Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 17
The Active Ingredient Catalpol in Rehmannia glutinosa Reduces Blood Glucose in Diabetic Rats via the AMPK Pathway
Authors Li Y, Chen Q, Sun HJ, Zhang JH, Liu X
Received 24 October 2023
Accepted for publication 15 March 2024
Published 16 April 2024 Volume 2024:17 Pages 1761—1767
DOI https://doi.org/10.2147/DMSO.S446318
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Konstantinos Tziomalos
Yang Li,* Qiang Chen,* Hong-Juan Sun, Jian-Hong Zhang, Xuan Liu
Pharmaceutical Preparation Section, the Fourth Central Hospital of Tianjin, Tianjin, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yang Li, Pharmaceutical preparation section, the Fourth Central Hospital of Tianjin, No. 1, Zhongshan Road, Hebei District, Tianjin, 300142, People’s Republic of China, Tel +86-022-26249316, Email [email protected]
Background: Type 2 diabetes mellitus (T2DM) poses a huge threat to population health globally, and more drugs need to be explored for treatment. In this study, we investigated the mechanism of active ingredient catalpol in Rehmannia glutinosa on reduces blood glucose in diabetic.
Methods: The T2DM model was constructed by intraperitoneal injection of streptozotocin into Sprague-Dawley (SD) rats, which were randomly grouped into diabetes model group, pioglitazone group, Rehmannia glutinosa group, catalpol high-dose group, catalpol low-dose group and normal control group.The intervention was continued for 28 d, and changes in body weight, fasting blood glucose, insulin and lipid levels were observed.
Results: Of all the drugs, pioglitazone had the most pronounced hypoglycemic effect, which began to decline after 2 weeks of treatment in the low-dose catalpol group and had no hypoglycemic effect in the high-dose catalpol group. Among them, Rehmannia glutinosa was able to increase serum triglyceride level, and pioglitazone effectively reduced total cholesterol level in rats. The low dose of catalpol decreased the concentration of low-density lipoprotein cholesterol (LDL), while the high dose of catalpol increased the concentration of LDL.
Conclusion: As an active ingredient in Rehmannia glutinosa, catalpol has the potential to lower blood glucose and improve blood lipids in diabetes treatment, and its action may be achieved by regulating the adenosine 5’-monophosphate (AMP)-activated protein kinase (AMPK) signaling pathway, which provides a new idea for the development of new diabetes therapeutic approaches.
Keywords: catalpol, Rehmannia glutinosa, type 2 diabetes, traditional Chinese medicine
Introduction
Type 2 diabetes mellitus (T2DM), known as a chronic metabolic disease, is showing a rapidly rising epidemic worldwide.1,2 According to the latest statistics, 537 million people worldwide will have diabetes as of 2021, and this number is expected to increase to 643 million by 2030.3,4 This trend has attracted widespread attention from the World Health Organization and the international community. Hyperglycemia, insulin resistance and relative insulin deficiency characterize T2DM, while its complications, such as cardiovascular disease and retinopathy, have a serious impact on patients’ quality of life and health.5 And the current major hypoglycemic drugs are mainly divided into insulin analogues, oral hypoglycemic drugs, insulin secretion enhancers, sodium-glucose cotransporter protein-2 inhibitors, and other categories.6,7 They have various mechanisms of action, including promoting insulin secretion, improving insulin sensitivity, and inhibiting the release of hepatic glycogen.8 In terms of clinical applications, glucose-lowering drugs are widely used in different types and severities of diabetes, significantly improving glycemic control, reducing symptoms, and lowering the risk of kidney disease and cardiovascular.9,10 However, they may also be accompanied by side effects and safety concerns, such as hypoglycemia and weight gain. Traditional Chinese medicine (TCM) exhibits potential hypoglycemic effects in diabetes management. This mechanism of action is mainly mediated through the active ingredients contained in TCM herbs and their pharmacological properties.11–14 These drugs may counteract the hyperglycemic state by increasing insulin sensitivity, reducing hepatic glycogen synthesis, and promoting insulin secretion.14–16
In addition, molecular pathways have a critical role in the development and complications of T2DM.17–19 Therapeutic studies for T2DM are increasingly focusing on the regulation of molecular pathways to find new therapeutic targets. Among them, the signaling pathway of adenosine 5’-monophosphate (AMP)-activated protein kinase (AMPK) has attracted a lot of attention, and AMPK plays an important role in insulin resistance, β-cell function, and complications of T2DM.20–22 In addition, other molecular pathways, such as miRNA-29, vascular endothelial protein tyrosine phosphatase, heat shock protein-90, Pdia4, and Hedgehog signaling, have also been strongly associated with the development and complications of T2DM.23–26 These findings provide strong evidence for finding new drug targets to improve the quality of life of T2DM patients and prevent the development of complications.
Catalpol, an iridoid glucoside obtained from the roots of Rehmannia glutinosa. This plant is traditionally used in China and Korea for the treatment of aging-related diseases and is extensively referred to as Di-Huang in TCM for treating diabetic disorders.27 Catalpol exerts a wide variety of biological activities including analgesic, sedative, liver protective, purgative, anti-inflammatory, anti-microbial, anti-tumour, and anti-apoptosis activity.28,29 In the last few years, catalpol has been extensively investigated and several studies have reported its multiple biological activities. The antioxidant and free radical scavenging activity of catalpol are the key mechanisms for exhibiting neuroprotection, anti-atherosclerosis, cardioprotective, and antidiabetic activity. Researchers reported that catalpol activated AMPK/PGC-1α/TFAM signaling, which augments mitochondrial biogenesis in skeletal muscle, thereby increasing glucose uptake and adenosine triphosphate production.30
However, the mechanism of how catalpol exerts its hypoglycemic effect is unclear. In this study, we explored the potential role of catalpol in diabetes treatment and its mechanism of action by exploring the AMPK signaling pathway. This study provides new ideas for the development of new diabetes therapeutics to improve the quality of life and prevent the development of complications in T2DM patients.
Materials and Methods
Experimental Animals
Animals 60 male Sprague-Dawley (SD) rats, body mass 180–200 g, were housed in the animal laboratory at a room temperature of 20 ± 2°C, relative humidity of 40–60%, and a light-dark cycle (12 h/12 h). During the experiment, the animals were fed freely. According to the grouping of animals, normal feed and high fat feed (HFD, 20% sucrose, 2.5% cholesterol, 10% lard, 1% sodium cholate, 66.5% basal feed) were given.
Grouping, Modeling and Observation
Male SD rats were acclimatized for 1 week, and 10 rats were randomly selected as the normal group and fed with normal chow. The remaining 50 rats were fed a high-fat diet for 4 weeks, then fasted for 12 h. Streptozotocin (STZ), a chemical widely used to induce diabetes in laboratory animals, and its cytotoxicity to the β-cells leads to a decrease in insulin release and a subsequent increase in blood glucose levels. STZ was injected intraperitoneally at 30 mg/kg. The control was injected with the same volume of citric acid-sodium citrate buffer at the same time. Fasting blood glucose in each rat was measured in triplicate at 72 h post-STZ injection; a sustained fasting blood glucose level ≥ 16.7 mmol/L was considered indicative of successful induction of diabetes mellitus modelling. After the modelling was completed, the animals were randomly grouped according to blood glucose and body weight, with diabetes model group, pioglitazone group (5 mg/kg-d), Rehmannia glutinosa group (6 g/kg-d,10 times the daily dose for human use), catalpol high-dose group (50 mg/kg-d), and catalpol low-dose group (25 mg/kg-d), 10 animals in each group.31 Juice the fresh Rehmannia glutinosa in a juicer and then add purified water until the ratio of the weight of the juice to the weight of the herbs reaches 1:1. Once the drug solution was ready, the drug was administered to the rats by gavage. The volume of gavage was the same in all groups. The original feeding method was continued during the experimental period, and the drug was administered for 4 weeks in total. The body weights of the rats were recorded before the start of the intervention, 2 and 4 weeks after the intervention, and fasting blood glucose levels were determined by blood collection from the tail. The protocol was approved by the Animal Ethics Committee (No. NKYY-DWLL-2023-194).
Animal Harvesting
At the end of 4 weeks of drug intervention, all rats were anesthetized with 1% sodium pentobarbital after fasted overnight 20 h fasting and 2 h water fasting, and blood was collected from abdominal aorta, 8–10 mL of blood was collected. The blood was allowed to stand at room temperature for 30 min, then centrifuged at 3000 rpm for 10 min at 4°C, and the supernatant was taken and stored in a refrigerator at −20°C. The liver was stored in liquid nitrogen and then transferred to −80°C for storage.
Measurement of Blood Lipid Levels
Serum total cholesterol (TC), triglyceride (TG), low-density lipoprotein cholesterol (LDL), and high-density lipoprotein cholesterol (HDL) levels were measured in rats using a blood biochemistry analyzer.
Western Blotting
Total proteins were extracted from trigeminal ganglion and nasal mucosa tissues using RIPA lysate containing protein phosphatase inhibitor. Western blotting was performed as previously described.32 After 3 washes in Tris-Buffered Saline Tween-20 (TBST), the PVDF membranes were incubated for 2h at room temperature using horseradish peroxidase (HRP)-labeled goat anti-rabbit IgG (Cell Signal Technology). After washing 3 times with TBST, ECL reagent was used for visualization and imaging was conducted by chemiluminescence imaging system.
Statistical Analysis
Statistical analysis of the data was done by SPSS Statistics 22.0 software. Differences between groups were determined by one way analysis of variance (One Way ANOVA), and the pairwise comparison between groups were performed using the Least Significance Difference method.
Results and Discussion
As an important part of TCM, herbal medicine has played an important role in the field of medicine since ancient times.33–36 They are widely used in medical practice and cover the treatment and regulation of many diseases, reflecting their unique therapeutic characteristics and theoretical system.33,37–39
In recent years, an increasing number of studies have focused on the potential of herbs in the treatment of diabetes. Clinical studies have shown that some herbs can significantly improve glycemic control and insulin sensitivity in diabetic patients. For example, a clinical trial of bitter melon found that bitter melon extract significantly reduced fasting blood glucose and glycated hemoglobin levels in diabetic patients.40,41 In addition, a study on yam found that yam polysaccharides significantly reduced blood glucose levels and improved insulin resistance in diabetic rats.42,43 These clinical findings provide preliminary evidence for the use of herbs in the treatment of diabetes.
Catalpol in Rehmannia glutinosa Reduces Blood Glucose
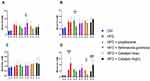
After 4 weeks of treatment, changes in body weight, blood glucose and serum insulin were determined in rats. All the rats in diabetic model group showed a significant decrease in body weight compared to the initial body weight when compared to the healthy control. There was no significant difference between each drug intervention group and the model group alone (Figure 1A). The pioglitazone group showed a significant decrease in fasting blood glucose after 2 weeks of treatment compared to the start of treatment, and the diabetic intervention group started to show a decrease in blood glucose at 4 weeks after treatment. The blood glucose of catalpol low dose group started decreasing at 2 weeks, whereas the catalpol high dose group showed no glucose lowering effect (Figure 1B). After 4 weeks of treatment, the insulin content of rats in the pioglitazone treatment group and Rehmannia glutinosa group increased, whereas the insulin content of the catalpol low-dose and high-dose groups decreased. Among all the drugs, pioglitazone treatment group had the highest serum insulin concentration compared to the HFD group after 4 weeks of treatment (18.50 ± 4.485 mU/L). However, there was no difference in serum insulin levels between the two groups (Figure 1C).
Effect of Rehmannia glutinosa and Catalpol on Blood Lipids in Diabetes
After 4 weeks of treatment, changes in serum TG, TC, HDL and LDL were determined. TG, relevant to the accumulation of lipid repository in the liver, is concerned with metabolic syndrome and T2DM. It was found that Rehmannia glutinosa was able to increase the serum TG level and pioglitazone was effective in lowering the TC level in the rats. There was no significant change in blood HDL levels among the groups. For LDL concentration, low dose of catalpol decreased significantly after 4 weeks of administration, and high dose of catalpol significantly increased lipoprotein LDL concentration. (Figure 2).
Hypoglycemic Effect of AMPK Pathway in Rehmannia glutinosa and Catalpol
AMPK, the AMP-activated protein kinase, is an important regulatory molecule for intracellular energy metabolism. It has been reported in the literature that the expression of both AMPK and its activated form, p-AMPK is significantly decreased in the liver of diabetes rats, is a key enzyme in the fatty acid synthesis process.44 AMPK knockdown resulted in a significant decrease in phosphorylation levels.45,46 Overexpression of recombinantly activated AMPK negatively reduces malonyl coenzyme A content in hepatocytes, thereby inhibiting fatty acid synthesis, and enhances mitochondrial utilization and oxidation of fatty acids, reduces lipid deposition in peripheral tissues, and thereby increases insulin sensitivity.47–49 The results of Western blotting showed that diabetes was able to increase the level of the p-AMPK pathway in the liver and decrease the concentration of AMPK. Pioglitazone reversed this trend, decreasing the level of the p-AMPK pathway and increasing the concentration of AMPK in the liver. Unexpectedly, Rehmannia glutinosa appeared to exacerbate the elevation of p-AMPK andAMPK pathway associated with diabetes. This suggests that the mechanism by which Rehmannia glutinosa ameliorates T2DM may be to reduce the activation of nuclear factor kappa-B by increasing the expression level of p-AMPK/AMPK. And then control the inflammatory response mediated by the activation of PYD domains-containing protein 3 inflammasome.50 For low concentration of catalpol treatment, it had no effect on AMPK concentrations, but was able to reduce p-AMPK concentrations. High concentration of catalpol inhibited both p-AMPK and AMPK concentrations in the liver (Figure 3).
Molecular mechanisms and pharmacological studies have revealed the role of herbs in regulating glucose metabolism and insulin signaling pathways.47,51 For example, it was found that bitter melanin was able to increase insulin sensitivity by activating the AMPK signaling pathway, thereby promoting glucose uptake and utilization.52,53 Panax ginseng saponin, on the other hand, can improve insulin resistance by inhibiting inflammatory responses and oxidative stress.48,54 These findings provide a theoretical basis for the mechanism of herbal medicine in diabetes treatment. In summary, this study found that catalpol in Rehmannia glutinosa can affect blood glucose by affecting the AMPK pathway, thus lowering the blood glucose of T2DM rats. Its effect is comparable to that of pioglitazone, indicating that Rehmannia glutinosa is able to activate AMPK and play the role of protecting liver function, improving liver lipid metabolism in rats with T2DM, and then treating T2DM through the AMPK signal transduction pathway. However, there are some limitations to this study. Due to significant biological differences between species, rat-based animal studies may be affected by species when extrapolated to populations. For a long-term chronic disease as diabetes, further studies are still needed to investigate the safety and efficacy of long-term medication.
Conclusion
T2DM, known as a chronic metabolic disease, poses a huge threat to population health globally, and more drugs need to be explored for treatment. Our findings suggest that catalpol, the active ingredient in Rehmannia glutinosa, has a certain therapeutic effect in the STZ-induced diabetic rats. It was found that a low dose of 25 mg/kg-d was found to be able to lower blood glucose and effectively improve lipid levels, while high doses above 50 mg/kg-d were ineffective. Exploration of the AMPK pathway also suggests that catalpol may produce hypoglycemic effects by modulating the AMPK pathway.
Declarations
The study protocol was approved by the Animal Ethical and Welfare Committee of NanKai Hospital (No. NKYY-DWLL-2023-194). All experiments were performed following the Animal Ethics and Welfare Committee of Nankai Hospital, as well as the Animal Protection Law and the Animal management regulations.
Funding
Tianjin Municipal Health Commission and Tianjin Municipal Administration of Traditional Chinese Medicine Research Project on Integrated Traditional Chinese and Western Medicine, Project No. 2021194.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Su Y, Ye L, Hu C, Zhang Y, Liu J, Shao L. Periodontitis as a promoting factor of T2D: current evidence and mechanisms. Int J Oral Sci. 2023;15(1):25. doi:10.1038/s41368-023-00227-2
2. Ling C. Epigenetic regulation of insulin action and secretion - role in the pathogenesis of type 2 diabetes. J Intern Med. 2020;288(2):158–167. doi:10.1111/joim.13049
3. Spinelli R, Baboota RK, Gogg S, et al. Increased cell senescence in human metabolic disorders. J Clin Invest. 2023;133(12):e169922. doi:10.1172/JCI169922
4. Eid SA, Rumora AE, Beirowski B, et al. New perspectives in diabetic neuropathy. Neuron. 2023;111(17):2623–2641. doi:10.1016/j.neuron.2023.05.003
5. Jin X, Qiu T, Li L, et al. Pathophysiology of obesity and its associated diseases. Acta Pharm Sin B. 2023;13(6):2403–2424. doi:10.1016/j.apsb.2023.01.012
6. Packer M, Wilcox CS, Testani JM. Critical Analysis of the Effects of SGLT2 Inhibitors on Renal Tubular Sodium, Water and Chloride Homeostasis and Their Role in Influencing Heart Failure Outcomes. Circulation. 2023;148(4):354–372. doi:10.1161/CIRCULATIONAHA.123.064346
7. Sacks DB, Arnold M, Bakris GL, et al. Guidelines and Recommendations for Laboratory Analysis in the Diagnosis and Management of Diabetes Mellitus. Diabetes Care. 2023;46(10):e151–e199. doi:10.2337/dci23-0036
8. Scheen AJ. Pragmatic trials, a step forward to assess cardiovascular efficacy of new glucose-lowering agents. Lancet Diabetes Endocrinol. 2023;11(9):626–627. doi:10.1016/S2213-8587(23)00161-4
9. Woo V, Connelly K, Lin P, McFarlane P. The role of sodium glucose cotransporter-2 (SGLT-2) inhibitors in heart failure and chronic kidney disease in type 2 diabetes. Curr Med Res Opin. 2019;35(7):1283–1295. doi:10.1080/03007995.2019.1576479
10. Galindo RJ, de Boer IH, Neumiller JJ, Tuttle KR. Continuous Glucose Monitoring to Optimize Management of Diabetes in Patients with Advanced CKD. Clin J Am Soc Nephrol. 2023;18(1):130–145. doi:10.2215/CJN.04510422
11. Liu D, Jia XB. “中药成分”相关研究现状分析与探讨 [Analysis and discussion about current development of relevant studies on “traditional Chinese medicine components”]. Zhongguo Zhong Yao Za Zhi. 2014;39(2):171–174. Chinese.
12. Liu Y, Cheng Y. Combined development of traditional Chinese medicine and interventional medicine. J Interv Med. 2021;4(3):136–138. doi:10.1016/j.jimed.2021.07.002
13. Wang S, Li XY, Shen L. Modulation effects of Dendrobium officinale on gut microbiota of type 2 diabetes model mice. FEMS Microbiol Lett. 2021;368(5):fnab020. doi:10.1093/femsle/fnab020
14. He WJ, Hu YN, Zhang YL, et al. 饮片性状与成分性状自相似关系研究 [Study on self-similarity relationship between decoction pieces property and component property]. Zhongguo Zhong Yao Za Zhi. 2014;39(13):2375–2377. Chinese.
15. Feng J, Zhou Y, Liao L, Yu L, Yuan P, Zhang J. Network Pharmacology and Transcriptomics Reveal the Mechanism of GuaLouQuMaiWan in Treatment of Type 2 Diabetes and Its Active Small Molecular Compound. J Diabetes Res. 2022;2022:2736504. doi:10.1155/2022/2736504
16. Chu N, Chan JCN, Chow E. Pharmacomicrobiomics in Western Medicine and Traditional Chinese Medicine in Type 2 Diabetes. Front Endocrinol. 2022;13:857090. doi:10.3389/fendo.2022.857090
17. Zhang AH, Qiu S, Xu HY, Sun H, Wang XJ. Metabolomics in diabetes. Clin Chim Acta. 2014;429:106–110. doi:10.1016/j.cca.2013.11.037
18. Aregbesola A, Voutilainen S, Virtanen JK, Aregbesola A, Tuomainen TP. Serum hepcidin concentrations and type 2 diabetes. World J Diabetes. 2015;6(7):978–982. doi:10.4239/wjd.v6.i7.978
19. Vishal K, Bhuiyan P, Qi J, et al. Unraveling the Mechanism of Immunity and Inflammation Related to Molecular Signatures Crosstalk Among Obesity, T2D, and AD: insights From Bioinformatics Approaches. Bioinform Biol Insights. 2023;17:11779322231167977. doi:10.1177/11779322231167977
20. Zheng Z, Ma T, Guo H, et al. 4-O-methylhonokiol protects against diabetic cardiomyopathy in type 2 diabetic mice by activation of AMPK-mediated cardiac lipid metabolism improvement. J Cell Mol Med. 2019;23(8):5771–5781. doi:10.1111/jcmm.14493
21. Zheng S, Liu J, Han Q, et al. Metformin induces renal medullary interstitial cell apoptosis in type 2 diabetic mice. J Diabetes. 2014;6(2):132–146. doi:10.1111/1753-0407.12105
22. Dihingia A, Ozah D, Ghosh S, et al. Vitamin K1 inversely correlates with glycemia and insulin resistance in patients with type 2 diabetes (T2D) and positively regulates SIRT1/AMPK pathway of glucose metabolism in liver of T2D mice and hepatocytes cultured in high glucose. J Nutr Biochem. 2018;52:103–114. doi:10.1016/j.jnutbio.2017.09.022
23. Coughlan KA, Valentine RJ, Ruderman NB, Saha AK. AMPK activation: a therapeutic target for type 2 diabetes? Diabetes Metab Syndr Obes. 2014;7:241–253. doi:10.2147/DMSO.S43731
24. Day EA, Ford RJ, Steinberg GR. AMPK as a Therapeutic Target for Treating Metabolic Diseases. Trends Endocrinol Metab. 2017;28(8):545–560. doi:10.1016/j.tem.2017.05.004
25. Khoramipour K, Bejeshk MA, Rajizadeh MA, Najafipour H, Dehghan P, Farahmand F. High-Intensity Interval Training Ameliorates Molecular Changes in the Hippocampus of Male Rats with the Diabetic Brain: the Role of Adiponectin. Mol Neurobiol. 2023;60(6):3486–3495. doi:10.1007/s12035-023-03285-z
26. Demaré S, Kothari A, Calcutt NA, Fernyhough P. Metformin as a potential therapeutic for neurological disease: mobilizing AMPK to repair the nervous system. Expert Rev Neurother. 2021;21(1):45–63. doi:10.1080/14737175.2021.1847645
27. Shieh JP, Cheng KC, Chung HH, Kerh YF, Yeh CH, Cheng JT. Plasma glucose lowering mechanisms of catalpol, an active principle from roots of Rehmannia glutinosa, in streptozotocin-induced diabetic rats. J Agric Food Chem. 2011;59(8):3747–3753. doi:10.1021/jf200069t
28. Bhattamisra SK, Yap KH, Rao V, Choudhury H. Multiple Biological Effects of an Iridoid Glucoside, Catalpol and Its Underlying Molecular Mechanisms. Biomolecules. 2019;10(1):32. doi:10.3390/biom10010032
29. Bai Y, Zhu R, Tian Y, et al. Catalpol in Diabetes and its Complications: a Review of Pharmacology, Pharmacokinetics, and Safety. Molecules. 2019;24(18):3302. doi:10.3390/molecules24183302
30. Xu DQ, Li CJ, Jiang ZZ, et al. The hypoglycemic mechanism of catalpol involves increased AMPK-mediated mitochondrial biogenesis. Acta Pharmacol Sin. 2020;41(6):791–799. doi:10.1038/s41401-019-0345-2
31. Yuan H, Ni X, Zheng M, Han X, Song Y, Yu M. Effect of catalpol on behavior and neurodevelopment in an ADHD rat model. Biomed Pharmacother. 2019;118:109033. doi:10.1016/j.biopha.2019.109033
32. Li L, Li Q, Huang W, et al. Dapagliflozin Alleviates Hepatic Steatosis by Restoring Autophagy via the AMPK-mTOR Pathway. Front Pharmacol. 2021;12:589273. doi:10.3389/fphar.2021.589273
33. Luo Y, Wang CZ, Hesse-Fong J, Lin JG, Yuan CS. Application of Chinese Medicine in Acute and Critical Medical Conditions. Am J Chin Med. 2019;47(6):1223–1235. doi:10.1142/S0192415X19500629
34. Lin Z. Ganoderma (Lingzhi) in Traditional Chinese Medicine and Chinese Culture. Adv Exp Med Biol. 2019;1181:1–13. doi:10.1007/978-981-13-9867-4_1
35. Devi N, Rani K, Kharb P, Prasad M. Herbal Medicine for Urinary Tract Infections with the Blazing Nanotechnology. J Nanosci Nanotechnol. 2021;21(6):3495–3512. doi:10.1166/jnn.2021.19002
36. Liang XL, Ji MM, Chen L, et al. Traditional Chinese herbal medicine Astragalus Radix and its effects on intestinal absorption of aconite alkaloids in rats. Chin Herb Med. 2020;13(2):235–242. doi:10.1016/j.chmed.2020.09.005
37. Hoffman RD, Li CY, He K, et al. Chinese Herbal Medicine and Its Regulatory Effects on Tumor Related T Cells. Front Pharmacol. 2020;11:492. doi:10.3389/fphar.2020.00492
38. Li Z, He X, Liu F, Wang J, Feng J. A review of polysaccharides from Schisandra chinensis and Schisandra sphenanthera: properties, functions and applications. Carbohydr Polym. 2018;184:178–190. doi:10.1016/j.carbpol.2017.12.058
39. Peng Z, Xu R, You Q. Role of Traditional Chinese Medicine in Bone Regeneration and Osteoporosis. Front Bioeng Biotechnol. 2022;10:911326. doi:10.3389/fbioe.2022.911326
40. Kim SK, Jung J, Jung JH, et al. Hypoglycemic efficacy and safety of Momordica charantia (bitter melon) in patients with type 2 diabetes mellitus. Complement Ther Med. 2020;52:102524. doi:10.1016/j.ctim.2020.102524
41. Hsu PK, Pan FFC, Hsieh CS. mcIRBP-19 of Bitter Melon Peptide Effectively Regulates Diabetes Mellitus (DM) Patients’ Blood Sugar Levels. Nutrients. 2020;12(5):1252. doi:10.3390/nu12051252
42. Yu L, Zhang J, Jiao J, et al. Effect of nano yam polysaccharide on the blood glucose and blood lipid in rats. Pak J Pharm Sci. 2020;33:565.
43. Lei X, Huo P, Wang Y, et al. Lycium barbarum Polysaccharides Improve Testicular Spermatogenic Function in Streptozotocin-Induced Diabetic Rats. Front Endocrinol. 2020;11:164. doi:10.3389/fendo.2020.00164
44. Fullerton MD, Galic S, Marcinko K, et al. Single phosphorylation sites in Acc1 and Acc2 regulate lipid homeostasis and the insulin-sensitizing effects of metformin. Nat Med. 2013;19(12):1649–1654. doi:10.1038/nm.3372
45. Shrikanth CB, Jagannath S, Chilkunda ND. AMPK differentially alters sulphated glycosaminoglycans under normal and high glucose milieu in proximal tubular cells. J Biochem. 2021;169(1):75–86. doi:10.1093/jb/mvaa094
46. Kim JH, Lee JO, Kim N, et al. Paclitaxel suppresses the viability of breast tumor MCF7 cells through the regulation of EF1α and FOXO3a by AMPK signaling. Int J Oncol. 2015;47(5):1874–1880. doi:10.3892/ijo.2015.3153
47. O’Neill HM, Lally JS, Galic S, et al. AMPK phosphorylation of ACC2 is required for skeletal muscle fatty acid oxidation and insulin sensitivity in mice. Diabetologia. 2014;57(8):1693–1702. doi:10.1007/s00125-014-3273-1
48. Marcinko K, Steinberg GR. The role of AMPK in controlling metabolism and mitochondrial biogenesis during exercise. Exp Physiol. 2014;99(12):1581–1585. doi:10.1113/expphysiol.2014.082255
49. Chen X, Shang L, Deng S, et al. Peroxisomal oxidation of erucic acid suppresses mitochondrial fatty acid oxidation by stimulating malonyl-CoA formation in the rat liver. J Biol Chem. 2020;295(30):10168–10179. doi:10.1074/jbc.RA120.013583
50. Meng X, Liu X, Ning C, et al. Rehmanniae Radix and Rehmanniae Radix Praeparata improve diabetes induced by high-fat diet coupled with streptozotocin in mice through AMPK-mediated NF-κB / NLRP3 signaling pathway. China J Chine Materia Medis. 2021;46(21):5627–5640. doi:10.19540/j.cnki.cjcmm.20210323.302
51. Zhang K, Shi Y, Huang C, et al. Activation of AMP-activated protein kinase signaling pathway ameliorates steatosis in laying hen hepatocytes. Poult Sci. 2021;100(3):100805. doi:10.1016/j.psj.2020.10.059
52. Li L, Jiang Z, Yao Y, Yang Z, Ma H. (-)-Hydroxycitric acid regulates energy metabolism by activation of AMPK - PGC1α - NRF1 signal pathway in primary chicken hepatocytes. Life Sci. 2020;254:117785. doi:10.1016/j.lfs.2020.117785
53. Liu Z, Qu CY, Li JX, et al. Hypoglycemic and Hypolipidemic Effects of Malonyl Ginsenosides from American Ginseng (Panax quinquefolius L.) on Type 2 Diabetic Mice. ACS Omega. 2021;6(49):33652–33664. doi:10.1021/acsomega.1c04656
54. Abdelazim A, Khater S, Ali H, et al. Panax ginseng improves glucose metabolism in streptozotocin-induced diabetic rats through 5’ adenosine monophosphate kinase up-regulation. Saudi J Biol Sci. 2019;26(7):1436–1441. doi:10.1016/j.sjbs.2018.06.001
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.