gingerol and 6-shogaol, which reduce stomach contraction and enhance gastrointestinal motility and spontaneous peristaltic activity.37 According to the current study findings, the ginger extract contains phenolic compounds such as shogaols, gingerols, and paradols. It demonstrated excellent DPPH radical scavenging activities. These findings imply that ginger extract might be used as an ingredient in the food and also in the pharmaceutical industry.
Conclusions
This study showed that Zingiber officinale could inhibit certain bacteria in-vitro, including Streptococcus mutans, Enterococcus faecalis, Staphylococcus spp., and Lactobacillus spp. However, further research is needed in this field to verify its efficacy in-vivo and to examine the plant’s toxicity, medication interactions, and adverse effects. Future in-vitro and in-vivo studies should be conducted to examine the cytotoxicity, medication interactions, plant side effects, and the standardization of extraction methods. Ginger may be utilized in toothpaste, mouthwash, and ointments for medical and dental applications in the future.
Data Availability Statement
Any data related to the study can be provided on reasonable request.
Ethics Statement
Institutional Review Board Statement: The study was conducted according to the guidelines of the Declaration of Helsinki, and ethical approval was obtained from the Institutional review board of Saveetha University, Chennai, Tamil Nadu, India with ethical clearance code: IHEC-SDC-FACULTY/21/ENDO/199.
Informed Consent Statement
A written informed consent form was obtained from patients to collect the oral swabs to identify and isolate bacterial isolates and from the healthy individual blood donor.
Acknowledgments
The present research work was supported by Taif University Researchers support project number TURSP-2020/102, Taif University, P.O Box-11099, Taif 21944, Saudi Arabia.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conceptualization, study design, execution, acquisition of data, data analysis and interpretation, or in all these areas; they took part in writing, revising or critically reviewing the manuscript; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for the contents of the article.
Supplementary Materials
The Supplementary data consist of tables and figures. The effects of extracts on Streptococcus mutans and other selective oral microbes (Table S1). The percentage scavenging effects of IC50 of antioxidant assays for CME and Fractions (Table S2). Figures showing the HPLC chromatogram of Ethanol, Water, n-Hexane, n-Butanol, Ethyl Acetate, Chloroform, extracts of Ginger (Figures S1‒S6, respectively).
Disclosure
The authors report no conflicts of interest in relation to this work.
References
1. Khan M, Ullah N, Azhar M, Komal W, Muhammad W. A mini-review on the therapeutic potential of Zingiber officinale (ginger). Nat Prod. 2019;15:125.
2. Wan-Nadilah WA, Hasima MN, Manaf AA. A review of medicinal plants and daily foods used in Southeast Asia possessing antidiabetic activity. J Agrobiotechnol. 2019;10:17–35.
3. Munda S, Dutta S, Haldar S, Lal M. Chemical analysis and therapeutic uses of ginger (Zingiber officinale Rosc.) essential oil: a review. J Essent Oil-Bear Plants. 2018;21(4):994–1002. doi:10.1080/0972060X.2018.1524794
4. Mahboubi M. Zingiber officinale Rosc. essential oil, a review on its composition and bioactivity. Clin Phytoscience. 2019;5(1):1–12. doi:10.1186/s40816-018-0097-4
5. Mao -Q-Q, Xu X-Y, Cao S-Y, Gan R-Y, Corke H, Li H-B. Bioactive compounds and bioactivities of ginger (Zingiber officinale Roscoe). Foods. 2019;8(6):185. doi:10.3390/foods8060185
6. Sharma Y. Ginger (Zingiber officinale)-an elixir of life a review. Pharm Innov. 2017;6:22.
7. Srinivasan K. Ginger rhizomes (Zingiber officinale): a spice with multiple health beneficial potentials. PharmaNutrition. 2017;5(1):18–28. doi:10.1016/j.phanu.2017.01.001
8. Tanweer S, Mehmood T, Zainab S, Ahmad Z, Shehzad A. Comparison and HPLC quantification of antioxidant profiling of ginger rhizome, leaves and flower extracts. Clin Phytoscience. 2020;6(1):1–12. doi:10.1186/s40816-020-00158-z
9. Handayani H, Achmad H, Suci AD, et al.. Analysis of antibacterial effectiveness of red ginger extract (Zingiber Officinale Var Rubrum) compared to white ginger extract (Zingiber Officinale Var. Amarum) in mouth cavity bacterial streptococcus mutans (in-vitro). J Int Dent Med Res. 2018;11:676–681.
10. Espinosa-Leal CA, Puente-Garza CA, García-Lara S. In vitro plant tissue culture: means for production of biological active compounds. Planta. 2018;248(1):1–18. doi:10.1007/s00425-018-2910-1
11. Philip N, Leishman S, Walsh L. Potential role for natural products in dental caries control. Oral Health Prev Dent. 2019;17:479–485. doi:10.3290/j.ohpd.a42741
12. Sivakumar A, Ravi V, Prasad A, Sivakumar J. Herbendodontics–Phytotherapy in endodontics: a review. Biomed Pharmacol J. 2018;11(2):1073–1082. doi:10.13005/bpj/1468
13. Egbuna C, Ifemeje J. Comparative studies on the phytochemical properties of five Nigerian medicinal plants. J Adv Med Pharm Sci. 2016;6(2):1–12. doi:10.9734/JAMPS/2016/21816
14. Gao L, Xu T, Huang G, Jiang S, Gu Y, Chen F. Oral microbiomes: more and more importance in oral cavity and whole body. Protein Cell. 2018;9(5):488–500. doi:10.1007/s13238-018-0548-1
15. Silva KRD, Damasceno JL, Inácio MDO, et al.. Antibacterial and cytotoxic activities of Pinus tropicalis and Pinus elliottii resins and of the diterpene dehydroabietic acid against bacteria that cause dental caries. Front Microbiol. 2019;10:987. doi:10.3389/fmicb.2019.00987
16. Philip N, Suneja B, Walsh LJ. Ecological approaches to dental caries prevention: paradigm shift or shibboleth?. Caries Res. 2018;52(1–2):153–165. doi:10.1159/000484985
17. Samiappan SC, Pandiyan R, Palanisamy S, Ramalingam S, Saravanan R, Hameed SA. Targeting the extracellular polysaccharide production (EPS) by biofilm forming bacteria from orthodontic brackets and wires through antiquorum sensing action of bioactive compounds from curcuma longa and Zingiber officinale. Biomed Pharmacol J. 2020;13(02):1037–1045. doi:10.13005/bpj/1973
18. Widyagarini A, Sutadi H, Budiardjo SB. Serotype c and e streptococcus mutans from dental plaque of child-mother pairs with dental caries. J Int Dent Med Res. 2016;9:339.
19. Matkowski A. Plant in vitro culture for the production of antioxidants—a review. Biotechnol Adv. 2008;26(6):548–560. doi:10.1016/j.biotechadv.2008.07.001
20. Brillatz T, Kubo M, Takahashi S, et al.. Metabolite profiling of Javanese ginger Zingiber purpureum and identification of antiseizure metabolites via a low-cost open-source Zebrafish Bioassay-Guided isolation. J Agric Food Chem. 2020;68(30):7904–7915. doi:10.1021/acs.jafc.0c02641
21. Kauser A, Shah SMA, Iqbal N, et al.. In vitro antioxidant and cytotoxic potential of methanolic extracts of selected indigenous medicinal plants. Prog Nutr. 2018;20:706–712.
22. Zubair S, Sawate A, Kshirsagar R, Agarkar B, Patil B. Studies on impact of different processing methods on phyto-chemical and antioxidant activity of dried ginger (Zingiber officinale L.) rhizome. J Pharmacogn Phytochem. 2020;9:3153–3158.
23. Arawande JO, Akinnusotu A, Alademeyin JO. Extractive value and phytochemical screening of ginger (Zingiber officinale) and turmeric (Curcuma longa) using different solvents. Int J Trad Nat Med. 2018;8:13–22.
24. Ling JWA, Chang LS, Mohd Khalid R, et al.. Sequential extraction of red button ginger (Costus woodsonii): phytochemical screening and antioxidative activities. J Food Process Preserv. 2020;44(10):e14776. doi:10.1111/jfpp.14776
25. Yahaya T, Mungadi A, Obadiah C. Phytoconstituent screening of roselle (Hibiscus sabdariffa), moringa (Moringa oleifera), ginger (Zingiber officinale) and fluted pumpkin (Telfairia occidentalis) leaves. J Appl Sci Environ Manag. 2017;21(2):253–256. doi:10.4314/jasem.v21i2.5
26. Othman L, Sleiman A, Abdel-Massih RM. Antimicrobial activity of polyphenols and alkaloids in Middle Eastern plants. Front Microbiol. 2019;10:911. doi:10.3389/fmicb.2019.00911
27. Osae R, Apaliya MT, Kwaw E, et al.. Drying techniques affect the quality and essential oil composition of Ghanaian ginger (Zingiber officinale Roscoe). Ind Crops Prod. 2021;172:114048. doi:10.1016/j.indcrop.2021.114048
28. Jain S, Jain A, Vaidya A, Kumar D, Jain V. Preliminary phytochemical, pharmacognostical and physico-chemical evaluation of Cedrus deodara heartwood. J Pharmacogn Phytochem. 2014;3:91–95.
29. Ali AMA, El-Nour MEM, Yagi SM. Total phenolic and flavonoid contents and antioxidant activity of ginger (Zingiber officinale Rosc.) rhizome, callus and callus treated with some elicitors. J Genet Eng Biotechnol. 2018;16(2):677–682. doi:10.1016/j.jgeb.2018.03.003
30. Rivero-Cruz JF, Granados-Pineda J, Pedraza-Chaverri J, et al.. Phytochemical constituents, antioxidant, cytotoxic, and antimicrobial activities of the ethanolic extract of Mexican brown propolis. Antioxidants. 2020;9(1):70. doi:10.3390/antiox9010070
31. Parveen S, Saqib S, Ahmed A, Shahzad A, Ahmed N. Prevalence of MRSA colonization among healthcare-workers and effectiveness of decolonization regimen in ICU of a tertiary care hospital, Lahore, Pakistan. J Adv Life Sci. 2020;8:38–41.
32. Aravind A, Aravind A, Aravind A, Dinatius P, Krishnan AV, Mathai M. Antimicrobial effect of ginger, garlic, honey, and lemon extracts on streptococcus mutans. J Contemp Dent Pract. 2017;18(11):1004–1008. doi:10.5005/jp-journals-10024-2165
33. Khalid A, Waseem A, Saadullah M, et al.. Antibacterial activity analysis of extracts of various plants against gram-positive and-negative bacteria. Afr J Pharmacy Pharmacol. 2011;5:887–893.
34. Clinical and Laboratory Standard Institute. Performance Standards for Antimicrobial Susceptibility Testing.
35. Pizon J, Nuñeza O, Uy M, Senarath W. In-vitro alpha-amylase inhibitory activity, antioxidant potential, and GC-MS analysis of crepe ginger (Costus speciosus (J. Koenig.) Sm) leaves. Int J Pharm Sci Res. 2018;9:4741–4749.
36. Tohma H, Gülçin İ, Bursal E, Gören AC, Alwasel SH, Köksal E. Antioxidant activity and phenolic compounds of ginger (Zingiber officinale Rosc.) determined by HPLC-MS/MS. J Food Meas Charact. 2017;11(2):556–566. doi:10.1007/s11694-016-9423-z
37. Yousfi F, Abrigach F, Petrovic J, Sokovic M, Ramdani M. Phytochemical screening and evaluation of the antioxidant and antibacterial potential of Zingiber officinale extracts. S Afr J Bot. 2021;142:433–440. doi:10.1016/j.sajb.2021.07.010
38. Si W, Chen YP, Zhang J, Chen Z-Y, Chung HY. Antioxidant activities of ginger extract and its constituents toward lipids. Food Chem. 2018;239:1117–1125. doi:10.1016/j.foodchem.2017.07.055
39. Saikaly SK, Saikaly TS, Saikaly LE. Recurrent aphthous ulceration: a review of potential causes and novel treatments. J Dermatol Treat. 2018;29(6):542–552. doi:10.1080/09546634.2017.1422079
40. Ezez D, Tefera M. Effects of solvents on total phenolic content and antioxidant activity of ginger extracts. J Chem. 2021;2021:1–5. doi:10.1155/2021/6635199
41. Mohammed WF, Saleh BH, Ibrahim RN, Hassan MB. Antibacterial activity of Zingiber officinale (Ginger) against clinical bacterial isolates. South Asian J Res Microbiol. 2019;1–7. doi:10.9734/sajrm/2019/v3i230080
42. Naseer M, Kamboh AA, Soho AB, Burriro R. In vitro antimicrobial efficacy of some plant extracts against multi-drug resistant Staphylococcus aureus and Streptococcus pyogenes isolated from Buffalo mastitic milk. Buffalo Bull. 2021;40:31–44.
43. Santo Grace U, Sankari M. Antimicrobial activity of ethanolic extract of Zingiber Officinale-an in vitro study. J Pharm Sci Res. 2017;9:1417.
44. Sawant D, Deshpande P, Shedge V, Phadke M, Sahani A, Godbole C. Antimicrobial activity of spices against gram positive and gram negative organisms. Afr J Biol Sci. 2021;3(3):37–40. doi:10.33472/AFJBS.3.3.2021.37-40
45. Tajbakhsh M, Soleimani N. Evaluation of the bactericidal effects of Zingiber officinale, Aloysia citrodora and Artemisia dracunculus on the survival of standard gram-positive and gram-negative bacterial strains. Jorjani Biomed J. 2018;6(1):22–32. doi:10.29252/jorjanibiomedj.6.1.22
46. Wang X, Shen Y, Thakur K, et al.. Antibacterial activity and mechanism of ginger essential oil against Escherichia coli and Staphylococcus aureus. Molecules. 2020;25(17):3955. doi:10.3390/molecules25173955
47. El-Hack A, Mohamed E, Alagawany M, et al.. Ginger and its derivatives as promising alternatives to antibiotics in poultry feed. Animals. 2020;10(3):452. doi:10.3390/ani10030452
48. Brum NF, Bezerra MS, Bezerra AS, de Souza GS, Marquezan PK. Antibacterial and antifungal activities herbácia Zingiber Officinale in dentistry: a literature review Atividade antibacteriana e antifúngica da herbácia Zingiber Officinale em odontologia: uma revisão de literatura Actividad antibacteriana y antifungica da herbaria Zingiber Officinale en la odontologia. Res Soc Dev. 2020;9:e6689109141–e6689109141.
49. Hossain S, De Silva DS, Wimalasena S, Pathirana P, Heo G. In vitro antibacterial effect of ginger (Zingiber officinale) essential oil against fish pathogenic bacteria isolated from farmed olive flounder (Paralichthys olivaceus) in Korea. Iran J Fish Sci. 2019;18:386–394.
50. Ali AM, El-Nour ME, Yagi SM, et al.. Cytotoxicity, phytochemical screening and genetic analysis of ginger (Zingiber officinale Rosc.) Callus and Rhizome. S Afr J Bot. 2021. doi:10.1016/j.sajb.2021.11.011
51. Ramzan M, Karobari MI, Heboyan A, et al.. Synthesis of silver nanoparticles from extracts of wild ginger (Zingiber zerumbet) with antibacterial activity against selective multidrug resistant oral bacteria. Molecules. 2007;2022(27):2007.
52. Aleem M, Khan MI, Shakshaz FA, Akbari N, Anwar D. Botany, phytochemistry and antimicrobial activity of ginger (Zingiber officinale): a review. Int J Herb Med. 2020;8(6):36–49. doi:10.22271/flora.2020.v8.i6a.705
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
