Back to Journals » Neuropsychiatric Disease and Treatment » Volume 19
Targeting the Arginine Vasopressin V1b Receptor System and Stress Response in Depression and Other Neuropsychiatric Disorders
Authors Kanes SJ, Dennie L, Perera P
Received 27 December 2022
Accepted for publication 23 March 2023
Published 12 April 2023 Volume 2023:19 Pages 811—828
DOI https://doi.org/10.2147/NDT.S402831
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Roger Pinder
Stephen J Kanes, Lara Dennie, Philip Perera
EmbarkNeuro, Oakland, CA, USA
Correspondence: Stephen J Kanes, EmbarkNeuro, Inc, 1111 Broadway, Suite 1300, Oakland, CA, 94607, USA, Tel +1 610 757 7821, Email [email protected]
Abstract: A healthy stress response is critical for good mental and overall health and promotes neuronal growth and adaptation, but the intricately balanced biological mechanisms that facilitate a stress response can also result in predisposition to disease when that equilibrium is disrupted. The hypothalamic-pituitary-adrenal (HPA) axis neuroendocrine system plays a critical role in the body’s response and adaptation to stress, and vasopressinergic regulation of the HPA axis is critical to maintaining system responsiveness during chronic stress. However, exposure to repeated or excessive physical or emotional stress or trauma can shift the body’s stress response equilibrium to a “new normal” underpinned by enduring changes in HPA axis function. Exposure to early life stress due to adverse childhood experiences can also lead to lasting neurobiological changes, including in HPA axis function. HPA axis impairment in patients with depression is considered among the most reliable findings in biological psychiatry, and chronic stress has been shown to play a major role in the pathogenesis and onset of depression and other neuropsychiatric disorders. Modulating HPA axis activity, for example via targeted antagonism of the vasopressin V1b receptor, is a promising approach for patients with depression and other neuropsychiatric disorders associated with HPA axis impairment. Despite favorable preclinical indications in animal models, demonstration of clinical efficacy for the treatment of depressive disorders by targeting HPA axis dysfunction has been challenging, possibly due to the heterogeneity and syndromal nature of depressive disorders. Measures of HPA axis function, such as elevated cortisol levels, may be useful biomarkers for identifying patients who may benefit from treatments that modulate HPA axis activity. Utilizing clinical biomarkers to identify subsets of patients with impaired HPA axis function who may benefit is a promising next step in fine-tuning HPA axis activity via targeted antagonism of the V1b receptor.
Keywords: allostatic overload, cortisol, HPA axis, major depressive disorder, neuroendocrine
Background
A healthy response to stress is critical for good mental and overall health and promotes neuronal growth and adaptation, but the intricately balanced biological mechanisms that facilitate a stress response can also result in a predisposition to disease when the equilibrium is disrupted.1–3 Allostasis is the biological process of ongoing adaptation to maintain homeostatic stability in response to challenges,2,3 and a key adaptive mechanism by which the body responds to stress is the hypothalamic-pituitary-adrenal (HPA) axis.4 The cumulative physiological impact of this adaptive response, or allostatic load, becomes allostatic overload when metabolic, hormonal, and neurotransmitter mediators of allostasis are overused or dysregulated as a result of stress, trauma, or abuse.2,3 It is at this point of allostatic overload and disrupted equilibrium that the cumulative impact of the body’s stress response system can shift from protective to damaging, leading to diseases such as depression and other neuropsychiatric disorders (Figure 1).1–3,5 Research suggests that arginine vasopressin (AVP) and vasopressin receptor subtype V1b play a key role in regulation of the HPA axis in response to stress, and therefore, targeting HPA axis dysregulation via modulation of the AVP-V1b receptor system may offer a novel therapeutic approach to treatment of these diseases.6
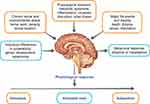 |
Figure 1 Allostasis and allostatic load. The brain perceives and responds to stimuli and stressors. The major function of cortisol and other mediators of allostasis is to promote adaptation. However, overuse and/or dysregulation among allostatic mediators can lead to allostatic load (or overload) and accelerate disease processes such as cardiovascular disease, diabetes, and affective disorders. Adapted from McEwen BS, Akil H. Revisiting the stress concept: implications for affective disorders. J Neurosci. 2020;40(1):12–21, with permission under the Creative Commons Attribution 4.0 International (CC BY 4.0) license.3 |
Role of the HPA Axis in Stress Response
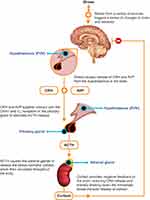
On a neural and molecular level, the HPA axis neuroendocrine system plays a critical role in the ability of an organism to cope with and adapt to stress.1,2,7 Activation of the HPA axis by a physical or emotional stressor triggers a signaling cascade from the hypothalamus (Figure 2), causing neuronal synthesis and release of AVP and corticotropin-releasing hormone (CRH) into the pituitary portal circulation, where they cooperate to trigger the release of adrenocorticotropic hormone (ACTH).3,8 AVP alone is a weak stimulus of ACTH secretion, but, upon simultaneous release of both AVP and CRH during stress, AVP potentiates the effect of CRH to stimulate ACTH secretion.8,9 After its release from the pituitary, ACTH acts on the adrenal cortex to stimulate production and release of glucocorticoids (cortisol in humans; corticosterone in rodents), which serve as key allostatic mediators that penetrate the blood-brain barrier to affect brain function and behavior as well as participate in negative feedback loops to influence release of CRH and AVP from the hypothalamus and ACTH from the pituitary gland.1,3,7,10 However, activation of the HPA axis, including relative levels of and sensitivity to each component and temporal pattern of response, varies according to the nature of the stressor: evidence suggests that acute stress triggers a primarily CRH-mediated, dynamic, rapid, and self-limited increase in ACTH and glucocorticoids, whereas chronic (repeated) stress results in blunted and/or sustained increases in ACTH and glucocorticoids mediated by AVP.2,5,9,11
Contribution of the AVP V1b Receptor System to the HPA Axis Stress Response
AVP activity is mediated through 3 vasopressin receptor subtypes: V1a, V1b, and V2.12 V1a receptors are located largely in vascular smooth muscle, and V2 receptors are located in the kidney; these receptors play key roles in vasoconstriction and fluid homeostasis, respectively, whereas V1b receptors are expressed in the anterior pituitary and limbic brain regions and are involved in HPA axis regulation, stress, and emotions.12–15 In rats, V1b receptor mRNA expression and V1b receptor protein have been shown in corticotrophs, the cells in the anterior pituitary that secrete ACTH during the HPA axis stress response,9,13,15 as well as in the cerebral cortex, hippocampus, amygdala, and hypothalamus.13,15–17 The limbic system, which includes the cerebral cortex, hippocampus, amygdala, and hypothalamus, contributes to emotion, cognition, behavior, and the stress response.9,18 The relative contributions of limbic and pituitary V1b receptors to the stress response is an area of ongoing study via selective V1b receptor inhibition in rodent models. Antidepressant- and/or anxiolytic-like effects can be achieved via local inhibition of V1b receptors in the amygdala or the lateral septum and by systemic inhibition in hypophysectomized rats,19–21 confirming the limbic role of V1b receptors in the stress response. Conversely, anxiolytic-like effects associated with systemic V1b receptor inhibition have been prevented via hypophysectomy,22 confirming the pituitary role of V1b receptors in the stress response. Thus, evidence suggests that the V1b receptor modulation of the stress response occurs via both pituitary-dependent and pituitary-independent pathways.
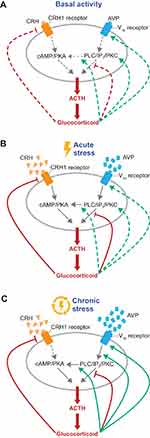
Altered AVP and V1b receptor responses to ongoing stress contribute to HPA axis dysfunction. During HPA axis homeostasis, basal CRH and AVP levels are regulated by glucocorticoid feedback inhibition as a protective mechanism to prevent inappropriate activation or overstimulation of the HPA axis in response to minor stimuli (Figure 3).9 During chronic stress, however, AVP becomes refractory to glucocorticoid feedback.9 Elevated glucocorticoid levels reduce expression of CRH and its receptor, whereas V1b receptor expression and its sensitivity to AVP are enhanced, suggesting that vasopressinergic regulation of the HPA axis is critical for sustaining corticotroph responsiveness during chronic stress in the presence of high levels of circulating glucocorticoids.9
Stress-Related Dysregulation of the HPA Axis
During a normal stress response, activation of the HPA axis promotes a mild state of anxiety, alters attention and memory, limits dysphoria, and alters pleasure and reward processing to allow for sufficient focus on the stressor.1 However, exposure to repeated or excessive physical or emotional stress or trauma shifts the body’s stress response equilibrium, or allostatic load, to a “new normal” that is underpinned by enduring changes in HPA axis function.2,3 In animal models of chronic stress, changes in structure, function, and connectivity within and between the hippocampus, amygdala, and prefrontal cortex are mediated by CRH and glucocorticoids, elements of the HPA axis.2,23,24 Moreover, similar effects have been observed in humans in the brain regions involved in regulation of emotion and stress response (Figure 4).25,26 Although the relative reversibility of these stress-induced effects suggests that they are the result of a system of adaptive neuroplasticity rather than of damage, a history of stress exposure can nevertheless lead to lasting neuroplastic dysregulation of stress reactivity.23,26 These changes and their clinical outcomes are influenced by the type and timing of stress, the environment, and genetic and epigenetic factors27–38 and can manifest as hyperactive or hypoactive impairment of the HPA axis.27,39
Neurobiological Changes Associated with Adverse Childhood Experiences
Exposure to early life stress (ELS) from, for example, adverse childhood experiences (ACEs) during windows of vulnerability in which the brain is still developing can lead to lasting neurobiological changes, including in HPA axis function, that significantly increase the risk of developing depression and other neuropsychiatric disorders.40–45 ACEs can include physical, psychological, or sexual abuse, physical or emotional neglect, or other traumatic events during childhood39 and are an important risk factor for many neuropsychiatric disorders, including depression.41,42 In a meta-analysis of more than 17,000 adults with depression, nearly half (46%) reported a history of childhood maltreatment, with an estimated prevalence of 43% for childhood emotional neglect, 37% for childhood emotional abuse, 36% for childhood physical neglect, 28% for childhood physical abuse, and 25% for childhood sexual abuse.43 Furthermore, patients with major depressive disorder (MDD) were almost 4 times more likely than healthy controls to have been mistreated as children, and those with persistent depressive disorder were almost 9 times more likely than healthy controls to have experienced multiple forms of abuse or neglect.44 Approximately 60% of adults with depression have experienced ≥1 ACE,44,45 and ELS is 4 times more prevalent in patients with depression than in healthy controls45 and is associated with earlier onset of depression,43,45 reduced response to depression treatment,43,45 and reduced life expectancy.42,46–49
During the heightened neuroplasticity of childhood, ELS or ACE exposure is also associated with substantial effects on brain structure, function, connectivity, and network architecture, including brain regions involved in regulation of emotion and stress response.25,50,51 Preclinical and clinical research have shown an association between ACEs and lasting changes to HPA axis function, which may contribute to the risk and course of depression.38,42,43,45 The nature of HPA axis impairment (eg, hyperactivity or hypoactivity) following an ACE is influenced by the type of ACE, psychosocial support, and genetic and epigenetic factors.38,52 Consequently, depression in patients with a history of ELS may be a distinct biologic endophenotype, with a unique clinical feature, course of illness, and response to therapy.31,38
HPA Axis Impairment in Depression, Neuropsychiatric Disorders, and Beyond
HPA axis impairment in patients with depression is considered among the most reliable findings in biological psychiatry.10 Alterations in activity of limbic regions that are regulated by the HPA axis may contribute to heightened anxiety, changes in attention and memory, dysphoria, and altered pleasure and reward processing.1 Stress has been shown to play a major role in the pathogenesis and onset of depression and to contribute to increased vulnerability to developing depressive symptoms.53,54 Polymorphisms in genes involved in HPA axis functioning have been shown to influence stress response, risk of depression,55–58 and response to antidepressant treatment,56,59 and several mechanisms of HPA axis impairment have been implicated in the pathogenesis of depression, including cortisol resistance, reduction in neurogenesis, increase in cytokines, and immune system activation.10,60 Significant HPA axis hyperactivity, as indicated by elevated serum, urinary, salivary, cerebral spinal fluid, and hair cortisol levels, occurs more often in patients with depression than in healthy controls,61,62 with nearly three-quarters of people with depression demonstrating elevated cortisol levels.61 Furthermore, elevated cortisol may be one of the few prospective predictors of MDD onset and relapse or recurrence63 and may correlate with the severity of depressive symptoms.61 For example, recent dramatic increases in stress and depressive symptoms associated with the COVID-19 pandemic and lockdown have been correlated with changes in cortisol levels,62,64,65 and elevated pre-pandemic cortisol levels were predictive of depressive symptoms during the pandemic (Box 1).62
 |
Box 1 Effects of the COVID-19 Pandemic on Global Mental Health |
Profound changes in HPA axis function during pregnancy and in the perinatal period may also contribute to postpartum depression.66 Under healthy conditions, significantly increased HPA axis activity is observed in the third trimester, followed by a decline in activity after the birth.67,68 Cortisol concentrations rise during pregnancy due in large part to placental CRH production, which stimulates production of and is subject to positive feedback by maternal and fetal cortisol.69 During and after birth, the HPA axis is subject to abrupt changes when the placental contribution of CRH ceases and cortisol concentrations decrease substantially during the postpartum period.69 Among women who develop perinatal or postpartum depression, elevated cortisol levels observed in some studies suggest that the HPA axis may not be adequately suppressed after birth.70–72 Compounding the effects of fluctuating cortisol, changes in estrogen during pregnancy may also alter HPA axis activity. In rats, estrogen induces remodeling of the CA1 region of the hippocampus, an area involved in suppression of the HPA axis stress response; in humans, estrogen alters HPA axis activity by increasing basal cortisol levels and blunting cortisol suppression by dexamethasone, similar to alterations observed in depression.24,73 Combined, these dramatic changes in hormone activity during and after pregnancy may impose a dysregulating effect on the HPA axis and result in postpartum depression.66 Likewise, MDD rates have been shown to increase 2- to 3-fold during the menopause transition, and research has suggested that hormonal changes leading to HPA axis impairment in cortisol reactivity may increase vulnerability to stress and depression in this population.74
Changes in HPA axis function have been implicated in a number of other neuropsychiatric conditions, as well. Reduced HPA axis activity appears to contribute to posttraumatic stress disorder (PTSD).75,76 While higher cortisol in children is predictive of PTSD, enhanced negative feedback inhibition of the HPA axis is observed in individuals experiencing PTSD, indicated by simultaneously reduced circulating cortisol and increased CRH coupled with enhanced cortisol suppression in response to dexamethasone challenge.76,77 In contrast, anxiety disorders are associated with hypercortisolemia and reduced feedback inhibition of the HPA axis.54,78 An increased susceptibility to anxiety due to ELS may be a consequence of HPA axis hyperactivity in the form of an imbalance between glucocorticoid receptor‒ and mineralocorticoid receptor‒mediated negative feedback.78 Excessive HPA axis activity is also a feature of bipolar disorder,79 with more robust hyperactivity observed in patients with severe manic symptoms80 or a history of suicidal behavior.81 In schizophrenia, changes in hair cortisol concentration are negatively associated with delusion severity, and evidence supports the use of measures of HPA axis activity as biomarkers for associated brain tissue loss.82,83 A dysregulated HPA axis response to stress is associated with anorexia and bulimia nervosa and is exacerbated by the added experience of childhood trauma.84–86 Attenuated HPA axis activity persisted following treatment for anorexia or bulimia and therefore may represent a risk factor for an eating disorder rather than a consequence thereof.86
One contributor to HPA axis impairment in patients living with these disorders is dysregulation of the AVP V1b receptor system, specifically.61,87,88 Elevated AVP levels have been shown in patients with depression,89–93 including anxious-retarded depression89 and melancholic-type depression,90 as well as in patients with bipolar disorder,91,93 obsessive compulsive disorder,94 bulimia nervosa,85 and PTSD,95 indicating overactivation of AVP in these patient populations.6 Involvement of the AVP-V1b receptor system HPA axis dysregulation in patients with depression is further supported by positive correlations demonstrated between AVP and cortisol levels,89,96 particularly among those who have attempted suicide.96 These data provide support for increased sensitivity of the V1b receptor to AVP regulation of the HPA axis stress response in the presence of elevated cortisol in patients with depression.88,97
The central role of the HPA axis in allostasis, allostatic load, and allostatic overload suggests broader implications beyond neuroplasticity for HPA axis dysfunction.4,98 Because the HPA axis neuroendocrine system dynamically influences a wide variety of physiological processes, changes in its function are associated with a broad range of long-term health consequences.3,61,99,100 Among patients with depression, HPA axis dysregulation as demonstrated by elevated cortisol levels is associated with increased risk of other medical conditions in which HPA axis dysregulation has been implicated, including diabetes, obesity, metabolic syndrome, cardiovascular disease, cognitive dysfunction, and osteoporosis.61,99–111 Overall, evidence suggests that HPA axis dysfunction underpins a bidirectional relationship between many of these comorbidities and depression.110,112–114
Cortisol as a Biomarker of HPA Axis Dysfunction
Depression is a clinically heterogenous condition comprising several subtypes that may be characterized by unique HPA axis profiles that range from hyperactivity to hypoactivity,68 but patients whose depression is associated with HPA axis impairment may benefit from treatment that selectively modulates a single target within the HPA axis.6 For example, melancholic depression (characterized by anhedonia, insomnia, loss of appetite, feelings of worthlessness, and diurnal mood variability) and psychotic depression (characterized by delusions or hallucinations) are associated with HPA axis impairment as demonstrated by elevated cortisol levels.61,115,116 Similarly, patients who are hospitalized for depression and older patients with depression are more likely than nonhospitalized patients and younger patients, respectively, to have HPA axis impairment as demonstrated by elevated cortisol levels.61
Conversely, some studies have shown a reduction in basal cortisol levels in depression and other neuropsychiatric disorders, indicating HPA axis hypoactivity, rather than hyperactivity, in some patients.61,68,77,117 For example, studies have suggested that patients with atypical depression (characterized by hypersomnia, fatigue, hyperphagia, weight gain, and emotional reactivity) may have lower cortisol levels than those with nonatypical depression and may not differ from healthy, nondepressed individuals.61,115 Furthermore, among patients exposed to chronic stressors, including patients with PTSD with or without MDD, reduced cortisol levels were directly proportional to the length of elapsed time between precipitating traumatic event and cortisol assessment.77,117,118 These observations suggest that, following exposure to chronic stress, HPA axis dysregulation follows a nonlinear course in which cortisol levels rise initially in response to the traumatic event or in anticipation of events, then taper over time with increasing chronicity until a state of hypocortisolism is reached.
Also important when considering the relationship between cortisol and depression are the diurnal (circadian) and ultradian (pulsatile) rhythms by which cortisol is released under basal conditions. Ultradian oscillations are characterized by pulsatile bursts of cortisol, CRH, AVP, and ACTH secretions.119,120 Mathematical modeling, confirmed in vivo in rats, supports the hypothesis that the ultradian pulses exchanged within the pituitary-adrenal system provide a dynamic feedforward-feedback regulation that can function independently of hypothalamic control.120,121 Ultradian rhythms contribute to the responsiveness of the HPA axis to stress, and changes in ultradian pulse amplitude and frequency are the foundation of circadian rhythm.119
In healthy individuals, the diurnal pattern is characterized by a marked rise in cortisol upon waking that peaks 50–100% higher than baseline 30–45 minutes later and returns to baseline approximately 1 hour after waking.122 Any point of the diurnal rhythm may be affected in depression, and the specific nature of cortisol changes can be related to disease severity or subtype.122,123 For example, flattened diurnal cortisol rhythms have been observed in severe depression, and distinguishable patterns have been identified in depressed patients with comorbid anxiety.124,125 Both higher and lower/blunted cortisol wakening responses have been observed in depression, with the former exhibiting a predictive relationship with major depressive episodes.122,126,127 Together, data from cortisol studies suggest that HPA axis impairment can result from either too much or too little cortisol, and HPA axis response and disease characteristics may depend on a variety of moderating influences such as features of the stressor, the person, and timing. As a measure of HPA axis function, therefore, cortisol may be a useful biomarker for identifying distinct types of patients with depression who may benefit from treatments that modulate HPA axis activity.68
Treating Depression and Other Neuropsychiatric Disorders by Targeted HPA Axis Modulation
In the approximately 4 years since the onset of the COVID-19 pandemic, global prevalence rates of depressive symptoms have increased from 1.3–11.5% to 18.3–33.7%.128–140 However, because only one-third of patients with depression achieve remission with their first antidepressant and a further third of patients fail to achieve remission with any antidepressant and will be considered treatment resistant, a significant unmet need still remains for new treatment approaches with novel mechanisms of action.141–144 To this end, modulating HPA axis activity with targeted treatments may be a promising approach for patients with depression and other neuropsychiatric disorders associated with HPA axis impairment.109,145 A meta-analysis of 16 randomized clinical trials and 7 open-label studies evaluating HPA axis–targeted therapies reported significant clinical benefits compared with controls, underscoring the potential of this approach for treating patients with depression.145 Also, some individual historical clinical trials of HPA axis–targeted therapies did not show clinical benefits in the overall study population, although post hoc subanalyses of these trials have shown benefit in some patient subgroups with HPA axis hyperactivity.75,146,147 Historically, failure of individual clinical trials to demonstrate efficacy of HPA axis–targeted therapies in patients with depression and other neuropsychiatric disorders may reflect the heterogeneity of the disorders and the broad patient populations enrolled.6,109,147,148 However, careful selection of patients with biomarkers reflecting HPA axis impairment, such as elevated cortisol levels, may be helpful in identifying which patients would benefit most from HPA axis–targeted treatment approaches.6,147
Research is ongoing to identify promising therapeutic targets within the HPA axis.149–151 Studies of CRH receptor antagonists have not reported significant improvements in depression, and the effects of glucocorticoid receptors in the treatment of depression are inconsistent.6,75,152 On the other hand, targeted antagonism of the V1b receptor is a promising treatment approach in patients with depression.6,146 In animal models, antagonism of the V1b receptor has been shown to attenuate depressive-like and anxiety-like behaviors, particularly in stressful situations.6,153 For example, the Brattleboro rat strain, which is characterized by a spontaneous AVP deficiency caused by a single nucleotide deletion in the AVP gene, exhibits reduced depressive-like and anxiety-like behaviors.153,154 Moreover, among Wistar rats that have been selectively bred for high anxiety-like behavior (HAB) or low anxiety-like behavior (LAB), the HAB lines exhibit higher AVP expression in the paraventricular nuclei of the hypothalamus than the LAB lines.155 In male HAB rats, dexamethasone suppression of the diurnal increase in circulating ACTH levels was significantly less efficient and subsequent CRH-stimulated plasma ACTH and corticosterone responses were significantly higher than in male LAB rats; pretreatment with a selective V1a/b receptor antagonist abolished the CRH-stimulated response in dexamethasone-pretreated male HAB rats, demonstrating that vasopressinergic activation accounts for the disrupted HPA axis response in male HAB rats.155 Additional animal model studies have shown that antagonism of the V1b receptor attenuates depressive-like and anxiety-like behaviors.6,19–22,146,153,156–171 Consistent effects of V1b receptor antagonism were not observed in 2 studies; although the reason for this discrepancy is unknown, the researchers speculated that methodological differences in the behavior assays used may have been a contributing factor.6,161,167
In humans, the V1b receptor antagonist ABT-436 has demonstrated reduction of HPA axis parameters such as plasma ACTH, serum and urine cortisol, and urine total glucocorticoids in healthy adults.172 In patients with MDD, research has suggested that ABT-436 was associated with reduced levels of plasma ACTH and cortisol, suggesting potential attenuation of HPA axis activity; further, this study showed statistically significant improvements with ABT-436 over placebo on 2 of the 5 Mood and Anxiety Symptom Questionnaire (MASQ) subscales (ie, subscales “General Distress-Depressive Symptoms” and “General Distress-Mixed Symptoms”) but not in Hamilton Depression Rating Scale [HDRS]) scores following 1 week of treatment.173 The V1b receptor antagonist SSR149415 failed to clearly demonstrate effective treatment of symptoms in patients with generalized anxiety disorder or MDD, although doses used in these trials may have been insufficient to block HPA axis activity and achieve therapeutic effects; these failures may also reflect the heterogeneity of the illness or the broad patient populations enrolled.6,61,174 Using doses determined based on V1b receptor occupancy and nonclinical behavioral models,175,176 adjunctive treatment with the V1b receptor antagonist TS-121 reduced depressive symptoms as assessed by the Montgomery-Åsberg Depression Rating Scale (MADRS), Clinical Global Impression of Severity (CGI-S), and Strengths and Difficulties Questionnaire (SDQ) measures in patients with MDD and inadequate response to their current antidepressant, although the number of patients analyzed was small and these reductions were not statistically significantly different from placebo.146 However, post hoc analyses showed that adjunctive treatment with TS-121 was associated with greater separation in efficacy outcomes compared with placebo among patients with MDD and higher cortisol levels consistent with elevated HPA axis activity.6,146 These observations suggest that, within the subset of patients with MDD who had been screened and met trial inclusion criteria, V1b receptor antagonists may be more efficacious in patients with elevated cortisol levels, consistent with HPA axis hyperactivity, relative to an unscreened population of patients with MDD. Based on these ANC-501 (formerly TS-121) findings and favorable ANC-501 safety and tolerability, a phase 2 trial of adjunctive ANC-501 (NCT05439603) is currently in progress in adults with MDD with history of inadequate response to standard antidepressants and disrupted HPA axis function as indicated by elevated cortisol levels.146,177,178 Based on the results from the phase 2 trial, a double-blind, placebo-controlled trial of ANC-501 is planned for 2023 in patients with depression.179
Although HPA axis dysfunction has been consistently demonstrated in patients with depression and other neuropsychiatric disorders, specific aspects of this dysfunction (eg, hyper- vs hypofunction) have differed across studies, which may be due to the unique pathological characteristics of different neuropsychiatric diseases and the heterogeneity and syndromal nature of many illnesses, as well as the methods employed to study them.61,180,181 Regarding V1b receptors specifically, their activity and the potential efficacy of antagonists in treating neuropsychiatric disorders may also depend upon contextual effects. In rats exposed to acute stress, V1b antagonism reduced ACTH response following lipopolysaccharide injection and restraint stress, but not noise stress.182 In addition, glucocorticoids are subject to regulation by both pituitary-dependent and -independent regulation of the adrenal gland: in a rat chronic stress model, increases in basal corticosterone levels and enhanced rapid corticosterone secretion following exposure to acute stress were both unaffected by CRH antagonism but were sensitive to sympathetic ganglion blockade.183 These findings suggest a role for the sympathetic nervous system in regulating stress-induced glucocorticoid levels.183 In those patients enrolled in the MDD trial of ANC-501 described above, the potential association of ANC-501 efficacy with the clinical biomarker of elevated cortisol may suggest that HPA axis–targeted therapies may only be able to demonstrate clinical treatment effects in patients with measurable HPA axis dysfunction.146 Thus, seemingly inconsistent findings across studies may indicate differences in the nature of HPA axis disturbances specific to the illness under investigation, the study design, or the influence of other contextual factors. Under those circumstances, differing results observed across clinical trials may be more indicative of inherent heterogeneity and the need to accurately identify appropriate testing conditions and more specific patient subgroups than of irregularities in V1b antagonist effects.
Unmet Need in Global Mental Health
In 2019, depressive disorders were among the 10 leading noninfectious drivers of increasing global disease burden.184 In 2020, the estimated global prevalence of MDD (unadjusted) was 193 million people, but many determinants of poor mental health outcomes were exacerbated that year by the emergence of the COVID-19 pandemic, increasing the resulting global MDD prevalence (adjusted) by 28% to 246 million people (Box 1).185 Among patients with depression who receive treatment, research has suggested that up to one-third do not achieve remission of symptoms, even after attempting up to 4 different sequential lines of therapy.186 Therefore, significant unmet needs remain not only for treatment of the global burden of depressive disorders, but also for new treatment approaches with novel mechanisms of action for patients with depression and other neuropsychiatric disorders.
Conclusions
Despite early favorable indications in animal models for targeting HPA axis dysfunction for the treatment of depressive disorders, translation of these findings into clinical efficacy has been challenging,6,23,148 particularly given the heterogeneity and syndromal nature of these diseases.3,187 Therefore, confronting this heterogeneity3 by utilizing an appropriate clinical biomarker,188 such as elevated cortisol, to identify the subset of patients with impaired HPA axis function is a promising next step in modulating HPA axis activity via targeted antagonism of the V1b receptor, facilitating a more tailored approach to the treatment of depression and other neuropsychiatric disorders.
Abbreviations
ACE, adverse childhood experience; ACTH, adrenocorticotropic hormone; AVP, arginine vasopressin; CGI-S, Clinical Global Impression of Severity; CRH, corticotropin-releasing hormone; ELS, early life stress; HAB, high anxiety-like behavior; HCC, hair cortisol concentration; HDRS, Hamilton Depression Rating Scale; HPA, hypothalamic-pituitary-adrenal; LAB, low anxiety-like behavior; MADRS, Montgomery-Åsberg Depression Rating Scale; MASQ, Mood and Anxiety Symptom Questionnaire; MDD, major depressive disorder; PTSD, post-traumatic stress disorder; SDQ, Strengths and Difficulties Questionnaire; V1a, V1b, and V2, vasopressin 1a, 1b, and 2 receptors.
Data Sharing Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Acknowledgments
Medical writing and editorial assistance were provided by Morgan C. Hill, PhD, CMPP, and Shannon Davis of Apollo Medical Communications and funded by EmbarkNeuro, Inc.
Author Contributions
All authors made substantial contributions to conception, design, and scope of this review article; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
Development of this manuscript was funded by EmbarkNeuro, Inc., which has initiated a phase 2 clinical trial of the V1b receptor antagonist ANC-501 as an adjunctive treatment in individuals with MDD (ClinicalTrials.gov identifier: NCT05439603).
Disclosure
S.J.K. and L.D. are employees of and P.P. is a consultant for EmbarkNeuro, Inc. In addition, S.J.K. has a patent “METHODS OF TREATING DEPRESSION WITH 1,2,4-TRIAZOLONE DERIVATIVES” pending to EmbarkNeuro. The authors report no other conflicts of interest in this work.
References
1. Gold PW. The organization of the stress system and its dysregulation in depressive illness. Mol Psychiatry. 2015;20(1):32–47. doi:10.1038/mp.2014.163
2. McEwen BS, Bowles NP, Gray JD, et al. Mechanisms of stress in the brain. Nat Neurosci. 2015;18(10):1353–1363. doi:10.1038/nn.4086
3. McEwen BS, Akil H. Revisiting the stress concept: implications for affective disorders. J Neurosci. 2020;40(1):12–21. doi:10.1523/JNEUROSCI.0733-19.2019
4. McEwen BS, Wingfield JC. The concept of allostasis in biology and biomedicine. Horm Behav. 2003;43(1):2–15. doi:10.1016/s0018-506x(02)00024-7
5. McEwen BS. Stressed or stressed out: what is the difference? J Psychiatry Neurosci. 2005;30(5):315–318.
6. Chaki S. Vasopressin V1B receptor antagonists as potential antidepressants. Int J Neuropsychopharmacol. 2021;24(6):450–463. doi:10.1093/ijnp/pyab013
7. Sandi C, Haller J. Stress and the social brain: behavioural effects and neurobiological mechanisms. Nat Rev Neurosci. 2015;16(5):290–304. doi:10.1038/nrn3918
8. Engelmann M, Landgraf R, Wotjak CT. The hypothalamic-neurohypophysial system regulates the hypothalamic-pituitary-adrenal axis under stress: an old concept revisited. Front Neuroendocrinol. 2004;25(3–4):132–149. doi:10.1016/j.yfrne.2004.09.001
9. Aguilera G, Rabadan-Diehl C. Vasopressinergic regulation of the hypothalamic-pituitary-adrenal axis: implications for stress adaptation. Regul Pept. 2000;96(1–2):23–29. doi:10.1016/s0167-0115(00)00196-8
10. Pariante CM, Lightman SL. The HPA axis in major depression: classical theories and new developments. Trends Neurosci. 2008;31(9):464–468. doi:10.1016/j.tins.2008.06.006
11. Duncan PJ, Sengul S, Tabak J, Ruth P, Bertram R, Shipston MJ. Large conductance Ca(2)(+)-activated K(+) (BK) channels promote secretagogue-induced transition from spiking to bursting in murine anterior pituitary corticotrophs. J Physiol. 2015;593(5):1197–1211. doi:10.1113/jphysiol.2015.284471
12. Peter J, Burbach H, Adan RA, et al. Molecular neurobiology and pharmacology of the vasopressin/oxytocin receptor family. Cell Mol Neurobiol. 1995;15(5):573–595. doi:10.1007/BF02071318
13. Lolait SJ, O’Carroll AM, Mahan LC, et al. Extrapituitary expression of the rat V1b vasopressin receptor gene. Proc Natl Acad Sci U S A. 1995;92(15):6783–6787. doi:10.1073/pnas.92.15.6783
14. Glavas M, Gitlin-Domagalska A, Debowski D, Ptaszynska N, Legowska A, Rolka K. Vasopressin and its analogues: from natural hormones to multitasking peptides. Int J Mol Sci. 2022;23(6). doi:10.3390/ijms23063068
15. Hernando F, Schoots O, Lolait SJ, Burbach JP. Immunohistochemical localization of the vasopressin V1b receptor in the rat brain and pituitary gland: anatomical support for its involvement in the central effects of vasopressin. Endocrinology. 2001;142(4):1659–1668. doi:10.1210/endo.142.4.8067
16. Corbani M, Marir R, Trueba M, et al. Neuroanatomical distribution and function of the vasopressin V1B receptor in the rat brain deciphered using specific fluorescent ligands. Gen Comp Endocrinol. 2018;258:15–32. doi:10.1016/j.ygcen.2017.10.011
17. Vaccari C, Lolait SJ, Ostrowski NL. Comparative distribution of vasopressin V1b and oxytocin receptor messenger ribonucleic acids in brain. Endocrinology. 1998;139(12):5015–5033. doi:10.1210/endo.139.12.6382
18. Catani M, Dellacqua F, Thiebaut de Schotten M. A revised limbic system model for memory, emotion and behaviour. Neurosci Biobehav Rev. 2013;37(8):1724–1737. doi:10.1016/j.neubiorev.2013.07.001
19. Griebel G, Simiand J, Serradeil-Le Gal C, et al. Anxiolytic- and antidepressant-like effects of the non-peptide vasopressin V1b receptor antagonist, SSR149415, suggest an innovative approach for the treatment of stress-related disorders. Proc Natl Acad Sci U S A. 2002;99(9):6370–6375. doi:10.1073/pnas.092012099
20. Salomé N, Stemmelin J, Cohen C, Griebel G. Differential roles of amygdaloid nuclei in the anxiolytic- and antidepressant-like effects of the V1b receptor antagonist, SSR149415, in rats. Psychopharmacology. 2006;187(2):237–244. doi:10.1007/s00213-006-0424-1
21. Stemmelin J, Lukovic L, Salome N, Griebel G. Evidence that the lateral septum is involved in the antidepressant-like effects of the vasopressin V1b receptor antagonist, SSR149415. Neuropsychopharmacology. 2005;30(1):35–42. doi:10.1038/sj.npp.1300562
22. Shimazaki T, Iijima M, Chaki S. The pituitary mediates the anxiolytic-like effects of the vasopressin V1B receptor antagonist, SSR149415, in a social interaction test in rats. Eur J Pharmacol. 2006;543(1–3):63–67. doi:10.1016/j.ejphar.2006.06.032
23. Daskalakis NP, Meijer OC, de Kloet ER. Mineralocorticoid receptor and glucocorticoid receptor work alone and together in cell-type-specific manner: implications for resilience prediction and targeted therapy. Neurobiol Stress. 2022;18:100455. doi:10.1016/j.ynstr.2022.100455
24. McEwen BS. Stress, sex, and neural adaptation to a changing environment: mechanisms of neuronal remodeling. Ann N Y Acad Sci. 2010;1204(Suppl):E38–59. doi:10.1111/j.1749-6632.2010.05568.x
25. Teicher MH, Samson JA, Anderson CM, Ohashi K. The effects of childhood maltreatment on brain structure, function and connectivity. Nat Rev Neurosci. 2016;17(10):652–666. doi:10.1038/nrn.2016.111
26. McEwen BS, Nasca C, Gray JD. Stress effects on neuronal structure: hippocampus, amygdala, and prefrontal cortex. Neuropsychopharmacology. 2016;41(1):3–23. doi:10.1038/npp.2015.171
27. Fogelman N, Canli T. Early life stress and cortisol: a meta-analysis. Horm Behav. 2018;98:63–76. doi:10.1016/j.yhbeh.2017.12.014
28. Dattilo V, Amato R, Perrotti N, Gennarelli M. The emerging role of SGK1 (serum- and glucocorticoid-regulated kinase 1) in major depressive disorder: hypothesis and mechanisms. Front Genet. 2020;11:826. doi:10.3389/fgene.2020.00826
29. Gonda X, Petschner P, Eszlari N, et al. Genetic variants in major depressive disorder: from pathophysiology to therapy. Pharmacol Ther. 2019;194:22–43. doi:10.1016/j.pharmthera.2018.09.002
30. Normann C, Buttenschon HN. Gene-environment interactions between HPA-axis genes and stressful life events in depression: a systematic review. Acta Neuropsychiatr. 2019;31(4):186–192. doi:10.1017/neu.2019.16
31. Silva RC, Maffioletti E, Gennarelli M, Baune BT, Minelli A. Biological correlates of early life stressful events in major depressive disorder. Psychoneuroendocrinology. 2021;125:105103. doi:10.1016/j.psyneuen.2020.105103
32. Bakusic J, Vrieze E, Ghosh M, Bekaert B, Claes S, Godderis L. Increased methylation of NR3C1 and SLC6A4 is associated with blunted cortisol reactivity to stress in major depression. Neurobiol Stress. 2020;13:100272. doi:10.1016/j.ynstr.2020.100272
33. Farrell C, Doolin K, O’Leary N, et al. DNA methylation differences at the glucocorticoid receptor gene in depression are related to functional alterations in hypothalamic-pituitary-adrenal axis activity and to early life emotional abuse. Psychiatry Res. 2018;265:341–348. doi:10.1016/j.psychres.2018.04.064
34. Jiang S, Postovit L, Cattaneo A, Binder EB, Aitchison KJ. Epigenetic modifications in stress response genes associated with childhood trauma. Front Psychiatry. 2019;10:808. doi:10.3389/fpsyt.2019.00808
35. Juruena MF, Gadelrab R, Cleare AJ, Young AH. Epigenetics: a missing link between early life stress and depression. Prog Neuropsychopharmacol Biol Psychiatry. 2021;109:110231. doi:10.1016/j.pnpbp.2020.110231
36. Smart C, Strathdee G, Watson S, Murgatroyd C, McAllister-Williams RH. Early life trauma, depression and the glucocorticoid receptor gene--an epigenetic perspective. Psychol Med. 2015;45(16):3393–3410. doi:10.1017/S0033291715001555
37. Talarowska M. Epigenetic mechanisms in the neurodevelopmental theory of depression. Depress Res Treat. 2020;2020:6357873. doi:10.1155/2020/6357873
38. Nemeroff CB. Paradise lost: the neurobiological and clinical consequences of child abuse and neglect. Neuron. 2016;89(5):892–909. doi:10.1016/j.neuron.2016.01.019
39. Ceruso A, Martínez-Cengotitabengoa M, Peters-Corbett A, Diaz-Gutierrez MJ, Martínez-Cengotitabengoa M. Alterations of the HPA axis observed in patients with major depressive disorder and their relation to early life stress: a systematic review. Neuropsychobiology. 2020;79(6):417–427. doi:10.1159/000506484
40. Dube SR, Felitti VJ, Dong M, Giles WH, Anda RF. The impact of adverse childhood experiences on health problems: evidence from four birth cohorts dating back to 1900. Prev Med. 2003;37(3):268–277. doi:10.1016/s0091-7435(03)00123-3
41. Giano Z, Wheeler DL, Hubach RD. The frequencies and disparities of adverse childhood experiences in the U.S. BMC Public Health. 2020;20(1):1327. doi:10.1186/s12889-020-09411-z
42. Grummitt LR, Kreski NT, Kim SG, Platt J, Keyes KM, McLaughlin KA. Association of childhood adversity with morbidity and mortality in US adults: a systematic review. JAMA Pediatr. 2021;175(12):1269–1278. doi:10.1001/jamapediatrics.2021.2320
43. Nelson J, Klumparendt A, Doebler P, Ehring T. Childhood maltreatment and characteristics of adult depression: meta-analysis. Br J Psychiatry. 2017;210(2):96–104. doi:10.1192/bjp.bp.115.180752
44. Struck N, Krug A, Yuksel D, et al. Childhood maltreatment and adult mental disorders - the prevalence of different types of maltreatment and associations with age of onset and severity of symptoms. Psychiatry Res. 2020;293:113398. doi:10.1016/j.psychres.2020.113398
45. Williams LM, Debattista C, Duchemin AM, Schatzberg AF, Nemeroff CB. Childhood trauma predicts antidepressant response in adults with major depression: data from the randomized international study to predict optimized treatment for depression. Transl Psychiatry. 2016;6:e799. doi:10.1038/tp.2016.61
46. Jackisch J, Brännström L, Almquist YB. Troubled childhoods cast long shadows: childhood adversity and premature all-cause mortality in a Swedish cohort. SSM Popul Health. 2019;9:100506. doi:10.1016/j.ssmph.2019.100506
47. Johnson J, Chaudieu I, Ritchie K, Scali J, Ancelin ML, Ryan J. The extent to which childhood adversity and recent stress influence all-cause mortality risk in older adults. Psychoneuroendocrinology. 2020;111:104492. doi:10.1016/j.psyneuen.2019.104492
48. Lee C, Ryff CD. Pathways linking combinations of early-life adversities to adult mortality: tales that vary by gender. Soc Sci Med. 2019;240:112566. doi:10.1016/j.socscimed.2019.112566
49. Rod NH, Bengtsson J, Budtz-Jørgensen E, et al. Trajectories of childhood adversity and mortality in early adulthood: a population-based cohort study. Lancet. 2020;396(10249):489–497. doi:10.1016/S0140-6736(20)30621-8
50. Marrocco J, Gray JD, Kogan JF, et al. Early life stress restricts translational reactivity in CA3 neurons associated with altered stress responses in adulthood. Front Behav Neurosci. 2019;13:157. doi:10.3389/fnbeh.2019.00157
51. Takesian AE, Hensch TK. Balancing plasticity/stability across brain development. Prog Brain Res. 2013;207:3–34. doi:10.1016/B978-0-444-63327-9.00001-1
52. Teicher MH, Samson JA. Childhood maltreatment and psychopathology: a case for ecophenotypic variants as clinically and neurobiologically distinct subtypes. Am J Psychiatry. 2013;170(10):1114–1133. doi:10.1176/appi.ajp.2013.12070957
53. Parker KJ, Schatzberg AF, Lyons DM. Neuroendocrine aspects of hypercortisolism in major depression. Horm Behav. 2003;43(1):60–66. doi:10.1016/s0018-506x(02)00016-8
54. Tafet GE, Nemeroff CB. The links between stress and depression: psychoneuroendocrinological, genetic, and environmental interactions. J Neuropsychiatry Clin Neurosci. 2016;28(2):77–88. doi:10.1176/appi.neuropsych.15030053
55. Davis EG, Keller J, Hallmayer J, et al. Corticotropin-releasing factor 1 receptor haplotype and cognitive features of major depression. Transl Psychiatry. 2018;8(1):5. doi:10.1038/s41398-017-0051-0
56. Papiol S, Arias B, Gastó C, Gutiérrez B, Catalán R, Fañanás L. Genetic variability at HPA axis in major depression and clinical response to antidepressant treatment. J Affect Disord. 2007;104(1–3):83–90. doi:10.1016/j.jad.2007.02.017
57. Schatzberg AF, Keller J, Tennakoon L, et al. HPA axis genetic variation, cortisol and psychosis in major depression. Mol Psychiatry. 2014;19(2):220–227. doi:10.1038/mp.2013.129
58. van Rossum EF, Binder EB, Majer M, et al. Polymorphisms of the glucocorticoid receptor gene and major depression. Biol Psychiatry. 2006;59(8):681–688. doi:10.1016/j.biopsych.2006.02.007
59. O’Connell CP, Goldstein-Piekarski AN, Nemeroff CB, et al. Antidepressant outcomes predicted by genetic variation in corticotropin-releasing hormone binding protein. Am J Psychiatry. 2018;175(3):251–261. doi:10.1176/appi.ajp.2017.17020172
60. Zajkowska Z, Gullett N, Walsh A, et al. Cortisol and development of depression in adolescence and young adulthood - a systematic review and meta-analysis. Psychoneuroendocrinology. 2022;136:105625. doi:10.1016/j.psyneuen.2021.105625
61. Stetler C, Miller GE. Depression and hypothalamic-pituitary-adrenal activation: a quantitative summary of four decades of research. Psychosom Med. 2011;73(2):114–126. doi:10.1097/PSY.0b013e31820ad12b
62. Feeney J, Kenny RA. Hair cortisol as a risk marker for increased depressive symptoms among older adults during the COVID-19 pandemic. Psychoneuroendocrinology. 2022;143:105847. doi:10.1016/j.psyneuen.2022.105847
63. Kennis M, Gerritsen L, van Dalen M, Williams A, Cuijpers P, Bockting C. Prospective biomarkers of major depressive disorder: a systematic review and meta-analysis. Mol Psychiatry. 2020;25(2):321–338. doi:10.1038/s41380-019-0585-z
64. Ibar C, Fortuna F, Gonzalez D, et al. Evaluation of stress, burnout and hair cortisol levels in health workers at a University Hospital during COVID-19 pandemic. Psychoneuroendocrinology. 2021;128:105213. doi:10.1016/j.psyneuen.2021.105213
65. Šik Novak K, Bogataj Jontez N, Kenig S, et al. The effect of COVID-19 lockdown on mental health, gut microbiota composition and serum cortisol levels. Stress. 2022;25(1):246–257. doi:10.1080/10253890.2022.2082280
66. Yu Y, Liang HF, Chen J, et al. Postpartum depression: current status and possible identification using biomarkers. Front Psychiatry. 2021;12:620371. doi:10.3389/fpsyt.2021.620371
67. Corwin EJ, Pajer K. The psychoneuroimmunology of postpartum depression. J Womens Health. 2008;17(9):1529–1534. doi:10.1089/jwh.2007.0725
68. Glynn LM, Davis EP, Sandman CA. New insights into the role of perinatal HPA-axis dysregulation in postpartum depression. Neuropeptides. 2013;47(6):363–370. doi:10.1016/j.npep.2013.10.007
69. Dickens MJ, Pawluski JL. The HPA axis during the perinatal period: implications for perinatal depression. Endocrinology. 2018;159(11):3737–3746. doi:10.1210/en.2018-00677
70. Garcia-Leal C, De Rezende MG, Corsi-Zuelli FMDG, De Castro M, Del-Ben CM. The functioning of the hypothalamic-pituitary-adrenal (HPA) axis in postpartum depressive states: a systematic review. Expert Rev Endocrinol Metab. 2017;12(5):341–353. doi:10.1080/17446651.2017.1347500
71. Iliadis SI, Comasco E, Sylvén S, Hellgren C, Sundström Poromaa I, Skalkidou A. Prenatal and postpartum evening salivary cortisol levels in association with peripartum depressive symptoms. PLoS One. 2015;10(8):e0135471. doi:10.1371/journal.pone.0135471
72. Pedersen CA, Stern RA, Pate J, Senger MA, Bowes WA, Mason GA. Thyroid and adrenal measures during late pregnancy and the puerperium in women who have been major depressed or who become dysphoric postpartum. J Affect Disord. 1993;29(2–3):201–211. doi:10.1016/0165-0327(93)90034-h
73. Bloch M, Daly RC, Rubinow DR. Endocrine factors in the etiology of postpartum depression. Compr Psychiatry. 2003;44(3):234–246. doi:10.1016/S0010-440X(03)00034-8
74. Gordon JL, Girdler SS, Meltzer-Brody SE, et al. Ovarian hormone fluctuation, neurosteroids, and HPA axis dysregulation in perimenopausal depression: a novel heuristic model. Am J Psychiatry. 2015;172(3):227–236. doi:10.1176/appi.ajp.2014.14070918
75. Dunlop BW, Binder EB, Iosifescu D, et al. Corticotropin-releasing factor receptor 1 antagonism is ineffective for women with posttraumatic stress disorder. Biol Psychiatry. 2017;82(12):866–874. doi:10.1016/j.biopsych.2017.06.024
76. Yehuda R. Status of glucocorticoid alterations in post-traumatic stress disorder. Ann N Y Acad Sci. 2009;1179:56–69. doi:10.1111/j.1749-6632.2009.04979.x
77. Schumacher S, Niemeyer H, Engel S, et al. HPA axis regulation in posttraumatic stress disorder: a meta-analysis focusing on potential moderators. Neurosci Biobehav Rev. 2019;100:35–57. doi:10.1016/j.neubiorev.2019.02.005
78. Juruena MF, Eror F, Cleare AJ, Young AH. The role of early life stress in HPA axis and anxiety. Adv Exp Med Biol. 2020;1191:141–153. doi:10.1007/978-981-32-9705-0_9
79. Belvederi Murri M, Prestia D, Mondelli V, et al. The HPA axis in bipolar disorder: systematic review and meta-analysis. Psychoneuroendocrinology. 2016;63:327–342. doi:10.1016/j.psyneuen.2015.10.014
80. Zhang M, Zhao S, Chen Y, et al. Chronic stress in bipolar disorders across the different clinical states: roles of HPA axis and personality. Neuropsychiatr Dis Treat. 2022;18:1715–1725. doi:10.2147/NDT.S372358
81. Juruena MF, Cleare AJ, Young AH. Neuroendocrine stress system in bipolar disorder. Curr Top Behav Neurosci. 2021;48:149–171. doi:10.1007/7854_2020_184
82. Ji E, Weickert CS, Purves-Tyson T, et al. Cortisol-dehydroepiandrosterone ratios are inversely associated with hippocampal and prefrontal brain volume in schizophrenia. Psychoneuroendocrinology. 2021;123:104916. doi:10.1016/j.psyneuen.2020.104916
83. Yang F, Cao X, Sun X, Wen H, Qiu J, Xiao H. Hair cortisol is associated with social support and symptoms in schizophrenia. Front Psychiatry. 2020;11:572656. doi:10.3389/fpsyt.2020.572656
84. Monteleone AM, Patriciello G, Ruzzi V, et al. Deranged emotional and cortisol responses to a psychosocial stressor in anorexia nervosa women with childhood trauma exposure: evidence for a “maltreated ecophenotype”? J Psychiatr Res. 2018;104:39–45. doi:10.1016/j.jpsychires.2018.06.013
85. Demitrack MA, Kalogeras KT, Altemus M, Pigott TA, Listwak SJ, Gold PW. Plasma and cerebrospinal fluid measures of arginine vasopressin secretion in patients with bulimia nervosa and in healthy subjects. J Clin Endocrinol Metab. 1992;74(6):1277–1283. doi:10.1210/jcem.74.6.1592871
86. Het S, Vocks S, Wolf JM, Herpertz S, Wolf OT. Treatment-resistant blunted HPA activity, but reversible cardiovascular stress reactivity in young women with eating disorders. Front Psychiatry. 2020;11:726. doi:10.3389/fpsyt.2020.00726
87. Abramova O, Zorkina Y, Ushakova V, Zubkov E, Morozova A, Chekhonin V. The role of oxytocin and vasopressin dysfunction in cognitive impairment and mental disorders. Neuropeptides. 2020;83:102079. doi:10.1016/j.npep.2020.102079
88. O’Keane V, Frodl T, Dinan TG. A review of atypical depression in relation to the course of depression and changes in HPA axis organization. Psychoneuroendocrinology. 2012;37(10):1589–1599. doi:10.1016/j.psyneuen.2012.03.009
89. de Winter RF, van Hemert AM, DeRijk RH, et al. Anxious-retarded depression: relation with plasma vasopressin and cortisol. Neuropsychopharmacology. 2003;28(1):140–147. doi:10.1038/sj.npp.1300002
90. Meynen G, Unmehopa UA, van Heerikhuize JJ, Hofman MA, Swaab DF, Hoogendijk WJ. Increased arginine vasopressin mRNA expression in the human hypothalamus in depression: a preliminary report. Biol Psychiatry. 2006;60(8):892–895. doi:10.1016/j.biopsych.2005.12.010
91. Purba JS, Hoogendijk WJ, Hofman MA, Swaab DF. Increased number of vasopressin- and oxytocin-expressing neurons in the paraventricular nucleus of the hypothalamus in depression. Arch Gen Psychiatry. 1996;53(2):137–143. doi:10.1001/archpsyc.1996.01830020055007
92. van Londen L, Goekoop JG, van Kempen GM, et al. Plasma levels of arginine vasopressin elevated in patients with major depression. Neuropsychopharmacology. 1997;17(4):284–292. doi:10.1016/S0893-133X(97)00054-7
93. Zhou JN, Riemersma RF, Unmehopa UA, et al. Alterations in arginine vasopressin neurons in the suprachiasmatic nucleus in depression. Arch Gen Psychiatry. 2001;58(7):655–662. doi:10.1001/archpsyc.58.7.655
94. Altemus M, Pigott T, Kalogeras KT, et al. Abnormalities in the regulation of vasopressin and corticotropin releasing factor secretion in obsessive-compulsive disorder. Arch Gen Psychiatry. 1992;49(1):9–20. doi:10.1001/archpsyc.1992.01820010009002
95. de Kloet CS, Vermetten E, Geuze E, Wiegant VM, Westenberg HG. Elevated plasma arginine vasopressin levels in veterans with posttraumatic stress disorder. J Psychiatr Res. 2008;42(3):192–198. doi:10.1016/j.jpsychires.2006.11.009
96. Inder WJ, Donald RA, Prickett TC, et al. Arginine vasopressin is associated with hypercortisolemia and suicide attempts in depression. Biol Psychiatry. 1997;42(8):744–747. doi:10.1016/s0006-3223(97)00301-6
97. Dinan TG, O’Brien S, Lavelle E, Scott LV. Further neuroendocrine evidence of enhanced vasopressin V3 receptor responses in melancholic depression. Psychol Med. 2004;34(1):169–172. doi:10.1017/s0033291703001004
98. McEwen BS. Allostasis and allostatic load: implications for neuropsychopharmacology. Neuropsychopharmacology. 2000;22(2):108–124. doi:10.1016/S0893-133X(99)00129-3
99. Brown ES, Varghese FP, McEwen BS. Association of depression with medical illness: does cortisol play a role? Biol Psychiatry. 2004;55(1):1–9. doi:10.1016/s0006-3223(03)00473-6
100. Charles ST, Mogle J, Piazza JR, Karlamangla A, Almeida DM. Going the distance: the diurnal range of cortisol and its association with cognitive and physiological functioning. Psychoneuroendocrinology. 2020;112:104516. doi:10.1016/j.psyneuen.2019.104516
101. Salzmann S, Salzmann-Djufri M, Euteneuer F. Childhood emotional neglect and cardiovascular disease: a narrative review. Front Cardiovasc Med. 2022;9:815508. doi:10.3389/fcvm.2022.815508
102. Gragnoli C. Hypothesis of the neuroendocrine cortisol pathway gene role in the comorbidity of depression, type 2 diabetes, and metabolic syndrome. Appl Clin Genet. 2014;7:43–53. doi:10.2147/TACG.S39993
103. Hoogendoorn CJ, Roy JF, Gonzalez JS. Shared dysregulation of homeostatic brain-body pathways in depression and type 2 diabetes. Curr Diab Rep. 2017;17(10):90. doi:10.1007/s11892-017-0923-y
104. Joseph JJ, Golden SH. Cortisol dysregulation: the bidirectional link between stress, depression, and type 2 diabetes mellitus. Ann N Y Acad Sci. 2017;1391(1):20–34. doi:10.1111/nyas.13217
105. Marazziti D, Rutigliano G, Baroni S, Landi P, Dell’Osso L. Metabolic syndrome and major depression. CNS Spectr. 2014;19(4):293–304. doi:10.1017/S1092852913000667
106. Vogelzangs N, Suthers K, Ferrucci L, et al. Hypercortisolemic depression is associated with the metabolic syndrome in late-life. Psychoneuroendocrinology. 2007;32(2):151–159. doi:10.1016/j.psyneuen.2006.11.009
107. Björntorp P. Do stress reactions cause abdominal obesity and comorbidities? Obes Rev. 2001;2(2):73–86. doi:10.1046/j.1467-789x.2001.00027.x
108. Martins LB, Monteze NM, Calarge C, Ferreira AVM, Teixeira AL. Pathways linking obesity to neuropsychiatric disorders. Nutrition. 2019;66:16–21. doi:10.1016/j.nut.2019.03.017
109. Mikulska J, Juszczyk G, Gawrońska-Grzywacz M, Herbet M. HPA axis in the pathomechanism of depression and schizophrenia: new therapeutic strategies based on its participation. Brain Sci. 2021;11(10):1298. doi:10.3390/brainsci11101298
110. Stawski RS, Almeida DM, Lachman ME, Tun PA, Rosnick CB, Seeman T. Associations between cognitive function and naturally occurring daily cortisol during middle adulthood: timing is everything. J Gerontol B Psychol Sci Soc Sci. 2011;66(Suppl 1):i71–i81. doi:10.1093/geronb/gbq094
111. Rosenblat JD, Gregory JM, Carvalho AF, McIntyre RS. Depression and disturbed bone metabolism: a narrative review of the epidemiological findings and postulated mechanisms. Curr Mol Med. 2016;16(2):165–178. doi:10.2174/1566524016666160126144303
112. Göthe F, Enache D, Wahlund LO, et al. Cerebrovascular diseases and depression: epidemiology, mechanisms and treatment. Panminerva Med. 2012;54(3):161–170.
113. Villa RF, Ferrari F, Moretti A. Post-stroke depression: mechanisms and pharmacological treatment. Pharmacol Ther. 2018;184:131–144. doi:10.1016/j.pharmthera.2017.11.005
114. Wang Y, Wang H, Sun W, et al. Higher concentration of adrenocorticotropic hormone predicts post-stroke depression. Clin Interv Aging. 2022;17:417–427. doi:10.2147/CIA.S356361
115. Lamers F, Vogelzangs N, Merikangas KR, de Jonge P, Beekman AT, Penninx BW. Evidence for a differential role of HPA-axis function, inflammation and metabolic syndrome in melancholic versus atypical depression. Mol Psychiatry. 2013;18(6):692–699. doi:10.1038/mp.2012.144
116. Nelson JC, Davis JM. DST studies in psychotic depression: a meta-analysis. Am J Psychiatry. 1997;154(11):1497–1503. doi:10.1176/ajp.154.11.1497
117. Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychol Bull. 2007;133(1):25–45. doi:10.1037/0033-2909.133.1.25
118. Morris MC, Compas BE, Garber J. Relations among posttraumatic stress disorder, comorbid major depression, and HPA function: a systematic review and meta-analysis. Clin Psychol Rev. 2012;32(4):301–315. doi:10.1016/j.cpr.2012.02.002
119. Walker JJ, Terry JR, Lightman SL. Origin of ultradian pulsatility in the hypothalamic-pituitary-adrenal axis. Proc Biol Sci. 2010;277(1688):1627–1633. doi:10.1098/rspb.2009.2148
120. Walker JJ, Romano N. Fast dynamics in the HPA axis: insight from mathematical and experimental studies. Curr Opin Endocr Metab Res. 2022;27:100403. doi:10.1016/j.coemr.2022.100403
121. Churilov AN, Milton JG. Modeling pulsativity in the hypothalamic-pituitary-adrenal hormonal axis. Sci Rep. 2022;12(1):8480. doi:10.1038/s41598-022-12513-w
122. Dedovic K, Ngiam J. The cortisol awakening response and major depression: examining the evidence. Neuropsychiatr Dis Treat. 2015;11:1181–1189. doi:10.2147/NDT.S62289
123. Keller J, Gomez R, Williams G, et al. HPA axis in major depression: cortisol, clinical symptomatology and genetic variation predict cognition. Mol Psychiatry. 2017;22(4):527–536. doi:10.1038/mp.2016.120
124. Hsiao FH, Yang TT, Ho RT, et al. The self-perceived symptom distress and health-related conditions associated with morning to evening diurnal cortisol patterns in outpatients with major depressive disorder. Psychoneuroendocrinology. 2010;35(4):503–515. doi:10.1016/j.psyneuen.2009.08.019
125. Xie Z, Deng Y, Xie C, Yao Y. Changes of adrenocorticotropic hormone rhythm and cortisol circadian rhythm in patients with depression complicated with anxiety and their effects on the psychological state of patients. Front Psychiatry. 2022;13:1030811. doi:10.3389/fpsyt.2022.1030811
126. Linnemann P, Friedrich N, Nauck M, Teismann H, Berger K. The relationship between cortisol awakening response and trait resilience in two patient cohorts and one population-based cohort. World J Biol Psychiatry. 2022;1–10. doi:10.1080/15622975.2022.2129445
127. Vrshek-Schallhorn S, Doane LD, Mineka S, Zinbarg RE, Craske MG, Adam EK. The cortisol awakening response predicts major depression: predictive stability over a 4-year follow-up and effect of depression history. Psychol Med. 2013;43(3):483–493. doi:10.1017/S0033291712001213
128. Blasco-Belled A, Tejada-Gallardo C, Fatsini-Prats M, Alsinet C. Mental health among the general population and healthcare workers during the COVID-19 pandemic: a meta-analysis of well-being and psychological distress prevalence. Curr Psychol. 2022;1–12. doi:10.1007/s12144-022-02913-6
129. Bueno-Notivol J, Gracia-García P, Olaya B, Lasheras I, López-Antón R, Santabárbara J. Prevalence of depression during the COVID-19 outbreak: a meta-analysis of community-based studies. Int J Clin Health Psychol. 2021;21(1):100196. doi:10.1016/j.ijchp.2020.07.007
130. Dattani S, Ritchie H, Roser M. Mental health; 2021. Available from: https://ourworldindata.org/mental-health.
131. Castaldelli-Maia JM, Marziali ME, Lu Z, Martins SS. Investigating the effect of national government physical distancing measures on depression and anxiety during the COVID-19 pandemic through meta-analysis and meta-regression. Psychol Med. 2021;51(6):881–893. doi:10.1017/S0033291721000933
132. Chekole YA, Abate SM. Global prevalence and determinants of mental health disorders during the COVID-19 pandemic: a systematic review and meta-analysis. Ann Med Surg. 2021;68:102634. doi:10.1016/j.amsu.2021.102634
133. Dragioti E, Li H, Tsitsas G, et al. A large-scale meta-analytic atlas of mental health problems prevalence during the COVID-19 early pandemic. J Med Virol. 2022;94(5):1935–1949. doi:10.1002/jmv.27549
134. Necho M, Tsehay M, Birkie M, Biset G, Tadesse E. Prevalence of anxiety, depression, and psychological distress among the general population during the COVID-19 pandemic: a systematic review and meta-analysis. Int J Soc Psychiatry. 2021;67(7):892–906. doi:10.1177/00207640211003121
135. Nochaiwong S, Ruengorn C, Thavorn K, et al. Global prevalence of mental health issues among the general population during the coronavirus disease-2019 pandemic: a systematic review and meta-analysis. Sci Rep. 2021;11(1):10173. doi:10.1038/s41598-021-89700-8
136. Phiri P, Ramakrishnan R, Rathod S, et al. An evaluation of the mental health impact of SARS-CoV-2 on patients, general public and healthcare professionals: a systematic review and meta-analysis. EClinicalMedicine. 2021;34:100806. doi:10.1016/j.eclinm.2021.100806
137. Salari N, Hosseinian-Far A, Jalali R, et al. Prevalence of stress, anxiety, depression among the general population during the COVID-19 pandemic: a systematic review and meta-analysis. Global Health. 2020;16(1):57. doi:10.1186/s12992-020-00589-w
138. Schafer KM, Lieberman A, Sever AC, Joiner T. Prevalence rates of anxiety, depressive, and eating pathology symptoms between the pre- and peri-COVID-19 eras: a meta-analysis. J Affect Disord. 2022;298(Pt A):364–372. doi:10.1016/j.jad.2021.10.115
139. US CDC National Center for Health Statistics. Anxiety and depression. Household Pulse Survey; 2022. Available from: https://www.cdc.gov/nchs/covid19/pulse/mental-health.htm.
140. Wu T, Jia X, Shi H, et al. Prevalence of mental health problems during the COVID-19 pandemic: a systematic review and meta-analysis. J Affect Disord. 2021;281:91–98. doi:10.1016/j.jad.2020.11.117
141. Sackeim HA, Aaronson ST, Bunker MT, et al. The assessment of resistance to antidepressant treatment: rationale for the Antidepressant Treatment History Form: Short Form (ATHF-SF). J Psychiatr Res. 2019;113:125–136. doi:10.1016/j.jpsychires.2019.03.021
142. Trivedi MH, Rush AJ, Wisniewski SR, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163(1):28–40. doi:10.1176/appi.ajp.163.1.28
143. Elias E, Zhang AY, Manners MT. Novel pharmacological approaches to the treatment of depression. Life. 2022;12(2). doi:10.3390/life12020196
144. Sinyor M, Schaffer A, Levitt A. The sequenced treatment alternatives to relieve depression (STAR*D) trial: a review. Can J Psychiatry. 2010;55(3):126–135. doi:10.1177/070674371005500303
145. Ding Y, Wei Z, Yan H, Guo W. Efficacy of treatments targeting hypothalamic-pituitary-adrenal systems for major depressive disorder: a meta-analysis. Front Pharmacol. 2021;12:732157. doi:10.3389/fphar.2021.732157
146. Kamiya M, Sabia HD, Marella J, et al. Efficacy and safety of TS-121, a novel vasopressin V1B receptor antagonist, as adjunctive treatment for patients with major depressive disorder: a randomized, double-blind, placebo-controlled study. J Psychiatr Res. 2020;128:43–51. doi:10.1016/j.jpsychires.2020.05.017
147. Menke A. Is the HPA axis as target for depression outdated, or is there a new hope? Front Psychiatry. 2019;10:101. doi:10.3389/fpsyt.2019.00101
148. Kokras N, Hodes GE, Bangasser DA, Dalla C. Sex differences in the hypothalamic-pituitary-adrenal axis: an obstacle to antidepressant drug development? Br J Pharmacol. 2019;176(21):4090–4106. doi:10.1111/bph.14710
149. Ben-Zvi A, Vernon SD, Broderick G. Model-based therapeutic correction of hypothalamic-pituitary-adrenal axis dysfunction. PLoS Comput Biol. 2009;5(1):e1000273. doi:10.1371/journal.pcbi.1000273
150. Karin O, Raz M, Tendler A, et al. A new model for the HPA axis explains dysregulation of stress hormones on the timescale of weeks. Mol Syst Biol. 2020;16(7):e9510. doi:10.15252/msb.20209510
151. Shipston MJ. Glucocorticoid action in the anterior pituitary gland: insights from corticotroph physiology. Curr Opin Endocr Metab Res. 2022;25:100358. doi:10.1016/j.coemr.2022.100358
152. Schüle C, Baghai TC, Eser D, Rupprecht R. Hypothalamic-pituitary-adrenocortical system dysregulation and new treatment strategies in depression. Expert Rev Neurother. 2009;9(7):1005–1019. doi:10.1586/ern.09.52
153. Varga J, Fodor A, Klausz B, Zelena D. Anxiogenic role of vasopressin during the early postnatal period: maternal separation-induced ultrasound vocalization in vasopressin-deficient Brattleboro rats. Amino Acids. 2015;47(11):2409–2418. doi:10.1007/s00726-015-2034-x
154. Mlynarik M, Zelena D, Bagdy G, Makara GB, Jezova D. Signs of attenuated depression-like behavior in vasopressin deficient Brattleboro rats. Horm Behav. 2007;51(3):395–405. doi:10.1016/j.yhbeh.2006.12.007
155. Keck ME, Wigger A, Welt T, et al. Vasopressin mediates the response of the combined dexamethasone/CRH test in hyper-anxious rats: implications for pathogenesis of affective disorders. Neuropsychopharmacology. 2002;26(1):94–105. doi:10.1016/S0893-133X(01)00351-7
156. Serradeil-Le Gal C, Wagnon J, Simiand J, et al. Characterization of (2S,4R)-1-[5-chloro-1-[(2,4-dimethoxyphenyl)sulfonyl]-3-(2-methoxy-phenyl)-2-oxo-2,3-dihydro-1H-indol-3-yl]-4-hydroxy-N,N-dimethyl-2-pyrrolidine carboxamide (SSR149415), a selective and orally active vasopressin V1b receptor antagonist. J Pharmacol Exp Ther. 2002;300(3):1122–1130. doi:10.1124/jpet.300.3.1122
157. Alonso R, Griebel G, Pavone G, Stemmelin J, Le Fur G, Soubrié P. Blockade of CRF(1) or V(1b) receptors reverses stress-induced suppression of neurogenesis in a mouse model of depression. Mol Psychiatry. 2004;9(3):278–286, 224. doi:10.1038/sj.mp.4001464
158. Iijima M, Chaki S. Separation-induced ultrasonic vocalization in rat pups: further pharmacological characterization. Pharmacol Biochem Behav. 2005;82(4):652–657. doi:10.1016/j.pbb.2005.11.005
159. Overstreet DH, Griebel G. Antidepressant-like effects of the vasopressin V1b receptor antagonist SSR149415 in the Flinders Sensitive Line rat. Pharmacol Biochem Behav. 2005;82(1):223–227. doi:10.1016/j.pbb.2005.07.021
160. Louis C, Cohen C, Depoortere R, Griebel G. Antidepressant-like effects of the corticotropin-releasing factor 1 receptor antagonist, SSR125543, and the vasopressin 1b receptor antagonist, SSR149415, in a DRL-72 s schedule in the rat. Neuropsychopharmacology. 2006;31(10):2180–2187. doi:10.1038/sj.npp.1301036
161. Hodgson RA, Higgins GA, Guthrie DH, et al. Comparison of the V1b antagonist, SSR149415, and the CRF1 antagonist, CP-154,526, in rodent models of anxiety and depression. Pharmacol Biochem Behav. 2007;86(3):431–440. doi:10.1016/j.pbb.2006.12.021
162. Iijima M, Chaki S. An arginine vasopressin V1b antagonist, SSR149415 elicits antidepressant-like effects in an olfactory bulbectomy model. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(3):622–627. doi:10.1016/j.pnpbp.2006.12.008
163. Surget A, Saxe M, Leman S, et al. Drug-dependent requirement of hippocampal neurogenesis in a model of depression and of antidepressant reversal. Biol Psychiatry. 2008;64(4):293–301. doi:10.1016/j.biopsych.2008.02.022
164. Bessa JM, Ferreira D, Melo I, et al. The mood-improving actions of antidepressants do not depend on neurogenesis but are associated with neuronal remodeling. Mol Psychiatry. 2009;14(8):764–773, 739. doi:10.1038/mp.2008.119
165. Breuer ME, van Gaalen MM, Wernet W, et al. SSR149415, a non-peptide vasopressin V1b receptor antagonist, has long-lasting antidepressant effects in the olfactory bulbectomy-induced hyperactivity depression model. Naunyn Schmiedebergs Arch Pharmacol. 2009;379(1):101–106. doi:10.1007/s00210-008-0336-1
166. Litvin Y, Murakami G, Pfaff DW. Effects of chronic social defeat on behavioral and neural correlates of sociality: vasopressin, oxytocin and the vasopressinergic V1b receptor. Physiol Behav. 2011;103(3–4):393–403. doi:10.1016/j.physbeh.2011.03.007
167. Hodgson RA, Mullins D, Lu SX, et al. Characterization of a novel vasopressin V1b receptor antagonist, V1B-30N, in animal models of anxiety-like and depression-like behavior. Eur J Pharmacol. 2014;730:157–163. doi:10.1016/j.ejphar.2014.02.027
168. Iijima M, Yoshimizu T, Shimazaki T, et al. Antidepressant and anxiolytic profiles of newly synthesized arginine vasopressin V1B receptor antagonists: TASP0233278 and TASP0390325. Br J Pharmacol. 2014;171(14):3511–3525. doi:10.1111/bph.12699
169. Bayerl DS, Hönig JN, Bosch OJ. Vasopressin V1a, but not V1b, receptors within the PVN of lactating rats mediate maternal care and anxiety-related behaviour. Behav Brain Res. 2016;305:18–22. doi:10.1016/j.bbr.2016.02.020
170. Poretti MB, Sawant RS, Rask-Andersen M, et al. Reduced vasopressin receptors activation mediates the anti-depressant effects of fluoxetine and venlafaxine in bulbectomy model of depression. Psychopharmacology. 2016;233(6):1077–1086. doi:10.1007/s00213-015-4187-4
171. Hernández-Pérez OR, Crespo-Ramírez M, Cuza-Ferrer Y, et al. Differential activation of arginine-vasopressin receptor subtypes in the amygdaloid modulation of anxiety in the rat by arginine-vasopressin. Psychopharmacology. 2018;235(4):1015–1027. doi:10.1007/s00213-017-4817-0
172. Katz DA, Liu W, Locke C, Dutta S, Tracy KA. Clinical safety and hypothalamic-pituitary-adrenal axis effects of the arginine vasopressin type 1B receptor antagonist ABT-436. Psychopharmacology. 2016;233(1):71–81. doi:10.1007/s00213-015-4089-5
173. Katz DA, Locke C, Greco N, Liu W, Tracy KA. Hypothalamic-pituitary-adrenal axis and depression symptom effects of an arginine vasopressin type 1B receptor antagonist in a one-week randomized phase 1b trial. Brain Behav. 2017;7(3):e00628. doi:10.1002/brb3.628
174. Griebel G, Beeské S, Stahl SM. The vasopressin V(1b) receptor antagonist SSR149415 in the treatment of major depressive and generalized anxiety disorders: results from 4 randomized, double-blind, placebo-controlled studies. J Clin Psychiatry. 2012;73(11):1403–1411. doi:10.4088/JCP.12m07804
175. Koga K, Nagai Y, Hanyu M, et al. High-contrast PET imaging of vasopressin V1B receptors with a novel radioligand, (11)C-TASP699. J Nucl Med. 2017;58(10):1652–1658. doi:10.2967/jnumed.116.188698
176. Inatani S, Mizuno-Yasuhira A, Kamiya M, Nishino I, Sabia HD, Endo H. Prediction of a clinically effective dose of THY1773, a novel V(1B) receptor antagonist, based on preclinical data. Biopharm Drug Dispos. 2021;42(5):204–217. doi:10.1002/bdd.2273
177. US National Institutes of Health. ANC-501 in the treatment of adults with major depressive disorder (NCT05439603). Available from: https://www.clinicaltrials.gov/ct2/show/NCT05439603.
178. Kanes SJ, Dennie L. ANC-501: a novel V1b receptor antagonist for major depressive disorder.
179. EmbarkNeuro. EmbarkNeuro announces initiation of phase 2 trial of ANC-501, a first-in-class, V1b receptor antagonist for the personalized treatment of depression [press release]; 2022 [November 21]. Available from: https://embarkneuro.com/companynews/embarkneuro-announces-initiation-of-phase-2-trial-of-anc-501-A-first-in-class-v1b-receptor-antagonist-for-The-personalized-treatment-of-depression/.
180. Juruena MF, Bocharova M, Agustini B, Young AH. Atypical depression and non-atypical depression: is HPA axis function a biomarker? A systematic review. J Affect Disord. 2018;233:45–67. doi:10.1016/j.jad.2017.09.052
181. Kunugi H, Hori H, Ogawa S. Biochemical markers subtyping major depressive disorder. Psychiatry Clin Neurosci. 2015;69(10):597–608. doi:10.1111/pcn.12299
182. Spiga F, Harrison LR, Wood S, et al. Blockade of the V(1b) receptor reduces ACTH, but not corticosterone secretion induced by stress without affecting basal hypothalamic-pituitary-adrenal axis activity. J Endocrinol. 2009;200(3):273–283. doi:10.1677/JOE-08-0421
183. Lowrance SA, Ionadi A, McKay E, Douglas X, Johnson JD. Sympathetic nervous system contributes to enhanced corticosterone levels following chronic stress. Psychoneuroendocrinology. 2016;68:163–170. doi:10.1016/j.psyneuen.2016.02.027
184. GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1204–1222. doi:10.1016/S0140-6736(20)30925-9
185. COVID-Mental Disorders Collaborators. Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID-19 pandemic. Lancet. 2021;398(10312):1700–1712. doi:10.1016/S0140-6736(21)02143-7
186. Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163(11):1905–1917. doi:10.1176/ajp.2006.163.11.1905
187. Goldberg D. The heterogeneity of “major depression”. World Psychiatry. 2011;10(3):226–228. doi:10.1002/j.2051-5545.2011.tb00061.x
188. Cook D, Brown D, Alexander R, et al. Lessons learned from the fate of AstraZeneca’s drug pipeline: a five-dimensional framework. Nat Rev Drug Discov. 2014;13(6):419–431. doi:10.1038/nrd4309
189. Coiro MJ, Watson KH, Ciriegio A, et al. Coping with COVID-19 stress: associations with depression and anxiety in a diverse sample of U.S. adults. Curr Psychol. 2021:1–13. doi:10.1007/s12144-021-02444-6
190. Daimer S, Mihatsch LL, Neufeld SAS, Murray GK, Knolle F. Investigating the relationship of COVID-19 related stress and media consumption with schizotypy, depression, and anxiety in cross-sectional surveys repeated throughout the pandemic in Germany and the UK. Elife. 2022;11. doi:10.7554/eLife.75893
191. Ettman CK, Abdalla SM, Cohen GH, Sampson L, Vivier PM, Galea S. Prevalence of depression symptoms in US adults before and during the COVID-19 pandemic. JAMA Netw Open. 2020;3(9):e2019686. doi:10.1001/jamanetworkopen.2020.19686
192. Ettman CK, Cohen GH, Abdalla SM, et al. Persistent depressive symptoms during COVID-19: a national, population-representative, longitudinal study of U.S. adults. Lancet Reg Health Am. 2022;5:100091. doi:10.1016/j.lana.2021.100091
193. MacDonald JJ, Baxter-King R, Vavreck L, et al. Depressive symptoms and anxiety during the COVID-19 pandemic: large, longitudinal, cross-sectional survey. JMIR Ment Health. 2022;9(2):e33585. doi:10.2196/33585
194. Monistrol-Mula A, Felez-Nobrega M, Domènech-Abella J, et al. The impact of COVID-related perceived stress and social support on generalized anxiety and major depressive disorders: moderating effects of pre-pandemic mental disorders. Ann Gen Psychiatry. 2022;21(1):7. doi:10.1186/s12991-022-00385-3
195. Taylor S, Landry CA, Paluszek MM, Fergus TA, McKay D, Asmundson GJG. COVID stress syndrome: concept, structure, and correlates. Depress Anxiety. 2020;37(8):706–714. doi:10.1002/da.23071
196. Daly M, Sutin AR, Robinson E. Depression reported by US adults in 2017–2018 and March and April 2020. J Affect Disord. 2021;278:131–135. doi:10.1016/j.jad.2020.09.065
197. Perlis R, Green J, Simonson M, et al. The COVID States Project: a 50-state COVID-19 survey. Report #54: mental health in the US; 2021. Available from: https://www.covidstates.org/reports/mental-health-in-the-united-states.
198. Feneberg AC, Forbes PAG, Piperno G, et al. Diurnal dynamics of stress and mood during COVID-19 lockdown: a large multinational ecological momentary assessment study. Proc Biol Sci. 2022;289(1975):20212480. doi:10.1098/rspb.2021.2480
199. Antoni FA. Magnocellular vasopressin and the mechanism of “glucocorticoid escape”. Front Endocrinol. 2019;10:422. doi:10.3389/fendo.2019.00422
200. Gjerstad JK, Lightman SL, Spiga F. Role of glucocorticoid negative feedback in the regulation of HPA axis pulsatility. Stress. 2018;21(5):403–416. doi:10.1080/10253890.2018.1470238
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.