Back to Journals » Journal of Inflammation Research » Volume 17
TAK-3 Inhibits Lipopolysaccharide-Induced Neuroinflammation in Traumatic Brain Injury Rats Through the TLR-4/NF-κB Pathway
Authors Hou P, Yang Y , Li Z, Ye D, Chen L, Feng T, Zeng J, Wei L, Wang S
Received 4 January 2024
Accepted for publication 26 March 2024
Published 9 April 2024 Volume 2024:17 Pages 2147—2158
DOI https://doi.org/10.2147/JIR.S454099
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Pengwei Hou,1,* Yang Yang,2,* Ziqi Li,1,* Dan Ye,2 Li Chen,1 Tianshun Feng,3 Jiateng Zeng,4 Liangfeng Wei,1 Shousen Wang1,5
1Department of Neurosurgery, Fuzong Clinical Medical College of Fujian Medical University (The 900TH Hospital), Fuzhou, Fujian Province, People’s Republic of China; 2Fuzhou General Teaching Hospital of Fujian University of Traditional Chinese Medicine, Fuzhou, Fujian Province, People’s Republic of China; 3Department of Neurosurgery, Dongfang Affiliated Hospital of Xiamen University School of Medicine, Xiamen University, Xiamen, Fujian Province, People’s Republic of China; 4Department of Neurosurgery, Neurosurgery Research Institute, the First Affiliated Hospital, Fujian Medical University, Fuzhou, Fujian Province, People’s Republic of China; 5Fujian Provincial Clinical Medical Research Center for Minimally Invasive Diagnosis and Treatment of Neurovascular Diseases, Fuzhou, Fujian Province, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Liangfeng Wei; Shousen Wang, Department of Neurosurgery, Fuzong Clinical Medical College of Fujian Medical University (The 900TH Hospital), No. 156, Xierhuanbei Road, Fuzhou, Fujian Province, 350025, People’s Republic of China, Tel +8613960760177 ; +8613950482966, Email [email protected]; [email protected]
Purpose: The activation of the inflammatory response is regarded as a pivotal factor in the pathogenesis of TBI. Central nervous system infection often leads to the exacerbation of neuroinflammation following TBI, primarily caused by Gram-negative bacteria. This study aims to elucidate the effects of the novel anti-inflammatory drug TAK-3 on LPS-induced neuroinflammation in TBI rats.
Methods: In conjunction with the rat controlled cortical impact model, we administered local injections of Lipopolysaccharide to the impact site. Subsequently, interventions were implemented through intraperitoneal injections of TAK-3 and NF-κB activitor2 to modulate the TLR4/NF-κB axis The impact of LPS on neurological function was assessed using mNSS, open field test, and brain water content measurement. Inflammatory markers, including TNF-α, IL-1β, IL-6 and IL-10 were assessed to evaluate the condition of neuritis by Elisa. The activation of the TLR-4/NF-κB signaling pathway was detected by immunofluorescence staining and Western blot to assess the anti-inflammatory effects of TAK-3.
Results: The administration of LPS exacerbated neurological damage in rats with TBI, as evidenced by a reduction in motor activity and an increase in anxiety-like behavior. Furthermore, LPS induced disruption of the blood-brain barrier integrity and facilitated the development of brain edema. The activation of microglia and astrocytes by LPS at the cellular and molecular levels has been demonstrated to induce a significant upregulation of neuroinflammatory factors. The injection of TAK-3 attenuated the neuroinflammatory response induced by LPS.
Conclusion: The present study highlights the exacerbating effects of LPS on neuroinflammation in TBI through activation of the TLR-4/NF-κB signaling pathway. TAK-3 can modulate the activity of this signaling axis, thereby attenuating neuroinflammation and ultimately reducing brain tissue damage.
Keywords: NF-κB activator2, CNS infection, CCI, inflammation, TAK-3
Introduction
Traumatic Brain Injury (TBI) represents a significant neurological disorder that imposes substantial economic burdens on both society and families.1 TBI refers to injury of brain tissue caused by external forces, which is often characterized by damage to brain function or pathophysiological alterations in the brain tissue.2,3 In 2014, the United States witnessed a staggering number of emergency department visits (2.53 million), hospitalizations (288,000), and fatalities (56,800) attributed to TBI.4 TBI leads to profound neurological dysfunction and long-term impairment of quality of life. The pathophysiological mechanisms underlying TBI are intricate and diverse, with the activation of inflammatory response being recognized as a pivotal factor in its progression.5–8 After TBI, the excessive activation of the inflammatory response not only exacerbates neurological damage but also impairs the recovery process.9
Central nervous system infections can exacerbate neuroinflammation following TBI, thereby contributing to heightened morbidity and mortality.10,11 Irrespective of their habitat, Gram-negative bacteria exhibit a close association with human infectious diseases. For instance, Vibrio represents the most prevalent bacterial species in marine environments.12 The mortality rate associated with intracranial infection caused by Gram-negative bacteria surpasses that of other pathogenic bacteria.13,14 In a study, 11.3% of patients with penetrating traumatic brain injury experienced intracranial infections during hospitalization, with a Gram-negative bacterial infection rate of 41.6%.15 Escherichia coli is the most common bacteria causing TBI with CNS infection. E. coli meningitis is associated with high morbidity and mortality in both adult and pediatric populations worldwide.16,17 In a previous study conducted in our experimental group, rats with TBI and immersion in seawater also exhibited CNS infection caused by E. coli.
Lipopolysaccharide (LPS), being the principal constituent of Gram-negative bacterial cell walls, plays a pivotal role in the regulation of immunity and inflammation.18–20 It has been widely employed by researchers as an inducer of inflammation.19,20 LPS can induce significant activation of microglia in TBI rats, resulting in elevated expression of pro-inflammatory cytokines.21 The activation of the Nuclear Factor κB (NF-κB) signaling pathway and mitogen-activated protein kinase is induced by LPS through its binding to Toll-Like Receptor 4 (TLR4), thereby facilitating the release of pro-inflammatory cytokines such as IL-6 and TNF-α.20,22–24 This process plays a pivotal role in the regulation of inflammatory and immune responses.18,25–27
TAK-3 is a pharmacological agent that specifically antagonizes the TLR4 receptor, exerting its anti-inflammatory effects on intracranial cells through intraperitoneal administration.28 Although the mechanism of action of LPS in regulating inflammation has been extensively investigated, previous studies predominantly employed intraperitoneal injection to induce intracranial inflammation, with limited investigations conducted on the mode of intracranial injection.20,29,30 The underlying mechanism by which intracranial injection of LPS influences the inflammatory response in TBI rats remains poorly understood. The molecular basis through which bacterial LPS exacerbates post-TBI inflammation, resulting in heightened nerve injury and dysfunction, has not been fully elucidated.10,11
Therefore, the objective of this study is to investigate the exacerbation of inflammatory response by LPS through the TLR4/NFKB pathway in rats with TBI. By employing a systematic experimental design and analysis, we aim to elucidate the precise mechanism underlying LPS-induced inflammatory response in TBI rats, thereby providing a scientific foundation for comprehending the progression of TBI and formulating effective treatment strategies.
In this study, we will comprehensively elucidate the intricate interplay between LPS, TLR4/NF-κB signaling pathway, and the inflammatory response in TBI by employing intracranial administration of LPS, TLR4 inhibitor, and NF-κB agonist within a Controlled Cortical Impact (CCI) model using Sprague-Dawley (SD) rats. Our aim is to unravel the underlying mechanisms through which LPS influences the inflammatory response in TBI rats via this specific pathway. This investigation will significantly contribute to an enhanced understanding of the complex relationship between bacterial LPS and TBI while offering novel insights for managing TBI complicated with central nervous system infections.
Materials and Methods
Randomization and Blinding
All animals underwent randomization and surgical management and were included in the analysis. Personnel responsible for experimental procedures and data analysis were blinded throughout the procedure and were unaware of the group assignments.
Animals and Groups
The experiment utilized a total of 72 male SD rats, weighing between 250 and 280 g, which were procured from the Laboratory Animal Center of the 900th Hospital. All animals were housed in the isolation facility provided by the same hospital. Each group of three rats was housed together in the same cage. Throughout the experiment, all rats had ad libitum access to food and water, with room temperature maintained at 24–26°C and humidity controlled at 40%-70%, under a 10-hour light/14-hour dark cycle. The study protocol was approved by the Institutional Animal Care and Use Committee (IACUC) of the 900th Hospital under batch number 2020–051.
In the initial phase of the experiment, a random allocation was performed to distribute all Sprague-Dawley rats into four distinct groups: Sham group, Sham+LPS group, TBI group, and TBI+LPS group (LPS, #HY-D1056, MedChemExpress China). Each rat was randomly assigned to one of four subgroups. One subgroup (n=3) underwent neurological function assessment, and paraffin sections of brain tissue were prepared following monitoring. Another subgroup (n=3) underwent Western blot assay and enzyme-linked immunosorbent assay (ELISA). A third subgroup (n=3) underwent the Evans blue (EB) extravasation assay. The final subgroup (n=3) underwent the open field test, with subsequent brain edema monitoring.
In the second part of the experiment, all rats were randomly assigned to four groups: TBI group, LPS group (TBI+LPS), TLR-4 group, and NF-κB group. The TRL4 inhibitor (TAK-3, MedChemExpress, China)28 and NFkB agonist (NFkB activator-2 MCE, China)31 were utilized. Each group was further divided into two subgroups; one subgroup (n=3) was designated for fluorescence staining while the other subgroup (n=3) was allocated for Western blot assay and enzyme-linked immunosorbent assay (ELISA).
Produce of Animal Models
The details of the damage caused by controlled cortical impact (CCI) have been described previously.32 After rats were anesthetized with intraperitoneal injection of sodium pentobarbital (40 mg/kg), a 2 cm midline incision was made on the scalp, followed using a dental grinding drill to create a circular bone window measuring 5 mm in diameter, immediately succeeded by a controlled impact (Figure 1A). The impact parameters were set as follows: the diameter of the impact head was 3.0 mm, the depth of impact was 2.0 mm, the velocity of impact was 5 m/s, and the residence time was 0.85 ms. Then, rats in the TBI + LPS group received an injection of LPS (60 μg/kg, dissolved in PBS solution, MedChemExpress China) into the site of trauma, with extended injection duration to facilitate drug absorption.33 After completion of the injection, the skull cap was replaced, followed by suturing the scalp. The rats in the LPS, NF-κB, and TAK-3 groups underwent equivalent degrees of TBI modeling and LPS injection. In the TBI group, rats received CCI alongside an equivalent volume of PBS solution at the injury site. Rats in the Sham+LPS group underwent craniotomy surgery and received an LPS injection equivalent to that of the LPS group. Sham group rats underwent craniotomy surgery and received an equivalent volume of PBS solution injected into the brain tissue at the bone window. The treatment phase commenced 24 hours after the completion of modeling. Rats in the TLR-4 group received intraperitoneal injections of TAK-3 (4 mg/kg/day, MedChemExpress, China).28 To investigate the anti-neuroinflammatory effects of TAK-3 through the TLR-4/NF-κB axis, rats in the NFκB group were not only injected with an equivalent dose of TAK-3 as in the TLR-4 group but also received intraperitoneal injections of NF-κB activator 2 (1 mg/kg/day, MedChemExpress, China). The TBI group and the LPS group were administered an equal volume of PBS solution via intraperitoneal injection. (Figure 1B).
Neurological Function Score
The neurological deficits were evaluated using a modified neurological Impairment Severity Score (mNSS).6 The rats underwent examinations to assess their motor function (muscle status and presence of abnormal movements), sensory abilities (vision, touch, and balance), as well as reflexes. These assessments were conducted when tasks could not be completed or reflexes were absent. The mNSS test is scored on a scale ranging from 0 to 18, where a score of 0 indicates normal performance, scores between 1 and 6 indicate mild impairment, scores between 7 and 12 indicate moderate impairment, and scores between 13 and 18 indicate severe impairment. Neurological function was assessed at various time points (at 12 h, 24 h, 48 h, and 72 h post-modeling) by investigators who were blinded to the group assignments.
Open Field Test
The rats were acclimatized in the laboratory for a minimum duration of 1 hour prior to experimentation. During the experiment, the test room was maintained at a constant temperature and humidity of 24 °C and 55% relative humidity respectively, while ensuring a subdued lighting and quiet environment. The 100×100×40 cm black open field box was partitioned into a total of 16 grids, with the outermost 12 grids constituting the peripheral area and the remaining four grids forming the central area. The rat was gently placed in the central area of the open field box for testing and allowed to freely explore its surroundings for a duration of 5 minutes. The time spent in the central area and the total walking distance were recorded and analyzed using video tracking software (SMART v.3.0 software, RWD Life Science). Revealing elevated levels of anxiety, shorter dwell time and reduced distance traveled within the central zone were observed. At the conclusion of each trial, the rats were thoroughly cleansed of their excrement and the box was meticulously deodorized using 75% ethanol. Allow for complete evaporation of ethanol before commencing subsequent tests.
ELISA Experiments
After euthanasia, fresh brain tissue was collected from the rats and then lysed. Subsequently, the lysed brain tissue solution was centrifuged at 12,000 rpm for 15 minutes at 4°C. The levels of inflammatory cytokines TNF-α, IL-1β, IL-6, and IL-10 in brain tissue were quantified using ELISA kits (Sevier, Wuhan, China), with the measured optical density values converted to corresponding concentration values.
Brain Water Content and EB Staining
The brain water content was determined using the wet-dry weight method.6,7 Subsequently, following the mNSS test, the animals were euthanized and their cerebral cortex was carefully excised at the periphery of the cranial window (200±20 mg). The surface was treated with filter paper to eliminate blood and CSF, followed by measurement of the wet weight. The surface was treated with filter paper to eliminate blood and CSF, followed by measurement of the wet weight. Samples were then placed in an oven for 72 h at 90 °C and reweighed to determine the dry weight. The brain water content percentage was determined using the following formula: (wet weight - dry weight)/wet weight ×100%. The permeability of the blood-brain barrier (BBB) was assessed by evaluating the extent of Evans blue dye extravasation.8 Three days post-model completion, rats were intravenously administered Evans blue (2%, 5 mL/kg, Sigma-Aldrich, USA) 2 hours prior to sacrifice. Following sacrifice, the brains were removed, weighed, and homogenized in trichloroacetic acid. The samples were then centrifuged. The absorbance of the supernatant was measured using a spectrophotometer at a wavelength of 620 nm. The quantity of EB was calculated according to a standard curve and expressed as micrograms of EB per gram of brain tissue.
HE Staining
The paraffin-embedded specimens were sectioned into 5 μm thick slices, which were subsequently subjected to hematoxylin and eosin staining. An HE staining kit (Sevier, China) was employed for the staining procedure in accordance with the manufacturer’s instructions.
Immunofluorescence
The formalin-fixed specimens were embedded in paraffin, sectioned into 4 μm thick slices, deparaffinized using xylene, and rehydrated with a graded series of alcohol prior to antigen retrieval. Following completion of the retrieval process, the specimens were allowed to cool naturally. Subsequently, a circular seal was applied for serum preservation. After a 30-minute blocking step, the sections were incubated with antibodies targeting pNF-κB, NF-κB, Iba1, GAFP, and TLR4. Subsequently, the sections were placed flat in a wet box and incubated overnight at 4°C. The slides were then exposed to the corresponding secondary antibody at room temperature for 50 minutes in darkness. Finally, DAPI was used for nuclear counterstaining. Immunopositive cells within selected regions were quantified under a Nikon Eclipse C1 microscope (Japan) at ×20 magnification by an experimenter blinded to group allocation.
Western Blot Experiments
Rats were sacrificed 3 days after modeling, and tissue samples were collected from the cerebral cortex surrounding the injury and extracted using RIPA lysis buffer. Lysates were incubated on ice and the supernatants were collected after centrifugation. Protein concentrations were determined using the BCA Protein Assay kit (Abcam). Then, 30 ug of the protein was loaded onto the gel and separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Subsequently, proteins were transferred to polyvinyl difluoride membranes and detected with primary antibodies against TLR-4 (1:1000, ab22048, Abcam), NF-κB p65 (1:1000, ab239882, Abcam), p-NF-κB p65 (1:1000, ab86299, Abcam), followed by secondary antibody incubation. Immunoblotting was visualized using the ECL Protein Blot detection System (Millipore). Expression levels were normalized to GAPDH-10.
Statistical Analysis
The statistical analysis was performed using SPSS 26.0 software. Results were presented as mean ± standard deviation. Independent sample t-test was employed to compare the two groups, while one-way analysis of variance (ANOVA) was utilized to assess statistical differences among multiple groups. For the animal groups subjected to cross-time testing, we employed two-way repeated measures analysis of variance for statistical analysis of the data. Statistical significance was defined as a P < 0.05.
Results
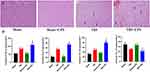
The Administration of LPS Exacerbated Neurological Deficits and Impaired Exploratory Abilities Following TBI
On day 3 after injury, the open field test was used to assess anxiety-like behavior and motor activity. The motor activity was quantified by measuring the total distance covered in an open field during a 5-minute period (Figure 1C). Compared to the TBI group, rats in the TBI+LPS group exhibited a significant decrease in the time spent in the center of the open field. (t = 11.95, P < 0.001) (Figure 1D). In our experiments, we observed a significant disparity in the total distance traversed within the open field between animals belonging to the TBI group and those in the TBI+LPS group (t = 4.26 P < 0.05) (Figure 1E). The data presented herein indicate that rats with traumatic brain injury (TBI) exhibited motor impairments and anxiety-like behavior, while LPS exacerbated TBI-induced anxiety-like behavior and attenuated locomotor activity. The neurological function scores in the Sham group remained unchanged at the corresponding time points (scores 0–2). The Sham+LPS group exhibited mild neurological deficits within 24 hours after modeling. However, at 12 hours post-TBI, neurological function was impaired (6.68±0.13). There was no significant difference in neurological function between the TBI group and the TBI+LPS group at this time point. Nevertheless, at 24 hours post-TBI, the neurological function of the TBI+LPS group exhibited a significantly greater impairment compared to that of the TBI group (t=8.49, P < 0.01) (Figure 1F).
The Administration of LPS Resulted in Further Impairment of the Blood-Brain Barrier and Exacerbated Brain Tissue Edema
BBB permeability was assessed through the quantification of Evans blue extravasation (EB). The results revealed a significant extravasation of Evans blue in the TBI group at 3 days post-injury, compared to both the Sham and Sham+LPS groups (t = 6.99, P < 0.05). The extravasation of Evans blue dye was notably more pronounced in the TBI+LPS group compared to the TBI group (Figure 2A). Brain water content serves as a crucial prognostic indicator for TBI outcomes. At 3 days post-injury, the TBI group exhibited significantly elevated brain water content (81.13%) compared to both the Sham and Sham+LPS groups (F (2, 6) = 324.4, P < 0.001). Furthermore, the TBI + LPS group demonstrated a significantly higher water content than the TBI group (t = 5.00, P < 0.05) (Figure 2B).
LPS Can Aggravate Neuroinflammation After Traumatic Brain Injury in Rats
The HE staining results revealed no evident brain tissue injury in the Sham group and Sham+LPS group of rats. The brain tissue of the TBI group and TBI+LPS group exhibited pronounced edema, with the latter group additionally presenting hemorrhage in the brain tissue. The magnification used was 200× (Figure 3A). The expression levels of inflammatory factors (TNF-α, IL-1β, IL-6, TL-10) were assessed using an ELISA kit to investigate their association with TBI. Our findings revealed significantly elevated expression levels of these inflammatory factors in the TBI group compared to both the Sham and Sham+LPS groups. Furthermore, LPS intervention further enhanced the TBI-induced expression of these factors while concurrently inhibiting the anti-inflammatory cytokine IL-10 (t = 2.78, P < 0.05) (Figure 3B).
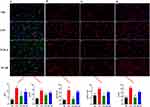
TAK-3 Suppresses TLR-4 Receptor to Alleviate LPS-Induced Acute Inflammation, Cellular Activation, and Neuroinflammation in Traumatic Brain Injury Rats, and this Effect is Reversible by the NF-κB
Immunofluorescence assay revealed significantly elevated levels of microglia and astrocyte activation in the LPS group compared to the TBI group following TBI (t=10.53, P<0.001) (Figure 4A). TAK-3 effectively suppressed LPS-induced activation of these cells, while NF-κB intervention also attenuated their activation. Notably, TAK-3 inhibited phosphorylation and nuclear translocation of P65, as well as reduced TLR-4 activity; conversely, NF-κB promoted TLR-4 expression and facilitated phosphorylation and nuclear translocation of P65 (Figure 4B-D).
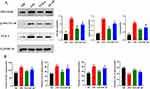
TAK-3 Inhibits LPS-Induced Neuroinflammation in TBI Rats Through the TLR-4/NF-κB Pathway
The Western blot analysis revealed that the inhibition of TLR-4 reduced the phosphorylation and expression of NF-κB P65 (t=7.44, P<0.01) (Figure 5A) (Supplementary Figure 1), as well as the levels of inflammatory factors in TBI rats following LPS intervention. However, this inhibitory effect was attenuated by NF-κB intervention (Figure 5B).
Discussion
This study aims to find the relationship between TAK-3 and LPS-induced neuroinflammation in TBI rats. In this experimental investigation, we observed that LPS administration at the site of TBI exacerbated neural damage. After 3 days of modeling, the LPS group exhibited an increase in EB leakage and brain water content, suggesting compromised integrity of blood-brain barrier (BBB). The BBB serves as a crucial three-dimensional interface between brain tissue and blood vessels, playing a pivotal role in maintaining brain homeostasis.34 It selectively permits the passage of substances from systemic circulation into the brain, ensuring its physiological integrity.6,35 The physical trauma associated with TBI and its resultant neuroinflammation often contribute to the disruption of the BBB.36,37
Feng et al investigated the effects of the toll-like receptor 4 antagonist, TAK-242, on TBI. Their findings suggest that TAK-242 may suppress autophagy and neuroinflammation activity via the NF-κB signaling pathway.38 Through our experimental findings, we have delineated the therapeutic effects of TAK-3 on LPS-induced neuroinflammation in TBI rats., encompassing the following key aspects: (1) LPS disrupts the integrity of BBB, exacerbating cerebral edema and neuroinflammation, thereby precipitating neurological impairments; (2) TAK-3 alleviates LPS-induced neuroinflammation; (3) TAK-3 improves neuroinflammation via the TLR-4/NF-κB pathway, corroborating previous observations made by other researchers.24,33
The neurological function score, open field test, and EB staining were employed to assess the impact of LPS on neurological function in rats with TBI, aiming to comprehend its effect comprehensively. The findings demonstrated that LPS significantly exacerbated neurological impairments in rats with TBI.
The TLR-4/NF-κB signaling pathway represents a pivotal mechanism for the activation of molecules induced by specific pathogens.18,22,25 NF-κB is intricately linked to the expression of a diverse array of cytokines and plays a pivotal role in mediating inflammatory responses, oxidative stress, apoptosis, and other pathological processes.31,37 The NF-κB family comprises five interconnected subunits, namely P50, P52, P65, RelB, and c-Rel. Previous research has indicated that the activation of the immune system occurs through TLR-4 binding to LPS.25,39 The expression of TLR-4 is predominantly observed in microglia and astrocytes within the brain. In our study, LPS induced the phosphorylation and subsequent nuclear translocation of NF-κB, thereby initiating gene expression and promoting the upregulation of tissue concentrations of TNF-α, IL-6, IL-1, and other cytokines. Interestingly, our experimental findings contradict the conclusions drawn by Frances Corrigan, who reported that LPS failed to enhance the inflammatory response at 1 day post injury in SD rats based on a mild repetitive traumatic brain injury model.30 We hypothesize that the inconsistent findings may be attributed to variations in the severity of TBI models, and our study’s rats exhibited a relatively pronounced disruption of the blood-brain barrier (BBB) following trauma, potentially contributing to the enhanced neuroinflammatory response induced by LPS.
To understand the role of LPS in TBI, we used TAK-3 intraperitoneal administration to antagonize TLR-4 receptor. TAK-3, as a selective blocking agent of TLR-4 receptor, can inhibit apoptosis and inflammation by blocking TLR-4 receptor.28 In the presence of a TAK-3, we observed a reduction in inflammatory cytokines, inflammatory cells, and neuronal damage in the brain. These findings suggest that the activation of TLR-4 receptors is closely associated with the critical role of LPS in secondary injury following TBI. Previous studies have shown that TLR-4 receptor activation induces phosphorylation of NF-κB and promotes neuroinflammation.28,30,40 To further investigate the role of the TLR-4/NF-κB pathway in mediating secondary TBI, we employed NF-κΒ activator 2 to augment the expression and activation of NF-κB.31 The findings demonstrated that the upregulation and activation of NF-κB led to an elevation in inflammatory cytokines and recruitment of inflammatory cells within the brain. After treatment with NF-κΒ activator 2, we observed a rebound in the inflammatory markers of the TLR-4 group after three days, indicating that the TLR-4/NF-κB pathway plays a crucial role in mediating LPS action in TBI.
However, this study also has certain limitations. Firstly, we investigated a specific pathway through which TAK-3 improves inflammation induced by LPS in rats, particularly the TLR-4/NF-κB pathway. Nevertheless, it is important to acknowledge that the involvement of LPS extends beyond its interaction with the TLR-4 receptor and encompasses other accessory molecules and receptors such as members of the LPS binding protein (LBP) family, cysteine proteases, TLR-2, etc.41,42 Given the predominant binding of LPS to the TLR-4 receptor and subsequent activation of NF-κB signaling, our investigation primarily focused on elucidating the TLR-4/NF-κB pathway following trauma.39 Secondly, the precise role of the TLR-4/NF-κB axis in exacerbating secondary injury following TBI, as well as the potential phenotypic changes occurring in microglia and astrocytes during this process, remain inconclusive. Therefore, further experimental studies or genetic phenotype detection are required to validate these findings. Finally, in clinical practice, the time window for diagnosing secondary intracranial infection after TBI is typically within 48 hours of admission. However, it remains uncertain whether the timing of LPS intervention has an impact on the functional outcome of TBI rats since we administered LPS immediately after completing the TBI model.
Conclusion
In conclusion, our study demonstrated that LPS exacerbates blood-brain barrier disruption, brain edema, and neuroinflammation following TBI. This effect is mediated by LPS-induced activation of the Toll-like receptor 4/nuclear factor kappa B signaling axis in microglia. TAK-3 can modulate the activity of this signaling axis, thereby attenuating neuroinflammation and ultimately reducing brain tissue damage.
Data Sharing Statement
All data sets and materials that support the conclusions of this article are provided with the manuscript.
Ethical Approval
The experimental protocol for this study, including all surgical procedures and use of animals, was in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals and was approved by the Laboratory Animal Ethics Committee of the 900th Hospital (Fuzhou, China). Ethics No.2020-051.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the 900th Hospital Special Treatment for Trauma (Grant number. 2022ZL01).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Feigin VL, Nichols E, Alam T., Collaborators GBDN. Global, regional, and national burden of neurological disorders, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(5):459–480. doi:10.1016/S1474-4422(18)30499-X
2. Menon DK, Schwab K, Wright DW, et al. Position Statement: definition of Traumatic Brain Injury. Arch Phys Med Rehabil. 2010;91(11):1637–1640. doi:10.1016/j.apmr.2010.05.017
3. Sharma R, Shultz SR, Robinson MJ, et al. Infections after a traumatic brain injury: the complex interplay between the immune and neurological systems. Brain Behav Immun. 2019;79:63–74. doi:10.1016/j.bbi.2019.04.034
4. Howlett JR, Nelson LD, Stein MB. Mental Health Consequences of Traumatic Brain Injury. Biol Psychiatry. 2022;91(5):413–420. doi:10.1016/j.biopsych.2021.09.024
5. Gao Y, Wang T, Cheng Y, et al. Melatonin ameliorates neurological deficits through MT2/IL-33/ferritin H signaling-mediated inhibition of neuroinflammation and ferroptosis after traumatic brain injury. Free Radic Biol Med. 2023;199:97–112. doi:10.1016/j.freeradbiomed.2023.02.014
6. Zheng S, Wang C, Lin L, et al. TNF-α Impairs Pericyte-Mediated Cerebral Microcirculation via the NF-κB/iNOS Axis after Experimental Traumatic Brain Injury. J Neurotrauma. 2023;40(3–4):349–364. doi:10.1089/neu.2022.0016
7. Chen X, Chen C, Fan S, et al. Omega-3 polyunsaturated fatty acid attenuates the inflammatory response by modulating microglia polarization through SIRT1-mediated deacetylation of the HMGB1/NF-kappaB pathway following experimental traumatic brain injury. J Neuroinflammation. 2018;15:116. doi:10.1186/s12974-018-1151-3
8. Hopp S, Nolte MW, Stetter C, et al. Alleviation of secondary brain injury, posttraumatic inflammation, and brain edema formation by inhibition of factor XIIa. J Neuroinflammation. 2017;14(1):39. doi:10.1186/s12974-017-0815-8
9. Hegdekar N, Sarkar C, Bustos S, et al. Inhibition of autophagy in microglia and macrophages exacerbates innate immune responses and worsens brain injury outcomes. Autophagy. 2023;19(7):2026–2044. doi:10.1080/15548627.2023.2167689
10. Lu G, Liu Y, Huang Y, et al. Prediction model of central nervous system infections in patients with severe traumatic brain injury after craniotomy. J Hosp Infect. 2023;136:90–99. doi:10.1016/j.jhin.2023.04.004
11. Hu Y, He W, Yao D, et al. Intrathecal or intraventricular antimicrobial therapy for post-neurosurgical intracranial infection due to multidrug-resistant and extensively drug-resistant Gram-negative bacteria: a systematic review and meta-analysis. Int J Antimicrob Agents. 2019;54:556–561. doi:10.1016/j.ijantimicag.2019.08.002
12. Baker-Austin C, Oliver JD, Alam M, et al. Vibrio spp. infections. Nature Reviews Disease Primers. 2018;4(1):1–19. doi:10.1038/s41572-018-0005-8
13. Pomar V, Benito N, Lopez-Contreras J, et al. Spontaneous gram-negative bacillary meningitis in adult patients: characteristics and outcome. BMC Infect Dis. 2013;13(1):451. doi:10.1186/1471-2334-13-451
14. Zhang Y, Zhou Y, Hou M, et al. Analysis of Cerebrospinal Fluid Routine Biochemical Level, Pathogenic Bacteria Distribution, and Risk Factors in Patients with Secondary Intracranial Infection after Brain Tumor Surgery. Evid Based Complement Alternat Med. 2022;2022:7716205. doi:10.1155/2022/7716205
15. Meister MR, Boulter JH, Yabes JM, et al. Epidemiology of cranial infections in battlefield-related penetrating and open cranial injuries. J Trauma Acute Care Surg. 2023;95(2S):S72–S78. doi:10.1097/TA.0000000000004018
16. Yang B, Yang R, Xu B, et al. miR-155 and miR-146a collectively regulate meningitic Escherichia coli infection-mediated neuroinflammatory responses. J Neuroinflammation. 2021;18(1):114. doi:10.1186/s12974-021-02165-4
17. Pons S, Frapy E, Sereme Y, et al. A high-throughput sequencing approach identifies immunotherapeutic targets for bacterial meningitis in neonates. EBioMedicine. 2023;88:104439. doi:10.1016/j.ebiom.2023.104439
18. Zhang Y, Liang X, Bao X, et al. Toll-like receptor 4 (TLR4) inhibitors: current research and prospective. Eur J Med Chem. 2022;235:114291. doi:10.1016/j.ejmech.2022.114291
19. Wang Y, Shan X, Dai Y, et al. Curcumin Analog L48H37 Prevents Lipopolysaccharide-Induced TLR4 Signaling Pathway Activation and Sepsis via Targeting MD2. J Pharmacol Exp Ther. 2015;353(3):539–550. doi:10.1124/jpet.115.222570
20. Hang CH, Shi JX, Tian J, et al. Effect of systemic LPS injection on cortical NF-kappaB activity and inflammatory response following traumatic brain injury in rats. Brain Res. 2004;1026:23–32. doi:10.1016/j.brainres.2004.07.090
21. Cai L, Gong Q, Qi L, et al. ACT001 attenuates microglia-mediated neuroinflammation after traumatic brain injury via inhibiting AKT/NFκB/NLRP3 pathway. Cell Commun Signal. 2022;20(1):56. doi:10.1186/s12964-022-00862-y
22. Munk M, Poulsen FR, Larsen L, et al. Cerebral Metabolic Changes Related to Oxidative Metabolism in a Model of Bacterial Meningitis Induced by Lipopolysaccharide. Neurocritical Care. 2018;29(3):496–503. doi:10.1007/s12028-018-0509-9
23. Perez-Dominguez M, Ávila-Muñoz E, Domínguez-Rivas E, et al. The detrimental effects of lipopolysaccharide-induced neuroinflammation on adult hippocampal neurogenesis depend on the duration of the pro-inflammatory response. Neural Regeneration Res. 2019;14.
24. Domínguez-Rivas E, Ávila-Muñoz E, Schwarzacher SW, et al. Adult hippocampal neurogenesis in the context of lipopolysaccharide-induced neuroinflammation: a molecular, cellular and behavioral review. Brain Behav Immun. 2021;97:286–302. doi:10.1016/j.bbi.2021.06.014
25. Fu YJ, Xu B, Huang SW, et al. Baicalin prevents LPS-induced activation of TLR4/NF-kappaB p65 pathway and inflammation in mice via inhibiting the expression of CD14. Acta Pharmacol Sin. 2021;42:88–96. doi:10.1038/s41401-020-0411-9
26. Zhang C, Wang X, Wang C, et al. Qingwenzhike Prescription Alleviates Acute Lung Injury Induced by LPS via Inhibiting TLR4/NF-kB Pathway and NLRP3 Inflammasome Activation. Front Pharmacol. 2021;12:790072. doi:10.3389/fphar.2021.790072
27. Wang N, Guo W, Liu T, et al. Toll-like receptors (TLR2 and TLR4) antagonist mitigates the onset of cerebral small vessel disease through PI3K/Akt/GSK3beta pathway in stroke-prone renovascular hypertensive rats. Biotechnol Genet Eng Rev;2023. 1–21. doi:10.1080/02648725.2023.2184961
28. Wang Y, Sadike D, Huang B, et al. Regulatory T cells alleviate myelin loss and cognitive dysfunction by regulating neuroinflammation and microglial pyroptosis via TLR4/MyD88/NF-κB pathway in LPC-induced demyelination. J Neuroinflammation. 2023;20(1). doi:10.1186/s12974-023-02721-0
29. Ventorp F, Bay-Richter C, Nagendra AS, et al. Exendin-4 Treatment Improves LPS-Induced Depressive-Like Behavior Without Affecting Pro-Inflammatory Cytokines. J Parkinsons Dis. 2017;7(2):263–273. doi:10.3233/JPD-171068
30. Corrigan F, Arulsamy A, Collins-Praino LE, et al. Toll like receptor 4 activation can be either detrimental or beneficial following mild repetitive traumatic brain injury depending on timing of activation. Brain Behav Immun. 2017;64:124–139. doi:10.1016/j.bbi.2017.04.006
31. Mathew B, Ruiz P, Dutta S, et al. Structure-activity relationship (SAR) studies of N-(3-methylpyridin-2-yl)-4-(pyridin-2-yl)thiazol-2-amine (SRI-22819) as NF-ҡB activators for the treatment of ALS. Eur J Med Chem. 2021;210:112952. doi:10.1016/j.ejmech.2020.112952
32. Xiong A, Li J, Xiong R, et al. Inhibition of HIF-1alpha-AQP4 axis ameliorates brain edema and neurological functional deficits in a rat controlled cortical injury (CCI) model. Sci Rep. 2022;12:2701. doi:10.1038/s41598-022-06773-9
33. Jakubs K, Bonde S, Iosif RE, et al. Inflammation Regulates Functional Integration of Neurons Born in Adult Brain. J Neurosci. 2008;28(47):12477–12488. doi:10.1523/JNEUROSCI.3240-08.2008
34. Guo ZN, Liu J, Chang J, et al. GAS6/Axl Signaling Modulates Blood-Brain Barrier Function Following Intravenous Thrombolysis in Acute Ischemic Stroke. Front Immunol. 2021;12:742359. doi:10.3389/fimmu.2021.742359
35. Alahmari A. Blood-Brain Barrier Overview: structural and Functional Correlation. Neural Plast. 2021;2021:6564585. doi:10.1155/2021/6564585
36. Vazquez-Rosa E, Shin MK, Dhar M, et al. P7C3-A20 treatment one year after TBI in mice repairs the blood-brain barrier, arrests chronic neurodegeneration, and restores cognition. Proc Natl Acad Sci U S A. 2020;117:27667–27675. doi:10.1073/pnas.2010430117
37. Kalra S, Malik R, Singh G, et al. Pathogenesis and management of traumatic brain injury (TBI): role of neuroinflammation and anti-inflammatory drugs. Inflammopharmacology. 2022;30(4):1153–1166. doi:10.1007/s10787-022-01017-8
38. Feng Y, Ju Y, Wu Q, Sun G, Yan Z. TAK-242, a toll-like receptor 4 antagonist, against brain injury by alleviates autophagy and inflammation in rats. Open Life Sci. 2023;18(1):20220662. doi:10.1515/biol-2022-0662
39. Mazgaeen L, Gurung P. Recent Advances in Lipopolysaccharide Recognition Systems. Int J Mol Sci. 2020;21. doi:10.3390/ijms22010021
40. Kawakita F, Fujimoto M, Liu L, et al. Effects of Toll-Like Receptor 4 Antagonists Against Cerebral Vasospasm After Experimental Subarachnoid Hemorrhage in Mice. Mol Neurobiol. 2017;54(8):6624–6633. doi:10.1007/s12035-016-0178-7
41. Gabarin RS, Li M, Zimmel PA, et al. Intracellular and Extracellular Lipopolysaccharide Signaling in Sepsis: avenues for Novel Therapeutic Strategies. J Innate Immun. 2021;13(6):323–332. doi:10.1159/000515740
42. Hsieh WT, Hsu MH, Lin WJ, et al. Ergosta-7, 9 (11), 22-trien-3β-ol Interferes with LPS Docking to LBP, CD14, and TLR4/MD-2 Co-Receptors to Attenuate the NF-κB Inflammatory Pathway In Vitro and Drosophila. Int J Mol Sci. 2021;22:6511. doi:10.3390/ijms22126511
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.