Back to Journals » Infection and Drug Resistance » Volume 16
Synthesis, Anti-Bacterial and Molecular Docking Studies of Arylated Butyl 2-Bromoisonicotinate Against Clinical Isolates of ESBL-Producing Escherichia coli ST405 and Methicillin-Resistant Staphylococcus aureus
Authors Naheed S, Din IU, Qamar MU , Rasool N, Ahmad M , Bilal M , Khalid A, Ahmad G , Al-Hussain SA , Zaki MEA
Received 12 April 2023
Accepted for publication 12 July 2023
Published 14 August 2023 Volume 2023:16 Pages 5295—5308
DOI https://doi.org/10.2147/IDR.S407891
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Shazia Naheed,1 Irum Umar Din,1 Muhammad Usman Qamar,2 Nasir Rasool,1 Matloob Ahmad,1 Muhammad Bilal,1 Aqsa Khalid,3 Gulraiz Ahmad,1 Sami A Al-Hussain,4 Magdi EA Zaki4
1Department of Chemistry, Government College University Faisalabad, Faisalabad, 38000, Pakistan; 2Institute of Microbiology, Faculty of Life Sciences, Government College University, Faisalabad, 38000, Pakistan; 3School of Interdisciplinary Engineering & Science (SINES), National University of Sciences and Technology (NUST), Islamabad, 44000, Pakistan; 4Department of Chemistry, Faculty of Science, Imam Mohammad Ibn Saud Islamic University, Riyad, 11623, Saudi Arabia
Correspondence: Nasir Rasool; Magdi E A Zaki, Email [email protected]; [email protected]
Introduction: Global public health concerns include the emergence and spread of methicillin-resistant Staphylococcus aureus (MRSA) and extended-spectrum beta-lactamase Escherichia coli (ESBL-E. coli). These pathogens cause infections that are difficult to treat, which can have fatal outcomes and require lengthy hospital stays. As a result, we created butyl 2-bromoisonicotinate and tested its antibacterial effectiveness against the ESBL-E. coli ST 405 and MRSA pathogens. Natural product discovery is complemented by synthetic compound synthesis because of the latter’s potential for superior characteristics, target specificity, scalability, intellectual advantages, and chemical diversity. Because of this, the potential for discovering new medicinal compounds is increased, and the constraints placed on natural sources are overcome. Natural items are tough to obtain since they are hard to isolate and synthesize. Therefore, modern science is actively searching for small molecules as therapeutic agents by applying sustainable techniques that can be commercialized.
Methods: Two patients’ blood samples were taken, and the BACTEC/Alert system was used to process them. On blood and MacConkey agar, the positive samples were subcultured and incubated aerobically at 37 °C. Using the VITEK 2 compact system, the isolates were subjected to isolate identification and MIC. MLST of the ESBL-E. coli was performed by PCR. Additionally, Fischer esterification was used to create butyl 2-bromoisonicotinate in excellent yields. A commercially available palladium catalyst was then used to arylate the compound, resulting in medium to good yields of arylated butyl 2-bromoisonicotinates. Using the agar well diffusion assay and the micro-broth dilution method, we assessed the in-vitro activities of the synthesized molecules (3, 5a-h) against clinically isolated ESBL-E. coli ST405, and MRSA. A molecular operating environment was used to carry out in silico validation of the synthesized compounds’ binding to the active site and to evaluate the stability of their molecular interactions with the target E. coli 2Y2T protein.
Results: MRSA and ESBL-producing E. coli were identified as the two clinical isolates. While MRSA was also resistant to beta-lactam drugs and least resistant to vancomycin, ESBL-producing E. coli belonged to ST405 and was resistant to cephalosporins and sensitive to carbapenems. Good yields of the desired compounds were produced by our effective and economical synthesis. By using a micro-broth dilution assay, the Molecules (3, 5a, and 5d) were most effective against both resistant strains. The Molecules (3, 5a, 5b, and 5d) also displayed good binding energies.
Conclusion: The butyl 2-bromoisonicotinate displayed antibacterial efficacy against ESBL-producing E. coli ST405 and MRSA strains. After the in-vivo trial, this substance might offer an alternative therapeutic option.
Keywords: ESBL, MRSA, MLST, Fischer esterification, Suzuki-Miyaura, docking studies
Introduction
The ultimate efficacy of antimicrobial drugs is still questioned due to the rising difficulty of treating emerging multi-drug resistant infections with currently available drugs.1 It is crucial to execute a comprehensive plan to stop the spread of microbial resistance and find new antimicrobial drugs.2 It is challenging to execute a comprehensive plan to stop the spread of bacterial resistance and to find new drugs.3 In 2050, if microbes resistant to antimicrobials predominate, a death will occur every three seconds, costing more than $100 trillion in economic output.4 The World Health Organization designated methicillin-resistant Staphylococcus aureus (MRSA) and extended spectrum-beta-lactamse producing Escherichia coli (ESBL-E. coli) as “High Priority Pathogens.”5 These bacteria cause wound infections, bacteremia, skin infections, respiratory tract infections, septicemia, nosocomial infections, and urinary tract infections. These bacteria have become resistant to different classes of drugs, such as quinolones, aminoglycosides, and beta-lactams. They can only be treated with antibiotics such as colistin, which is toxic to the human body.6 Drug resistance can arise through different processes, such as drug degradation, modification or mutation of the target site, and lower intracellular concentration brought on by reduced energy-dependent efflux and permeability. Many clinically significant Gram-negative bacteria acquired resistance or developed innate resistance as a consequence of the interaction between decreased absorption and medication efflux.7,8 Since infections and formerly used drugs have a declining impact on bacterial resistance, which are substantial risk factors for death and morbidity in both developing and developed countries, the invention of newer antimicrobial medications is still necessary.9–11 The Food and Drug Administration has approved the first lipid-lowering drug, nicotinic acid, which has been used for over 50 years for the treatment of dyslipidemia.12 The literature survey indicated that nicotinic acid derivatives show anti-tubular,13 anti-lipolytic14 and anti-viral activities.15 Suzuki-Miyaura cross-coupling (SMC) is an effective tool that involves organoboron nucleophiles and pseudohalide/organohalide electrophiles to form a carbon–carbon bond.16,17 Hence, we designed the synthesis of butyl 2-bromoisonicotinate (3) by the reaction of 2-bromo isonicotinic acid (1) with n-butanol (2) by F. esterification, and its derivatives (5a–h) were synthesized via SMC reaction.18 The agar-well diffusion method was used to evaluate the target molecules for anti-bacterial activity against ESBL-producing E. coli ST405 and MRSA. Following that, the zone inhibition, MIC, and MBC were analyzed and validated by docking studies.
Methodology
Ethical Consideration
Before beginning this study, the Ethical Review Committee (ERC) of Government College University, Faisalabad, obtained ethical approval following the Helsinki Declaration. Informed consent was obtained from each research participant, and they were allowed to hear and sign the informed consent form in a language they understood. They agreed to provide a sample and allow the isolates to be used for research. They were assured that the samples would only be used for research and that their personal information would be kept confidential.
General Information
All the chemicals used in this research were sourced from Alfa Aesar and Sigma-Aldrich (USA). The SMC reactions were carried out in an argon atmosphere. A rotary evaporator was used to dry the synthesized molecules or reaction mixture. Thin-layer chromatography (TLC) was used to monitor the progress of the reaction (silica gel 60 PF254 cards). Further, the required product was purified by flash column chromatography using silica gel with a mesh size of 230–400. Spectroscopic techniques like NMR (Bruker 500 MHz) were used to confirm the synthesized molecules and the presence of solvent-deuterated solvents.
General Procedure for the Synthesis of Molecules
Synthesis of Butyl 2-Bromoisonicotinate (3)
2-bromo isonicotinic acid (1 eq., 4.95 mmol) was dissolved in 12 mL of n-butanol, here, n-butanol acted as a solvent and a reactant. The catalytic amount of H2SO4 was then added, and the reaction mixture was refluxed for 18 hours. Thin-layer chromatography was used to monitor the completion of the reaction (TLC). Following the completion of the reaction, the reaction mixture was washed with a saturated solution of NaHCO3 and chloroform. The organic layer appeared in a separating funnel at the bottom and was then dried by rotary evaporator. The product was validated by spectroscopic techniques. For NMR spectrum of compound 3 see Supplementary Information (Figure S1).19
 |
Figure 1 Phenotypic Confirmation of MRSA. |
Arylation of Butyl 2-Bromoisonicotinate (3)
At room temperature, butyl 2-bromoisonicotinate (1.0 eq., 0.99 mmol) and 7 mol% tetrakis (triphenylphosphine)palladium(0) catalyst were added in Schlenk flask in 5 mL 1,4-dioxane under inert atmosphere and agitated for 30 minutes. Then, Het/aryl boronic acids (1.1 eq., 1.089 mmol), K3PO4 (2.0 eq., 1.98 mmol) base, and 0.5 mL water were added. Water is used to homogenize the reaction mixture in this case. For 8–18 hours, the reaction mixture was refluxed. TLC was used to monitor the progress of the reaction. Following the completion of the reaction, the reaction mixture was dried using a rotary evaporator and washed with ethyl acetate. Flash column chromatography was used to further purify the synthesized compounds. The synthesized product was then validated using spectroscopic techniques. For NMR spectra of compound 5a-h see Supplementary Information (Figures S2–S8).20
Chemical Characterization
Butyl 2-Bromoisonicotinate (3)
1HNMR (500 MHz, DMSO) δ 8.60–8.58 (m, 1H), 7.93 (d, J = 0.6 Hz, 1H), 7.84 (ddd, J = 6.4, 5.0, 1.4 Hz, 1H), 4.33 (t, J = 6.7 Hz, 2H), 1.60 (q, J = 6.8 Hz, 2H), 0.95–0.88 (m, 5H). 13C NMR (125 MHz, DMSO) δ 166.49, 148.30, 147.46, 144.20, 126.15, 119.66, 66.88, 31.15, 19.94, 14.02. Anal. Calcd. For C10H12BrNO2: C, 46.53; H, 4.69; N, 5.43. Found: C, 46.51; H, 4.72; N, 5.46%.
Butyl 2-(4-Methoxyphenyl)isonicotinate (5a)
1HNMR (500 MHz, DMSO) δ 8.82 (dd, J = 4.9, 0.6 Hz, 1H), 8.22 (s, 1H), 8.09 (d, J = 8.9 Hz, 2H), 7.71 (ddd, J = 6.4, 5.0, 1.4 Hz, 1H), 7.08 (d, J = 8.9 Hz, 2H), 4.38 (t, J = 6.7 Hz, 2H), 3.83 (s, 3H), 1.66 (q, J = 6.8 Hz, 2H), 1.00–0.92 (m, 5H). 13C NMR (125 MHz, DMSO) δ 166.49, 164.15, 161.92, 148.85, 141.80, 131.03, 131.03, 128.55, 121.32, 121.32, 114.03, 114.03, 66.88, 56.04, 31.15, 19.94, 14.02. Anal. Calcd. For C17H19NO3: C, 71.56; H, 6.71; N, 4.91. Found: C, 71.52; H, 6.70; N, 4.94%.
Butyl 2-(3,5-Difluorophenyl)isonicotinate (5c)
1HNMR (500 MHz, DMSO) δ 8.91–8.90 (m, 1H), 8.40–8.39 (m, 1H), 7.89–7.87 (m, 2H), 7.73–7.67 (m, 1H), 7.39 (ddd, J = 9.1, 5.7, 2.3 Hz, 1H), 4.40 (t, J = 6.8 Hz, 2H), 1.66 (dt, J = 14.9, 6.4 Hz, 2H), 1.0–0.93 (m, 5H). 13C NMR (125 MHz, DMSO) δ 166.49, 163.79, 163.56, 163.56, 148.49, 141.53, 138.44, 123.70, 122.08, 109.35, 109.35, 104.78, 66.88, 31.15, 19.94, 14.02. Anal. Calcd. For C16H15F2NO2: C, 65.97; H, 5.19; N, 4.81. Found: C, 65.95; H, 5.17; N, 4.85%.
Butyl 2-(4-(Methylthio)phenyl)isonicotinate (5d)
1HNMR (500 MHz, DMSO) δ 8.85 (t, J = 4.2 Hz, 1H), 8.25 (s, 1H), 8.07 (d, J = 8.2 Hz, 2H), 7.81–7.70 (m, 1H), 7.39 (d, J = 8.2 Hz, 2H), 4.38 (t, J = 6.7 Hz, 2H), 2.54 (s, 3H), 1.70 (ddd, J = 45.0, 13.4, 6.7 Hz, 2H), 1.00–0.93 (m, 5H). 13C NMR (125 MHz, DMSO) δ 166.49, 164.15, 148.85, 141.80, 140.99, 136.41, 129.75, 129.75, 128.10, 128.10, 121.32, 121.32, 66.88, 31.15, 19.94, 16.53, 14.02. Anal. Calcd. For C17H19NO2S: C, 67.70; H, 6.35; N, 4.65. Found: C, 67.74; H, 6.38; N, 4.68%.
Butyl 2-(4-(Methoxycarbonyl)phenyl)isonicotinate (5e)
1H NMR (500 MHz, DMSO) δ 8.95–8.93 (m, 1H), 8.39 (d, J = 0.8 Hz, 1H), 8.30 (d, J = 8.6 Hz, 2H), 8.11 (d, J = 8.6 Hz, 2H), 7.87 (ddd, J = 6.4, 5.0, 1.4 Hz, 1H), 4.42–4.25 (m, 2H), 3.90 (s, 3H), 1.78–1.65 (m, 2H), 0.96 (d, J = 6.6 Hz, 5H). 13C NMR (125 MHz, DMSO) δ 167.39, 166.49, 164.15, 148.85, 141.80, 137.90, 133.11, 129.38, 129.38, 128.45, 128.45, 121.32, 121.32, 66.88, 52.08, 31.15, 19.94, 14.02. Anal. Calcd. For C18H19NO4: C, 68.99; H, 6.11; N, 4.47. Found: C, 68.96; H, 6.08; N, 4.49%.
Butyl 2-(4-Chlorophenyl)isonicotinate (5f)
1H NMR (500 MHz, DMSO) δ 8.86 (dd, J = 4.5, 3.9 Hz, 1H), 8.26 (d, J = 0.7 Hz, 1H), 8.12 (d, J = 8.4 Hz, 2H), 7.79–7.77 (m, 1H), 7.55 (d, J = 8.4 Hz, 2H), 4.35 (q, J = 6.7 Hz, 2H), 1.66–1.57 (m, 2H), 0.95–0.91 (m, 5H). 13C NMR (125 MHz, DMSO) δ 166.49, 164.15, 148.85, 141.80, 136.07, 133.64, 129.56, 129.56, 129.43, 129.43, 121.32, 121.32, 66.88, 31.15, 19.94, 14.02. Anal. Calcd. For C16H16ClNO2: C, 66.32; H, 5.57; N, 4.83. Found: C, 66.29; H, 5.56; N, 4.83%.
Butyl 2-(3,5-Dimethylphenyl)isonicotinate (5g)
1H NMR (500 MHz, DMSO) δ 8.79 (d, J = 4.9 Hz, 1H), 8.17 (s, 1H), 7.69 (ddd, J = 5.0, 1.4, 0.7 Hz, 1H), 7.66 (s, 2H), 7.03 (s, 1H), 4.31 (t, J = 6.8 Hz, 2H), 2.31 (s, 6H), 1.65 (ddd, J = 54.0, 13.5, 6.8 Hz, 2H), 0.94–0.87 (m, 5H). 13C NMR (125 MHz, DMSO) δ 166.49, 163.79, 148.49, 141.88, 141.53, 137.33, 137.33, 130.52, 123.83, 123.83, 123.70, 122.08, 66.88, 31.15, 21.99, 21.99, 19.94, 14.02. Anal. Calcd. For C18H21NO2: C, 76.29; H, 7.44; N, 4.94. Found: C, 76.26; H, 7.47; N, 4.96%.
Butyl 2-(Thiophen-3-Yl)isonicotinate (5h)
1H NMR (500 MHz, DMSO) δ 8.81–8.79 (m, 1H), 8.34 (dd, J = 3.0, 1.3 Hz, 1H), 8.22 (dd, J = 1.4, 1.0 Hz, 1H), 7.81 (dd, J = 5.0, 1.3 Hz, 1H), 7.75–7.61 (m, 2H), 4.38 (t, J = 6.7 Hz, 2H), 1.66 (q, J = 6.8 Hz, 2H), 1.00–0.93 (m, 5H). 13C NMR (125 MHz, DMSO) δ 166.49, 155.52, 151.39, 144.19, 143.39, 136.11, 131.35, 129.35, 122.10, 121.95, 66.88, 31.15, 19.94, 14.02. Anal. Calcd. For C14H15NO2S: C, 64.34; H, 5.79; N, 5.36. Found: C, 64.30; H, 5.76; N, 5.40%.
Anti-Bacterial Activity
Isolates Identification
Clinical isolates of S. aureus and E. coli were obtained from blood samples. Mannitol salt agar was used to confirm the presence of S. aureus and UTI chrome agar was used to confirm the E. coli (Aldrich Sigma, UK). They were further confirmed by the VITEK 2® compact system (BioMerieux, France).
Antibiogram of the Isolates
The MIC (mg/L) was calculated using AST cards in the VITEK 2® compact system (BioMerieux, France). Ampicillin/sulbactam, penicillin, cefoxitin, clindamycin, amoxicillin/clavulanic acid, cefixime, piperacillin, cefuroxime, cefepime, ceftriaxone, meropenem, minocycline, chloramphenicol, amikacin, tetracycline, colistin, tigecycline, and trimethoprim were all tested. CLSI 2020 guidelines were used to assess susceptibility (Table 1).
 |
Table 1 MIC (mg/L) of Antibiotics Against ESBL-Producing E. coli ST405 and MRSA |
Phenotypic Confirmation of Extended Spectrum β-Lactamase Producing E. coli
The double-disc synergy test, as described in the CLSI guidelines 2020,21 was used to detect ESBL production. In brief, 0.5 McFarland E. coli suspension was prepared in sterile normal saline. The E. coli was swabbed on the Mueller Hinton agar (MHA) plate, and co-amoxiclav disc (20/10µg) was placed in the center of the MHA plate, followed by ceftriaxone (30µg), ceftazidime (30µg), and cefepime (30µg) antibiotics 20mm away from the co-amoxiclav disc. The plates were incubated at 37°C overnight aerobically. The result interpretation was carried out as per CLSI guidelines. If a zone of inhibition produced between the co-amoxiclav and cephalosporin discs, the isolate is an ESBL producer.
Phenotypic Confirmation of Methicillin Resistant Staphylococcus aureus
The MRSA was confirmed following CLSI 2020 guidelines. In brief, the 0.5McFarland MRSA suspension was prepared in sterile normal saline and swabbed on the MHA plate. Cefoxitin disc (30µg) was placed in the MHA, and the plate was incubated overnight at 37°C. If the bacteria showed zone of inhibition ≤ 17mm, the isolate is MRSA as described in the CLSI guidelines (Figure 1).
Multilocus Sequence Typing (MLST) of E. coli
The two-locus CH typing technique was used for E. coli MLST analysis. Each PCR amplicon was extracted from an agarose gel with a QIAquick Gel Extraction kit (Qiagen, Hilden, Germany) and sequenced by Eurofins Scientific (London, UK). For data analysis, the E. coli MLST database (http://mlst.warwick.ac.uk/mlst/dbs/Ecoli/) was used.
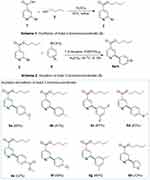 |
Figure 2 An overview of synthesis of butyl 2-bromoisonicotinate (3) and its derivatives (5a–5h) via SMC reaction. |
Anti-Bacterial Efficacy of Molecules
Agar Well Diffusion Assay of Various Molecules Against ESBL Producing E. coli ST 405 and MRSA
An agar well diffusion assay was used to test the anti-bacterial activity of the molecules (3, 5a, 5d–f, and 5h) against both pathogens. In brief, a 0.5 McFarland bacterial suspension was prepared in sterile normal saline and cultured on MHA plates and the wells were created on each plate using sterile 6 mm cork borers. One hundred microliters of each DMSO-diluted compound with concentration of 5 mg, 10 mg, 20 mg, 30 mg, 40 mg, and 50 mg was added to each well, and the plates were incubated at 37 °C overnight aerobically. A vernier caliper was used to measure the zone of inhibition (mm). The test was carried out three times.
The MIC of Various Molecules Against ESBL Producing E. coli ST405 and MRSA
The MIC (% w/v) of 3, 5a, 5d-f, and 5h molecules was determined using the micro-broth dilution test. In short, the bacterial suspension of both bacteria was diluted to 0.5 McFarland with sterile normal saline. Each well of microtiter plate (1–12) received 100 µL of LB broth. One hundred microliters of 0.5McFarland of bacteria were added in well 1–10 and 12 as positive control while 11 well (negative control) received LB broth. One hundred microliters of each molecule was double diluted from well 1–11. The microtiter plate was stored in a MaxQTM Mini overnight at 37°C. The MIC was calculated by comparing each well to the negative and positive control wells and the test was repeated for three times.
MBC Determination Against ESBL Producing E. coli ST405 and MRSA
The MBC is the lowest dilution required to prevent bacterial growth on an agar plate. To calculate the MBC, 10 µL was taken from each well of microtiter plate and inoculated on the MHA for bacterial count. The plates were incubated at 37°C overnight aerobically. The viability of the cells on the plates was determined, and any visible colonies were classified as bacterial growth or no growth. Each experiment was carried out in triplicate.
Molecular Docking Studies
Molecular docking was carried out to predict the interaction of synthesized molecules with the binding sites of E. coli CsgC in reduced form (PDB ID: 2Y2T) using Molecular Operating Environment (MOE version 2019.02). The 2D structures of chemical molecules, ie, 3, 5a–5h were drawn using ChemBioDraw Ultra [Chemical Structure Drawing Standard; Cambridge Soft Corporation, USA],22 and then optimized by adding polar hydrogen atoms followed by the energy minimization using Merck molecular force field (MMFF94)23 in MOE. The X-ray crystallographic structure of E. coli (PDB ID: 2Y2T) with a resolution of 2.30 Å24 was downloaded from Protein Data Bank (http://www.rcsb.org./pdb). The structure was prepared per the docking protocol by adding hydrogen atoms, removing hetero-atoms/water, and minimizing energy with the AMBER99 force field in MOE. Further, induced fit molecular docking of the chemical molecules was performed with the receptor site atoms with Alpha Triangle placement method using London dG scoring method.25 Additionally, 50 poses per molecule were generated to make the system flexible, and the best pose based on least energy value (E-score) was selected for protein ligand interaction fingerprint. Visualization of the docked pose was done with the help of MOE.
Results
Isolates Confirmation
The isolates were confirmed as ESBL-producing E. coli ST405 and MRSA after VITEK 2 compact system, phenotypic confirmation and MLST data analysis.
Chemistry of the Compounds
Butyl 2-bromoisonicotinate (3) was formed by the reaction of commercially available 2-bromo isonicotinic acid (1) with n-butanol (2) by F. esterification by using a catalytic amount of sulphuric acid (H2SO4) to form the product in 91% yield. Subsequently, the SMC reaction of butyl 2-bromoisonicotinate (3) with various heteroaryl and aryl boronic acids (4) in the presence of potassium phosphate as a base and commercially available tetrakis(triphenylphosphine) palladium(0) formed the targeted molecules (5a–h) in good to excellent yields (52–89%). The newly formed molecules were purified by flash column chromatography, characterized by spectroscopic techniques. The influence of substituents on boronic acids was previously investigated. Results showed that electron-rich groups were more crucial for effective yields than electron-deficient groups.26,27 Similarly, molecule (5e) has the lowest yield (52%) due to the presence of electron-poor and bulky groups (Figure 2).
Anti-Bacterial Activity of Compound
A single isolate of ESBL producing E. coli ST405 and MRSA resistant to commonly used antibiotics such as cephalosporins, β-lactam inhibitors, chloramphenicol, levofloxacin, and amikacin were used in this study. MRSA isolate was sensitive to vancomycin, whereas E. coli was only sensitive to colistin. The phenotypic test confirmed E. coli isolate as an ESBL producer, whereas Staphylococcus aureus was resistant to methicillin and hence designated MRSA. Upon testing of ESBL-producing E. coli, molecule (3) produced the maximum zone of inhibition (29 mm) at the concentration of 50 mg/mL followed by molecule (5a), which had 15mm zone, and molecule (5d) had 14mm zone inhibition. Further, for the MRSA isolate, molecule (3) had 18mm zone of inhibition, molecule (5a) 24 mm zone and molecule (5d) 21 mm zone inhibition at 50mg/mL concentration (Table 2 and 3). ESBL-producing E. coli was inhibited at the concentration of 0.78 mg/mL, molecule (3), followed by 3.12 mg/mL (5a), 6.25 mg/mL (5d), 25mg/mL (5e), 25mg/mL (5f), and 25mg/mL (5h). ESBL-producing E. coli were killed at the concentration of 1.56mg/mL, molecule (3), 6.25mg/mL (5a), 12.5mg/mL (5d), and 50mg/mL for each molecule (5e, 5f, and 5h).
 |
Table 2 Zone of Inhibition (mm) of ESBL-Producing E. coli ST405 Agar Well Diffusion Assay |
 |
Table 3 Zone of Inhibition (mm) of MRSA by Agar Well Diffusion Assay |
MRSA was inhibited at the concentration of 0.78mg/mL, molecule (3) followed by 1.56mg/mL (5a), 6.25mg/mL (5d), and 25 mg/mL for 5e, 5f, and 5h. However, these isolates were killed at a concentration of 1.56 mg/mL, molecule (3), 3.12mg/mL (5a) and 12.5mg/mL (5d) and 25mg/mL for each molecule 5e, 5f, and 5h (Table 4 and Table 5, Figures 3 and 4).
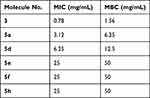 |
Table 4 MIC (w/v) and MBC (w/v) of Different Molecules Against ESBL-Producing E. coli ST405 |
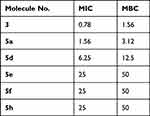 |
Table 5 MIC (w/v) and MBC (w/v) of Different Molecules Against MRSA |
 |
Figure 3 MIC of the ESBL-producing E. coli ST405 and MRSA isolate X-axis are the isolates, and Y-axis is the different concentrations of the molecules, -ve: negative control +ve: positive control. |
 |
Figure 4 MBC of the molecule (3) against ESBL producing E. coli ST405. |
Molecular Docking
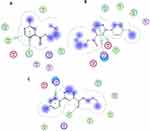
In this study, a series of butyl 2-bromoisonicotinate (3) and its derivatives (5a–h) were studied in silico to investigate the potential binding geometries and interaction modes with the active site of E. coli 2Y2T target protein using Molecular Operating Environment (MOE) version. 2019. According to the outcomes obtained from the docking studies, it has been observed that the parent molecule (3) and two analogs (5a, 5b and 5d) showed better binding interaction (hydrogen bonding and hydrophobic) with the common scaffold (2-bromoisonicotinate) and strongest binding energies (Table 6). Moreover, the pyridinyl group of molecule (3) displayed two hydrophobic interactions (H-pi: C—5ring, pi-H: 6ring---CG2) with Ile71 and the hydrophobic side chain of Trp95 amino acid residue of the receptor as shown in Figure 5A. Similarly, pyridinyl group of the molecule (5a) also showed two hydrophobic, ie, pi-H: 6-ring---CA and 6-ring---N interactions with Arg76 and Val77 amino acid residues, respectively, Figure 5B. Additionally, the molecule (5b) exhibited well-established hydrogen bonding (H-acceptor: O---N) and hydrophobic (Pi-H: 6ring---CD) with Arg76 and Val77, respectively, Figure 5C. However, for the rest of the molecules (5c, e–h), a weak or no interaction pattern (Figure S9) was observed which might be due to the bulky group substitution on the 2-bromoisonicotinate.
 |
Table 6 Binding Energies and Interaction Pattern of the Parent Molecule (3) and Its Analogues (5a–h) |
Discussion
The emergence of AMR pathogens is becoming a serious threat to the public health sector globally. Globally, around 4.9 million people’s deaths are associated with AMR pathogens (https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(21)02724-0/fulltext). By 2050, one person will die every three seconds if we not tackle the AMR now (https://www.dovepress.com/molecular-epidemiology-of-extensively-drug-resistant-acinetobacter-bau-peer-reviewed-fulltext-article-IDR). Therefore, novel therapeutic approaches are need of the hour to combat the global AMR issue. Metal-catalyzed cross-coupling reactions have become indispensable in various stages of drug development, particularly in the discovery phase where rapid access to a variety of chemical matter for biological evaluation is critical. The Suzuki-Miyaura coupling is one of the most widely used cross-coupling reactions in pharmaceutical synthesis due to its mild reaction conditions, functional group tolerance, and the stability, ease of preparation, and environmental friendliness of organoboron reagents. The rapid transmetalation using palladium(II) complexes allows for feasible scaling up of the process and low reagent cost, making it applicable to agrochemical, fine chemical, pharmaceutical, and modern material industries. The successful and adaptable application of SM coupling in constructing biaryl moieties has made it the “gold standard” in medicinal chemistry.28 It has also been utilized to modify and enhance the activity of natural products and pharmaceuticals in late stages to achieve better yields and chemoselectivity.29–31 Our research team employed SMC coupling to synthesize carboxamides and carboxylate derivatives, which were then tested for their in-vitro anti-bacterial properties against WHO-listed extremely resistant bacterial strains, aiming to identify potential therapeutic candidates.32–35 In the present study, both the ESBL producing E. coli and MRSA were resistant to commonly used antibiotics including penicillins, cephalosporins, and beta-lactam inhibitors. The anti-bacterial activity of butyl 2-bromoisonicotinate molecules and its derivatives against ESBL-producing E. coli ST405 and MRSA. Molecule (3) produced the maximum zone of inhibition (29 mm) at 50 mg/mL concentration against ESBL producing and 18mm against MRSA.
Our previous studies revealed that carboxamide is one of the significant scaffolds against resistant bacteria. Previously we studied the biofilm inhibitory activity against E. coli and B. subtilis and found that the aryl groups act as proliferative sites and show hydrophobic (non-polar) interactions. At the same time, the amides behave as a hydrophilic binding site against bacterial enzyme proteins, which may cause death or make static growth, leading to good inhibitory activity.36 Further, we studied the N, O, and S heterocycles carboxamides like N-(4-bromophenyl)furan-2-carboxamide,32 2-aryl-4-chlorophenyl-5-arylthiophene-2-carboxylates34 4-bromo-N-(5-methyl-1H-pyrazol-3-yl)benzamide,35 N-(4-methylpyridin-2-yl) Thiophene-2-Carboxamide37 to study the effectiveness of these scaffolds and found effectiveness against different resistant strains of E. coli, A. baumannii, S. enterica, E. cloacae, S. aureus and K. pneumoniae. We found that heterocyclic carboxamides are pivotal in anti-bacterial activities. Due to the importance of facile synthesized small molecules in drug discovery, there is a need for the modern world due to economic and environmental factors. So, we designed a molecule with a linear alkyl group that can let the compounds’ proliferation into the bacterial cell. N-heterocyclic amide moiety makes it hydrophilic to attach with the bacterial proteins.
ESBL-producing E. coli ST405 was inhibited at the MIC of 0.78 mg/mL of molecule (3), and 3.12 mg/mL with molecule 5a. However, MRSA was inhibited at the concentration of 0.78mg/mL with molecule (3) and 1.56mg/mL with 5a.
Herein, we used an approachable, conventional and friendly technique to make butyl 2-bromoisonicotinate (3) by Fischer esterification. The esterification reaction is commonly utilized to convert carboxylic acids into esters by using a strong acid catalyst and an excess of alcohol as the reactants to form ester from 2-bromo isonicotinic acid (1). Nicotinic acid has ability to show anti-bacterial activity as reported in the literature.38–41 So, we extend our study and make a functionalized series of compounds (5a–5h) from 2-bromoisonicotinate (3) using SMC.
The advancement of docking methodologies has led to more accurate predictions of the biological activity of molecules. Computational techniques and theoretical approaches have enabled the development of explanatory hypotheses on drug mechanisms of action. This work presents in-silico studies using the MOE software (Molecular Operating Environment), to validate the results. The results showed that the most of the interactions observed in the present in silico study are hydrophobic. Thus, we can propose that the hydrophobic cavity of the receptor (2Y2T) would be the better binding cavity for inhibiting the E. coli receptor involved in urinary and bloodstream infections. Furthermore, synthesized molecules (3, 5a, 5b and 5d) showed good binding energy (Table 6) toward the target protein ranging from −3.0 to −4.5 kJ mol−1, and interestingly, these findings are well aligned with the findings from the experimental assays for the prediction of inhibitory potency.
Conclusions
We successfully synthesized butyl-2-bromoisonicotinate by Fischer esterification and its analogues via SMC in medium to good yields. Fischer esterification is comparatively a greener approach as it has been done in water. Molecule (3) was found to be most effective against ESBL-producing E. coli ST405 with 29 mm zone inhibition, and molecules (5a and 5d) were most effective against MRSA strain with 24 mm and 21 mm zone inhibition, respectively. Furthermore, docking studies revealed that the hydrophobic cavity of the receptor (2Y2T) would be the better binding cavity for inhibiting the E. coli ST405 receptor. It showed that hydrophobic interactions play a key role in the attachment to the hydrophobic pocket. The synthesized molecules (3, 5a, 5b, and 5d) showed good binding energies. However, weak or no interaction pattern was observed for the rest of the molecules (5c, 5e–h).
Acknowledgments
The authors extend their appreciation to the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia for funding this research through the project number IFP-IMSIU-2023131. The authors also appreciate the Deanship of Scientific Research at Imam Mohammad Ibn Saud Islamic University (IMSIU) for supporting and supervising this project.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Fischbach MA, Walsh CT. Antibiotics for emerging pathogens. Science. 2009;325(5944):1089–1093. doi:10.1126/science.1176667
2. Smith RD, Coast J. Antimicrobial resistance: a global response. Bull World Health Organ. 2002;80:126–133.
3. Usman Qamar M, S Lopes B, Hassan B, et al. The present danger of New Delhi metallo-β-lactamase: a threat to public health. Future Microbiol. 2020;15:1759–1778. doi:10.2217/fmb-2020-0069
4. Aslam B, Wang W, Arshad MI, et al. Antibiotic resistance: a rundown of a global crisis. Infect Drug Resist. 2018;11:1645–1658. doi:10.2147/IDR.S173867
5. Asokan GV, Ramadhan T, Ahmed E, Sanad H. WHO global priority pathogens list: a bibliometric analysis of medline-PubMed for knowledge mobilization to infection prevention and control practices in Bahrain. Oman Med J. 2019;34(3):184–193. doi:10.5001/omj.2019.37
6. Ejaz H, Younas S, Qamar MU, et al. Molecular epidemiology of extensively drug-resistant mcr encoded colistin-resistant bacterial strains co-expressing multifarious β-lactamases. Antibiotics. 2021;10(4):467. doi:10.3390/antibiotics10040467
7. Liu W-T, Chen E-Z, Yang L, et al. Emerging resistance mechanisms for 4 types of common anti-MRSA antibiotics in Staphylococcus aureus: a comprehensive review. Microb Pathog. 2021;156:104915. doi:10.1016/j.micpath.2021.104915
8. Kumar A, Schweizer HP. Bacterial resistance to antibiotics: active efflux and reduced uptake. Adv Drug Deliv Rev. 2005;57(10):1486–1513. doi:10.1016/j.addr.2005.04.004
9. Gabriel GJ, Som A, Madkour AE, Eren T, Tew GN. Infectious disease: connecting innate immunity to biocidal polymers. Mater Sci Eng R Rep. 2007;57(1–6):28–64. doi:10.1016/j.mser.2007.03.002
10. Phillips DJ, Harrison J, Richards S-J, et al. Evaluation of the antimicrobial activity of cationic polymers against mycobacteria: toward antitubercular macromolecules. Biomacromolecules. 2017;18(5):1592–1599. doi:10.1021/acs.biomac.7b00210
11. Tuchilus CG, Nichifor M, Mocanu G, Stanciu MC. Antimicrobial activity of chemically modified dextran derivatives. Carbohydr Polym. 2017;161:181–186. doi:10.1016/j.carbpol.2017.01.006
12. Bruckert E, Labreuche J, Amarenco P. Meta-analysis of the effect of nicotinic acid alone or in combination on cardiovascular events and atherosclerosis. Atherosclerosis. 2010;210(2):353–361. doi:10.1016/j.atherosclerosis.2009.12.023
13. Eldehna WM, Fares M, Abdel-Aziz MM, Abdel-Aziz HA. Design, synthesis and antitubercular activity of certain nicotinic acid hydrazides. Molecules. 2015;20(5):8800–8815. doi:10.3390/molecules20058800
14. Dhalla AK, Santikul M, Smith M, Wong M-Y, Shryock JC, Belardinelli L. Antilipolytic activity of a novel partial A1 adenosine receptor agonist devoid of cardiovascular effects: comparison with nicotinic acid. J Pharmacol Exp Ther. 2007;321(1):327–333. doi:10.1124/jpet.106.114421
15. Narang R, Narasimhan B, Sharma S, et al. Synthesis, antimycobacterial, antiviral, antimicrobial activities, and QSAR studies of nicotinic acid benzylidene hydrazide derivatives. Med Chem Res. 2012;21(8):1557–1576. doi:10.1007/s00044-011-9664-7
16. Blangetti M, Rosso H, Prandi C, Deagostino A, Venturello P. Suzuki-Miyaura cross-coupling in acylation reactions, scope and recent developments. Molecules. 2013;18(1):1188–1213. doi:10.3390/molecules18011188
17. Han F-S. Transition-metal-catalyzed Suzuki–Miyaura cross-coupling reactions: a remarkable advance from palladium to nickel catalysts. Chem Soc Rev. 2013;42(12):5270–5298. doi:10.1039/c3cs35521g
18. Billingsley K, Buchwald SL. Highly efficient monophosphine-based catalyst for the palladium-catalyzed Suzuki−Miyaura reaction of heteroaryl halides and heteroaryl boronic acids and esters. J Am Chem Soc. 2007;129(11):3358–3366. doi:10.1021/ja068577p
19. Deshmukh MB, Patil SH, Shripanavar CS. Synthesis and insecticidal activity of some nicotinic acid derivatives. J Chem Pharm Res. 2012;4(1):326–332.
20. Kanwal I, Rasool N, Zaidi SHM, et al. Synthesis of functionalized thiophene based pyrazole amides via various catalytic approaches: structural features through computational applications and nonlinear optical properties. Molecules. 2022;27(2):360. doi:10.3390/molecules27020360
21. Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing.
22. Mills N. ChemDraw Ultra 10.0 Cambridgesoft, 100 CambridgePark Drive, Cambridge, MA 02140. Commercial Price: 1910fordownload, 2150 for CD-ROM; Academic Price: 710fordownload, 800 for CD-ROM. ACS Publications; 2006.
23. Hwang SB, Lee CJ, Lee S, et al. PMFF: development of a physics-based molecular force field for protein simulation and ligand docking. J Phys Chem B. 2020;124(6):974–989. doi:10.1021/acs.jpcb.9b10339
24. Taylor JD, Zhou Y, Salgado PS, et al. Atomic resolution insights into curli fiber biogenesis. Structure. 2011;19(9):1307–1316. doi:10.1016/j.str.2011.05.015
25. Li Y, Liu Z, Li J, et al. Comparative assessment of scoring functions on an updated benchmark: 1. Compilation of the test set. J Chem Inf Model. 2014;54(6):1700–1716. doi:10.1021/ci500080q
26. Malik A, Rasool N, Kanwal I, et al. Suzuki–Miyaura reactions of (4-bromophenyl)-4, 6-dichloropyrimidine through commercially available palladium catalyst: synthesis, optimization and their structural aspects identification through computational studies. Processes. 2020;8(11):1342. doi:10.3390/pr8111342
27. Imran HM, Rasool N, Kanwal I, et al. Synthesis of halogenated [1, 1′-biphenyl]-4-yl benzoate and [1, 1′: 3′, 1 ″-terphenyl]-4′-yl benzoate by palladium catalyzed cascade C–C coupling and structural analysis through computational approach. J Mol Struct. 2020;1222:128839. doi:10.1016/j.molstruc.2020.128839
28. Cooper TW, Campbell IB, Macdonald SJ. Factors determining the selection of organic reactions by medicinal chemists and the use of these reactions in arrays (small focused libraries). Angew Chem Int Ed. 2010;49(44):8082–8091. doi:10.1002/anie.201002238
29. McConnell CR, Liu S-Y. Late-stage functionalization of BN-heterocycles. Chem Soc Rev. 2019;48(13):3436–3453. doi:10.1039/C9CS00218A
30. Zhang J, Zhang P, Shao L, Wang R, Ma Y, Szostak M. Mechanochemical solvent‐free Suzuki–Miyaura cross‐coupling of amides via highly chemoselective N− C cleavage. Angew Chem Int Ed. 2022;61(7):e202114146.
31. Yang S, Zhou T, Poater A, Cavallo L, Nolan SP, Szostak M. Suzuki–Miyaura cross-coupling of esters by selective O–C (O) cleavage mediated by air-and moisture-stable [Pd (NHC)(μ-Cl) Cl] 2 precatalysts: catalyst evaluation and mechanism. Catal Sci Technol. 2021;11(9):3189–3197. doi:10.1039/D1CY00312G
32. Siddiqa A, Zubair M, Bilal M, et al. Synthesis of functionalized N-(4-Bromophenyl) furan-2-carboxamides via Suzuki-Miyaura cross-coupling: anti-bacterial activities against clinically isolated drug Resistant A. baumannii, K. pneumoniae, E. cloacae and MRSA and its validation via a computational approach. Pharmaceuticals. 2022;15(7):841. doi:10.3390/ph15070841
33. Arshad M, Rasool N, Qamar MU, Shah SAA, Zakaria ZA. Facile synthesis of functionalized phenoxy quinolines: antibacterial activities against ESBL producing Escherichia coli and MRSA, docking studies, and structural features determination through computational approach. Molecules. 2022;27(12):3732. doi:10.3390/molecules27123732
34. Mujahid A, Rasool N, Qamar MU, et al. Arylation of halogenated thiophene carboxylate via Suzuki–Miyaura reaction: anti-bacterial study against clinically isolated extensively drug resistant Escherichia coli sequence type 405 and computational investigation. Arab J Chem. 2022;15(3):103662. doi:10.1016/j.arabjc.2021.103662
35. Ahmad G, Rasool N, Qamar MU, et al. Facile synthesis of 4-aryl-N-(5-methyl-1H-pyrazol-3-yl) benzamides via Suzuki Miyaura reaction: antibacterial activity against clinically isolated NDM-1-positive bacteria and their docking studies. Arab J Chem. 2021;14(8):103270. doi:10.1016/j.arabjc.2021.103270
36. Ejaz S, Zubair M, Rasool N, et al. N‐([1, 1ʹ‐biaryl]‐4‐yl)‐1‐naphthamide‐based scaffolds synthesis, their cheminformatics analyses, and screening as bacterial biofilm inhibitor. J Basic Microbiol. 2022;62(9):1143–1155. doi:10.1002/jobm.202100288
37. Ahmad G, Khalid A, Qamar MU, et al. Antibacterial efficacy of N-(4-methylpyridin-2-yl) thiophene-2-carboxamide analogues against extended-spectrum-β-lactamase producing clinical strain of Escherichia coli ST 131. Molecules. 2023;28(7):3118. doi:10.3390/molecules28073118
38. Morjan RY, Mkadmh AM, Beadham I, et al. Antibacterial activities of novel nicotinic acid hydrazides and their conversion into N-acetyl-1, 3, 4-oxadiazoles. Bioorg Med Chem Lett. 2014;24(24):5796–5800. doi:10.1016/j.bmcl.2014.10.029
39. Ashma A, Yahya S, Subramani A, et al. Synthesis of new nicotinic acid hydrazide metal complexes: potential anti-cancer drug, supramolecular architecture, antibacterial studies and catalytic properties. J Mol Struct. 2022;1250:131860. doi:10.1016/j.molstruc.2021.131860
40. Osigbemhe IG, Louis H, Khan EM, et al. Antibacterial potential of 2-(-(2-Hydroxyphenyl)-methylidene)-amino) nicotinic acid: experimental, DFT studies, and molecular docking approach. Appl Biochem Biotechnol. 2022;194(12):5680–5701. doi:10.1007/s12010-022-04054-9
41. Asif M. Antimicrobial potential of nicotinic acid derivatives against various pathogenic microbes. Eur Rev Chem Res. 2014;1(1):10–21. doi:10.13187/ercr.2014.1.10
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.