Back to Journals » Journal of Pain Research » Volume 16
Sympathetic-Sensory Coupling as a Potential Mechanism for Acupoints Sensitization
Authors Cui X , Zhang Z, Xi H, Liu K, Zhu B, Gao X
Received 7 June 2023
Accepted for publication 15 August 2023
Published 30 August 2023 Volume 2023:16 Pages 2997—3004
DOI https://doi.org/10.2147/JPR.S424841
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Houman Danesh
Xiang Cui,1 Ziyi Zhang,1,2 Hanqing Xi,1 Kun Liu,1 Bing Zhu,1 Xinyan Gao1
1Department of Physiology, Institute of Acupuncture and Moxibustion, Academy of Chinese Medical Sciences, Beijing, 100700, People’s Republic of China; 2College of Acupuncture and Tuina, Shaanxi University of Chinese Medicine, Xianyang, Shaanxi Province, 712046, People’s Republic of China
Correspondence: Xiang Cui; Xinyan Gao, Department of Physiology, Institute of Acupuncture and Moxibustion, China Academy of Chinese Medical Sciences, Beijing, 100700, People’s Republic of China, Tel +86-10-64089422 ; +86-10-64089420, Email [email protected]; [email protected]
Abstract: A series of studies have demonstrated acupoint sensitization, in which acupoints can be activated in combination with sensory hypersensitivity and functional plasticity during visceral disorders. However, the mechanisms of acupoint sensitization remain unclear. Neuroanatomy evidence showed nociceptors innervated in acupoints contribute to the mechanism of acupoint sensitization. Increasing studies suggested sympathetic nerve plays a key role in modulating sensory transmission by sprouting or coupling with sensory neuron/nociceptor in the peripheral, forming the functional structure of the sympathetic-sensory coupling. Notably, the sensory inputs of the disease-induced sensitized acupoint contribute to the homeostatic regulation and also involve in delivering therapeutic information under acupuncture, hence, the role of sprouted sympathetic in acupoint function should be given attention. We herein reviewed the current knowledge of sympathetic and its sprouting in pain modulation, then discussed and highlighted the potential value of sympathetic-sensory coupling in acupoint functional plasticity.
Keywords: referred pain, hyperalgesia, acupuncture, nociceptor, Somato-sympathetic-viscera reflex
Introduction
Acupoints are critical places on the body for acupuncture treatment, which act an enhancing role in switching on and promoting the self-healing process under conditions.1 Yet, its mechanism remains unknown. Numerous studies have attempted to elucidate the nature of acupoints via somato-autonomic nerve reflex and neuroendocrine-immune networks.2,3 Existing evidence demonstrated that the neuroanatomical basis of acupoint function was attributed to the involvement of the nociceptors, skin microecology, immune cells, and the skin-brain axis.4–10 Acupoint sensitization has been described recently and primarily pertains to referred acupoint activation by visceral disease or tissue injury, accompanied by sensory hypersensitivity and functional enhancement.11–13 Neurogenic inflammation is the principal pathogenic feature of sensitized acupoints and is largely a consequence of nociceptor activation and neuroimmune interactions in sensitized acupoints.14–16 Meanwhile, nociceptor hyperexcitation was found to strengthen their ability to respond to needling manipulation and increase the level of acupuncture signals conveyed to the body.4,7,17 Therefore, when an visceral disorder or tissue injury may result in referred sensory hypersensitivity at an acupoint, activating nociceptors in the referred sensitized area can also enhance the therapeutic action of the acupoint by amplifying the delivery of acupuncture signals.
Previous research has suggested the role of the sympathetic nervous system in the encoding of nociceptive information during disease and that inhibiting sympathetic activity or pharmacologically blocking it greatly improves sensory hyperalgesia.18–20 The sympathetic nervous system, as a component of the autonomic nervous system, is extensively engaged in the functional regulation of the body’s organs. Sympathetic nerve hyperexcitation and terminal sprouting are the principal functional and morphological responses to visceral disease and pain.21–23 In the 1990s, McLachlan and Chung observed sympathetic nerve terminal sprouting in a neuropathic pain rat model; these sprouted fibers encircled dorsal root ganglion (DRG) neurons and formed basket structures, a phenomenon known as sympathetic-sensory coupling.24,25 Increasing evidence has indicated that peripheral sensory afferents are impacted by these functional connections, contributing to persistent pain. As of yet, it is unclear whether sympathetic-sensory coupling is involved in acupoint sensitization and drives a role in the enhancement of acupuncture signal. This review summarized recent advances in sympathetic sprouting and sympathetic-sensory coupling under pathological conditions and discussed the underlying role of these effects in the functional enhancement of sensitized acupoints, intending to provide a novel theoretical basis for studying the neural mechanisms of acupoints.
Acupoint Sensitization and Plasticity
Acupoint Sensitization
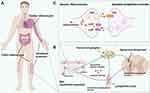
Uncovering the acupoints’ scientific basis has been the main goal of acupuncture researchers worldwide. Recent findings have demonstrated a high rates of overlap between the distribution of acupoints and referred hypersensitive areas on the body surface caused by visceral illnesses or tissue injury (Figure 1A). In 1893, the notable British physician Henry Head observed and summarized the distribution rules for referred pain areas associated with various visceral disorders.26–28 He also observed “sensitive spots” in these locations that, when stimulated, could alleviate visceral symptoms, a function quite similar to that of acupoints.29 Later, in 1895, Mackenzie methodically mapped the distribution of cardiac referred pain, which closely corresponds to the path of the Hand Shao Yin Heart Meridian.30 Recent clinical studies have revealed that colitis-related somatic sensitized points are predominantly located in the abdomen, low back, and lower extremities,31 whereas pulmonary disease-related somatic referred sensitized points are predominantly located in the neck and shoulders, upper chest, upper back, and upper extremities.32 Notably, these referred sensitive areas contain many acupoints that are commonly used in clinics for the treatment of diseased viscera. For example, the lower abdomen contains acupoints ST25 and SP14, which are used for the treatment of intestinal problems, while the upper back contains acupoints BL13 and CV17, which are used for the treatment of pulmonary diseases.
Additionally, some researchers have compared the locations of routine acupoints to those of referred hypersensitive areas caused by visceral dysfunction. Beissner et al reported largely overlap between the Head’s zone and the Shu- and Mu-acupoints.29 Other studies have reported overlap rates of 48.76% between heat-sensitive sites and acupoints, and 71–92% between the trigger and pressure points.33–35 Together, these findings show a strong relationship between the origins of acupoints and areas of referred hypersensitivity induced by visceral disease or tissue injury, which Bing and other scholars have termed “acupoint sensitization.” This notion refers to an acupoint as a “somatic referred point” generated by visceral diseases and tissue injury,36 which can exert a specific regulatory effect on visceral function via the soma-viscera connection established via biological evolution.
Progress in the Study of Acupoints and Their Functions
Sensitized acupoints manifest primarily through sensory alterations. Mechanical, heat, and pressure sensitization all indicate hypersensitive acupoints. The guiding principle for locating acupoints, as described in Huangdi Neijing, was “where there is pain, there is a way”, implying that sensory-sensitive points are the first option for needling. Notably, recent research has revealed increased treatment effects of sensitive acupoints in response to needling manipulation, a feature classified as “acupoint plasticity”. For example, Rong et al demonstrated significantly enlarged receptive fields of wide dynamic neurons in the spinal cord associated with acupoints ST36 and ST37 in rats following colitis,37 and that stimulating sensitized ST36 and ST37 evoked significantly more C fiber sciatic nerve discharge compared to non-sensitized acupoints.38 Furthermore, a recent study demonstrated that sensitized acupoints directly regulated visceral function. As neurogenic inflammation is the primary feature of sensitized acupoints, Li et al simulated disease conditions by subcutaneously injecting mustard oil, which activated the C nociceptor and resulted in neurogenic inflammation, into rat acupoint ST25.11 They observed that sensitized ST25 had a direct inhibitory effect on jejunal motility and that the electric current required to suppress jejunal motility was also greatly reduced compared to normal settings. Additionally, our recent study discovered that the inputs of sensitized acupoints caused by myocardial ischemia simulation contributed to the homeostatic regulation of heart function by boosting sympathetic hyperexcitation.1 Although these findings imply that sensitized acupoints directly drive the homeostatic process and have a more therapeutic effect on visceral function in response to needling manipulation, the underlying neural mechanism of acupoint plasticity remains elusive. Together with peripheral sensory nerves (nociceptors), mast cells, and immune cells, a recent study demonstrated the contributions of sympathetic nerves to sensory modulation. Visceral dysfunction and tissue injury can elicit the sprouting of sympathetic fiber terminals surrounding the primary sensory neurons to form a basket-like structure, a process termed sympathetic-sensory coupling that plays a critical role in sensory modulation by sensitizing peripheral sensory fibers. We hypothesized that sympathetic-sensory coupling may be a critical anatomical structure that enhances acupoint responsiveness in response to needling manipulation and may also modulate the neuronal mechanism of acupoint plasticity. The role of peripheral sympathetic fibers and sympathetic-sensory coupling in this process requires more in-depth study.
Sympathetic Sprouting and Sensory-Sympathetic Coupling Involved in Nociceptive Modulation
Distribution and Function of the Sympathetic Nervous System
The sympathetic and parasympathetic nervous systems constitute the autonomic nervous system, which manages visceral function and enables the body to respond rapidly to internal and external dangers or environmental changes.39–41 The cell bodies of the sympathetic preganglionic neurons are located in the lateral horn of the spinal cord, with the axons terminating in the sympathetic paravertebral or prevertebral ganglions. The axons of postganglionic neurons depart the ganglia and terminate in effector tissues and organs. As a single preganglionic fiber typically contains numerous branches that can connect to a variety of diverse postganglionic neurons, stimulating sympathetic nerves has a more comprehensive regulatory effect compared to that in the parasympathetic nervous system. In addition to regulating the body’s fight-or-flight response, the sympathetic nervous system is generally considered to be involved in the regulation of the activities of nearly all key organs and is critical for homeostasis regulation.42 Along with other duties, sympathetic nerves also play a physiological role in the control of heart rate, blood pressure, and circulation.43 However, accumulating evidence suggests a close relationship between the sympathetic nervous system and nociception and hyperalgesia under pathological situations such as visceral pain and somatic hyperalgesia.44–46
Sympathetic Sprouting and Sympathetic-Sensory Coupling Under Pathological Conditions
Generally, sensory sensitization can be classified as spontaneous pain, touch-sensitive pain (allodynia), and pain sensitization (hyperalgesia),47 all of which can be detected in sensitized acupoints.10,11,13–15 Although it has been established that ectopic discharge of sensory neurons caused by various injuries or inflammations contributes to sensory sensitization,21,48–50 recent research suggests that sprouted sympathetic fibers contribute to the peripheral mechanism of sensory hypersensitivity.51 To date, hyperexcitation and sympathetic postganglionic fiber sprouting have been identified as two pathological features of the sympathetic nerves, both of which have been implicated in the development of visceral pain and hyperalgesia.46 McLachlan et al defined the first specific case of sympathetic-sensory coupling in a rat model of neuropathic pain in the early twentieth century, in which postganglionic sympathetic fiber terminals sprouted into the DRG and surrounded the DRG sensory neurons, particularly those with ectopic discharge, forming “basket-like structures”24 This structure strengthens the connection between sympathetic and sensory neurons, increasing the magnitude of their sensitization, thereby expanding the neurons’ activation range and enhancing the efficiency with which injury information is transmitted to the central nervous system. Emerging evidence suggests that sympathetic-sensory coupling in the DRG engage into neuropathic, inflammation, chemotherapy-induced neuropathic pain, and visceral pain mechanisms. Current research has demonstrated the range of locations in which sympathetic-sensory coupling has been reported in pathological states. For instance, in a rat model of chronic knee arthritis, Nascimento et al observed sympathetic sprouting in the skin of an affected foot sole,52 Jimenez-Andrade et al observed sensory nerve fibers and sympathetic fibers sprouting in the synovial membrane and periosteum of the knee joint,53 and Grelik et al reported sympathetic sprouting in the lower lip skin following mental nerve ligation.54 In a rat model of keratitis, Marfurt et al reported sympathetic sprouting in the cornea.55 In a model of chronic myocardial ischemia, Yin et al observed sympathetic sprouting in the ischemic myocardium and the superior cervical ganglion.56,57
Given the overactivity of peripheral sensory neurons caused by sprouted sympathetic terminals, recent research indicates that suppressing sympathetic nerve activity can alleviate pain. Xie et al showed that cutting the gray ramus communications of the spinal nerve decreased sympathetic sprouting in the DRG and ameliorated pain behavior in a rat model of spinal nerve ligation (SNL).49 Francisney et al observed suppressed sympathetic sprouting and significantly improved plantar pain sensitivity 2 weeks following SNI surgery in rats administered guanethidine, a sympathetic blocker.52 Zhang et al discovered that the pre-injection of lidocaine reduced sympathetic sprouting and relieving pain in the DRG in a neuropathic pain model.58 In general, these findings indicate that sympathetic and sensory nerves can interact in a sympathetic-sensory coupling fashion. In terms of its role in sensory modulation in disease conditions, we proposed that sympathetic-sensory coupling also played a role in the sensory hypersensitivity of sensitized acupoints, contributing to functional plasticity by amplifying the inputs from needling manipulation.
The neuronal mechanism of sympathetic sprouting and the interplay of sympathetic-sensory coupling have been studied extensively. Physiologically, after the sympathetic preganglionic fibers are exchanged in the paravertebral or prevertebral ganglia, a small portion of the sympathetic postganglionic fibers attach to the dorsal ramus of the spinal nerves, but only a small proportion of the postganglionic fibers travel to innervate small vessels within the DRG.49 Alternatively, diverse pathological states result in the sprouting of sympathetic nerves in the DRG of injured and inflamed areas, where sprouted fiber-endings couple with sensory neurons or fibers, as previously described. Side branch and regenerative are two distinct forms of sympathetic fiber sprouting. Side branch sprouting primarily refers to sympathetic fibers that innervate the vasculature of DRG that sprouting toward the injured neurons in a nerve growth factor (NGF)-dependent way following remote nerve injury, termed NGF-dependent sympathetic fiber sprouting.59–61 Regenerative sprouting emerges if peripheral nerve injury hampers the sprouting of surrounding sympathetic fibers to distant sites (limb direction), resulting in sprouting along the initial path into the DRG, forming sympathetic-sensory coupling following surrounding sensory neurons. Given the absence of NGF, this pattern of regeneration sprouting is termed NGF-independent sympathetic sprouting. Ectopic sensory neuronal activity acts as a trigger, driving sympathetic postganglionic fibers to sprout into the DRG.62 While sensory neurons in the DRG rarely exhibit spontaneous activity under normal settings, pathological conditions such as nerve injury, compression, or inflammation cause an increase in the spontaneous discharge of C and Aβ DRG neurons.50,63 Simultaneously, lesioned sensory neurons release NGF, which can bind to TrkA channels expressed on sensory neurons, resulting in hyperexcitation and exacerbating spontaneous firing of these neurons.64 Increased NGF concentrations induce the sprouting of sympathetic postganglionic fibers, especially in neurons with ectopic firing,48 which then interact with these injured neurons or fibers to form coupling structures in DRG or the skin. Simultaneously, the structure of sprouted sympathetic terminals is dilated and extracellularly releases ATP and norepinephrine (NE),65,66 which can bind with P2 receptors and adrenergic α2 receptors expressed on neurons,65 respectively, with the diffusion of satellite glia.18,66 Increased neuron activation leads to increased noxious peripheral inputs at the spinal and supraspinal levels,24,67 implying a role in sensory sensitization.68
Receptor Mechanisms Mediating Sympathetic-Sensory Coupling
Sprouted sympathetic terminals primarily act by activating adrenoceptors (ADRs) on the surface of sensory neurons or terminals. ADRs can be classified into two subtypes: ADRA (adrenoceptor alpha) and ADRB (adrenoceptor beta). Additionally, ADRA is subdivided into two subtypes, ADRA1 and ADRA2, whereas ADRB is subdivided into three subtypes, ADRB1, ADRB2, and ADRB3.61 Although prior research revealed the involvement of ADRA1 and ADRB2 in the sensory-sympathetic coupling in the DRG,21,23 additional evidence indicated that ADRA2, expressed on sensory neurons, is the major receptor involved in the sympathetic-sensory coupling relationship,18,19,51,69–71 ADRA2 activation lowered the concentration of PKC (adenylyl cyclase) via the intracellular Gi route, resulting in decreased intracellular ATP hydrolysis and increased extracellular ATP concentration. Extracellular ATP then binds to P2X receptors and activates sensory neurons, resulting in pain.72 In contrast, the facilitating role of ADRA2 in nociceptive pathways remains unclear and may be related to the molecule subtypes.
ADRA2 has three subtypes: ADRA2A, ADRA2B, and ADRA2C. The distributions and functional alterations of these subtypes in DRG under healthy and pathological settings have been documented. Physiologically, DRG neurons mostly express ADRA2C receptors (80%), followed by ADRA2A receptors (20%) and nearly no ADRA2B receptors.73 In neuropathic pain, ADRA2A receptor expression increases significantly (45%) whereas ADRA2C receptor expression decreases somewhat. However, the expression of the three receptor subtypes did not change significantly in carrageenan-induced inflammatory pain.73 Meanwhile, the silencing of ADRA2A receptors dramatically improved plantar hyperalgesia in a neuropathic pain model.71 These findings imply that ADRA2A receptors on the surface of sensory neurons may contribute to the functional interplay of sympathetic and sensory neurons.
Underlying Role of Sympathetic-Sensory Coupling in Acupoint Sensitization
As previously stated, sympathetic sprouting and sympathetic-sensory coupling contribute to local responsiveness and transmission effectiveness in acupuncture manipulation. Recent research in a rat model of chronic myocardial ischemia (MI) demonstrated that MI resulted in the sprouting of sympathetic postganglionic fibers in the skin of cardiac referred pain, as well as T1–T5 DRGs in the spinal segment proximal to the heart, where sympathetic-sensory coupling also formed 7 days after MI modeling.1 Notably, the cardiac referred region in acupuncture therapy includes multiple acupoints associated with heart illness, including PC6 (Neiguan). Given the role of the peripheral sensory fibers in acupoints in processing signals of acupuncture manipulation, we hypothesized that the interplay of sympathetic-sensory coupling contributes to the enhancement of acupoint therapeutic activity. Notably, this study demonstrated that sensory inputs from a cardiac-referred hypersensitive location (sensitized acupoint, PC6) could enhance sympathetic hyperexcitation, implying that referred pain or acupoint sensitization induced by visceral illness can directly drive the homeostatic process through sympathetic activity. Furthermore, electroacupuncture (EA) stimulation of PC6 produced a significant cardioprotective effect that was diminished after the subcutaneous injection of an ADRA2 pre- and post-synaptic blocker, indicating the involvement of ADRA2-mediated sympathetic-sensory coupling in the EA-induced cardioprotective effect. Another study detected sympathetic-sensory coupling in the L6 DRG of rats with colitis. Consecutive 7-days EA treatment on the ST36-ST37 acupoints greatly decreased sympathetic terminal sprouting and sympathetic-sensory coupling in the DRG.74 However, why sympathetic-sensory coupling either enhances the therapeutic efficacy of acupoints or is diminished by acupuncture treatment remains unknown. Additional research is necessary to elucidate the neurological and molecular mechanisms.
Conclusion
Cutting-edge research techniques enable us to systemically understand the nature of acupoint. As sympathetic-sensory coupling has been implicated in the mechanism of sensory modulation, this review provides a unique perspective on the neurological substrate of the acupoint effect in response to acupoint intervention. Although recent research has identified a correlation between sympathetic-sensory coupling and acupoints and demonstrated the contributions of ADRA2-mediated sympathetic-sympathetic coupling to the therapeutic effect of sensitized acupoints, the underlying neurobiological mechanisms in the peripheral and central nervous systems remain unresolved (Figure 1B and C). Researchers in acupuncture field should use new techniques, including in-vivo calcium imaging, optogenetic, and chemogenetic methods, to demonstrate the role of sympathetic-sensory coupling in sensitized acupoints and elucidate the acupoint’s instinct and neural basis.
Abbreviations
ADR, adrenoceptor; ADRA, adrenoceptor alpha; ADRB, adrenoceptor beta; EA, electroacupuncture; MI, myocardial ischemia; NE, norepinephrine; NGF, nerve growth factor; SNL, spinal nerve ligation.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81904309, 81973963), Scientific and technological innovation project of China Academy of Chinese Medical Sciences (No. CI2021A03402), the Fundamental Research Funds for the Central public welfare research institutes (No. ZZ15-YQ-049).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Cui X, Sun G, Cao H, et al. Referred somatic hyperalgesia mediates cardiac regulation by the activation of sympathetic nerves in a rat model of myocardial ischemia. Neurosci Bull. 2022;38(4):386–402. doi:10.1007/s12264-022-00841-w
2. Liu S, Wang Z, Su Y, et al. A neuroanatomical basis for electroacupuncture to drive the vagal-adrenal axis. Nature. 2021;598(7882):641–645. doi:10.1038/s41586-021-04001-4
3. Ulloa L. Electroacupuncture activates neurons to switch off inflammation. Nature. 2021;598(7882):573–574. doi:10.1038/d41586-021-02714-0
4. Cui X, Liu K, Gao X, Zhu B. Advancing the understanding of acupoint sensitization and plasticity through cutaneous C-nociceptors. Front Neurosci. 2022;16. doi:10.3389/fnins.2022.822436
5. Chen YY, Wang SY, Gao XY, Zhu B. Association between skin microecology and acupoint sensitization. Zhen Ci Yan Jiu. 2021;46(7):625–630. doi:10.13702/j.1000-0607.200945
6. Gong Y, Li N, Lv Z, et al. The neuro-immune microenvironment of acupoints-initiation of acupuncture effectiveness. J Leukoc Biol. 2020;108(1):189–198. doi:10.1002/jlb.3ab0420-361rr
7. Zhang M, Guo H, Ma Y, et al. Acupoint sensitization is associated with increased excitability and hyperpolarization-activated current (I(h)) in C- but not Aδ-type neurons. Neuroscience. 2019;404:499–509. doi:10.1016/j.neuroscience.2019.02.028
8. Yuan W, Yue J-X, Wang Q, et al. Role of peptidergic neurons in modulating acupoint sensitization caused by neck acute inflammatory pain in rats. World J Acupunct Moxibustion. 2023;33(1):44–50. doi:10.1016/j.wjam.2022.12.005
9. Zhang Z-Y, Wan H-Y, Jing X-H. Acupoint effects and neuro-immune modulation. World J Acupunct Moxibustion. 2023. doi:10.1016/j.wjam.2023.05.013
10. Fan Y, Kim D-H, Ryu Y, et al. Neuropeptides SP and CGRP underlie the electrical properties of acupoints. original research. Front Neurosci. 2018;2018(12):907. doi:10.3389/fnins.2018.00907
11. Li W, Xie XY, Tang YW, et al. Acupoint sensitization enhances inhibitory effect of electroacupuncture on jejunum mobility in rats. Zhen Ci Yan Jiu. 2021;46(1):27–32. doi:10.13702/j.1000-0607.200778
12. Y-c Y, Tang O-F, Wu Y-Z, et al. Exploration of the therapeutic effect and clinical outcomes of acupuncture at pain-sensitive points to treat chronic nonspecific low back pain: application of the acupoint sensitization theory. World J Acupunct Moxibustion. 2021;31(4):270–274. doi:10.1016/j.wjam.2021.08.002
13. Huang S, Li L, Liu J, et al. The preventive value of acupoint sensitization for patients with stable angina pectoris: a randomized, double-blind, positive-controlled, multicentre trial. Evid Based Complement Alternat Med. 2021;2021:7228033. doi:10.1155/2021/7228033
14. He W, Wang XY, Shi H, et al. Cutaneous neurogenic inflammation in the sensitized acupoints induced by gastric mucosal injury in rats. BMC Complement Altern Med. 2017;17(1):141. doi:10.1186/s12906-017-1580-z
15. Kim DH, Ryu Y, Hahm DH, et al. Acupuncture points can be identified as cutaneous neurogenic inflammatory spots. Sci Rep. 2017;7(1):15214. doi:10.1038/s41598-017-14359-z
16. Wu ML, Xu DS, Bai WZ, et al. Local cutaneous nerve terminal and mast cell responses to manual acupuncture in acupoint LI4 area of the rats. J Chem Neuroanat. 2015;68:14–21. doi:10.1016/j.jchemneu.2015.06.002
17. Chang S, Kwon OS, Bang SK, et al. Peripheral sensory nerve tissue but not connective tissue is involved in the action of acupuncture. Front Neurosci. 2019;13. doi:10.3389/fnins.2019.00110
18. Wu JR, Chen H, Zhang DX, et al. Local injection to sciatic nerve of DEX reduces pain behaviors, SGCs activation, NGF expression and sympathetic sprouting in CCI rats. Brain Res Bull. 2017;132:118–128. doi:10.1016/j.brainresbull.2017.04.016
19. Ogon I, Takebayashi T, Miyakawa T, et al. Attenuation of pain behaviour by local administration of alpha-2 adrenoceptor antagonists to dorsal root ganglia in a rat radiculopathy model. Eur J Pain. 2016;20(5):790–799. doi:10.1002/ejp.804
20. Xie W, Chen S, Strong JA, Li A-L, Lewkowich IP, Zhang J-M. Localized sympathectomy reduces mechanical hypersensitivity by restoring normal immune homeostasis in rat models of inflammatory pain. J Neurosci. 2016;36(33):8712–8725. doi:10.1523/JNEUROSCI.4118-15.2016
21. Zheng Q, Xie W, Lückemeyer DD, et al. Synchronized cluster firing, a distinct form of sensory neuron activation, drives spontaneous pain. Neuron. 2021;S0896–6273(21):00834–00835. doi:10.1016/j.neuron.2021.10.019
22. Raja SN. Role of the sympathetic nervous system in acute pain and inflammation. Ann Med. 1995;27(2):241–246. doi:10.3109/07853899509031966
23. Shen S, Tiwari N, Madar J, Mehta P, Qiao LY. Beta 2-adrenergic receptor mediates noradrenergic action to induce cyclic adenosine monophosphate response element-binding protein phosphorylation in satellite glial cells of dorsal root ganglia to regulate visceral hypersensitivity. Pain. 2022;163(1):180–192. doi:10.1097/j.pain.0000000000002330
24. Mclachlan EM, Jänig W, Devor M, Michaelis M. Peripheral nerve injury triggers noradrenergic sprouting within dorsal root ganglia. Nature. 1993;363(6429):543–546. doi:10.1038/363543a0
25. Chung K, Kim HJ, Na HS, Park MJ, Chung JM. Abnormalities of sympathetic innervation in the area of an injured peripheral nerve in a rat model of neuropathic pain. Neurosci Lett. 1993;162(1–2):85. doi:10.1016/0304-3940(93)90566-4
26. Head H. On disturbances of sensation with especial reference to the pain of visceral disease 1. Brain. 1893;16(1–2):1–133. doi:10.1093/brain/16.1-2.1
27. Head H. On disturbances of sensation with especial reference to the pain of visceral disease part II.—head and neck. Brain. 1894;17(3):339–480. doi:10.1093/brain/17.3.339
28. Head H. On disturbances of sensation with especial reference to the pain of visceral disease part III.—pain in diseases of the heart and lungs. Brain. 1896;19(2–3):153–276. doi:10.1093/brain/19.2-3.153
29. Beissner F, Henke C, Unschuld PU. Forgotten features of head zones and their relation to diagnostically relevant acupuncture points. Evid Based Complement Alternat Med. 2011;2011:240653. doi:10.1093/ecam/nen088
30. James Mackenzie E. Heart pain and sensory disorders associated with heart failure. Lancet. 1895;145(3723):16–22. doi:10.1016/S0140-6736(02)06365-1
31. Cui X, Zhang W, Sun JH, et al. Correlation between referred pain distribution and acupoint sensitization in patients with intestinal diseases. Zhongguo Zhen Jiu. 2019;39(11):1193–1198. doi:10.13703/j.0255-2930.2019.11.016
32. Shi J, Wang J, Wang Y, et al. Correlation between pain region and sensitized acupoints in patients with lung diseases. J Tradit Chin Med. 2020;35(12):6029–6032.
33. Birch S. Trigger point--acupuncture point correlations revisited. J Altern Complement Med. 2003;9(1):91–103. doi:10.1089/107555303321222973
34. Dorsher PT. Can classical acupuncture points and trigger points be compared in the treatment of pain disorders? Birch’s analysis revisited. J Altern Complement Med. 2008;14(4):353–359. doi:10.1089/acm.2007.0810
35. Melzack R, Stillwell DM, Fox EJ. Trigger points and acupuncture points for pain: correlations and implications. Pain. 1977;3(1):3–23. doi:10.1016/0304-3959(77)90032-X
36. Zhu B. On the acupoint and its specificity. Zhongguo Zhen Jiu. 2021;41(9):943–950. doi:10.13703/j.0255-2930.20210701-k0002
37. Rong PJ, Li S, Ben H, et al. Peripheral and spinal mechanisms of acupoint sensitization phenomenon. Evid Based Complement Alternat Med. 2013;2013:742195. doi:10.1155/2013/742195
38. Xu JF, Wu Q, Lin RZ, et al. Electrophysiological characteristics of sensitized acupoints after acute intestinal mucosal injury in rats. Zhen Ci Yan Jiu. 2015;40(3):180–185.
39. Porges SW. The polyvagal perspective. Biol Psychol. 2007;74(2):116. doi:10.1016/j.biopsycho.2006.06.009
40. Porges SW. The polyvagal theory: new insights into adaptive reactions of the autonomic nervous system. Cleve Clin J Med. 2009;76(Suppl_2):S86. doi:10.3949/ccjm.76.s2.17
41. Porges SW. The polyvagal theory: phylogenetic substrates of a social nervous system. Int J Psychophysiol. 2001;42(2):123. doi:10.1016/S0167-8760(01)00162-3
42. Jänig W, Häbler HJ. Specificity in the organization of the autonomic nervous system: a basis for precise neural regulation of homeostatic and protective body functions. Prog Brain Res. 2000;122(19):351–367.
43. Jainig W. The sympathetic nervous system in pain: physiology and pathophysiology. Pain Symp Nerv Syst. 1990;1990:17–89.
44. Kim SH, Chung JM. Sympathectomy alleviates mechanical allodynia in an experimental animal model for neuropathy in the rat. Neurosci Lett. 1991;134(1):131–134. doi:10.1016/0304-3940(91)90524-W
45. Loh L, Nathan PW. Painful peripheral states and sympathetic blocks. J Neurol Neurosurg Psychiatry. 1978;41(7):664. doi:10.1136/jnnp.41.7.664
46. Gil DW, Wang J, Gu C, Donello JE, Cabrera S, Al-Chaer ED. Role of sympathetic nervous system in rat model of chronic visceral pain. Neurogastroenterol Motil. 2016;28(3):423. doi:10.1111/nmo.12742
47. Cohen SP, Mao J. Neuropathic pain: mechanisms and their clinical implications. BMJ. 2014;348(feb05 6):f7656. doi:10.1136/bmj.f7656
48. Xie W, Strong JA, Mao J, Zhang JM. Highly localized interactions between sensory neurons and sprouting sympathetic fibers observed in a transgenic tyrosine hydroxylase reporter mouse. Mol Pain. 2011;7(1):53. doi:10.1186/1744-8069-7-53
49. Xie W, Strong JA, Zhang JM. Increased excitability and spontaneous activity of rat sensory neurons following in vitro stimulation of sympathetic fiber sprouts in the isolated dorsal root ganglion. Pain. 2010;151(2):447–459. doi:10.1016/j.pain.2010.08.006
50. Liu X, Eschenfelder S, Blenk KH, Jänig W, Häbler H. Spontaneous activity of axotomized afferent neurons after L5 spinal nerve injury in rats. Pain. 2000;84(2):309–318. doi:10.1016/S0304-3959(99)00211-0
51. Pertovaara A. Noradrenergic pain modulation. Prog Neurobiol. 2006;80(2):53–83. doi:10.1016/j.pneurobio.2006.08.001
52. Nascimento FP, Magnussen C, Yousefpour N, Ribeirodasilva A. Sympathetic fibre sprouting in the skin contributes to pain-related behaviour in spared nerve injury and cuff models of neuropathic pain. Mol Pain. 2015;11(1):59. doi:10.1186/s12990-015-0062-x
53. Jimenezandrade JM, Mantyh PW. Sensory and sympathetic nerve fibers undergo sprouting and neuroma formation in the painful arthritic joint of geriatric mice. Arthritis Res Ther. 2012;14(3):R101. doi:10.1186/ar3826
54. Grelik C, Bennett GJ, Ribeiro-da-silva A. Autonomic fibre sprouting and changes in nociceptive sensory innervation in the rat lower lip skin following chronic constriction injury. Eur J Neurosci. 2005;21(9):2475. doi:10.1111/j.1460-9568.2005.04089.x
55. Marfurt CF, Ellis LC, Jones MA. Sensory and sympathetic nerve sprouting in the rat cornea following neonatal administration of capsaicin. Somatosens Mot Res. 1993;10(4):377–398. doi:10.3109/08990229309028845
56. Yin J, Wang Y, Hu H, et al. P2X 7 receptor inhibition attenuated sympathetic nerve sprouting after myocardial infarction via the NLRP3/IL-1β pathway. J Cell Mol Med. 2017;21(4):2695–2710. doi:10.1111/jcmm.13185
57. Liu J, Li G, Peng H, et al. Sensory–sympathetic coupling in superior cervical ganglia after myocardial ischemic injury facilitates sympathoexcitatory action via P2X 7 receptor. Purinergic Signal. 2013;9(3):463–479. doi:10.1007/s11302-013-9367-2
58. Zhang JM, Li H, Munir MA. Decreasing sympathetic sprouting in pathologic sensory ganglia: a new mechanism for treating neuropathic pain using lidocaine. Pain. 2004;109(1):143–149. doi:10.1016/j.pain.2004.01.033
59. Ramer MS, Bisby MA. Adrenergic innervation of rat sensory ganglia following proximal or distal painful sciatic neuropathy: distinct mechanisms revealed by anti-NGF treatment. Eur J Neurosci. 1999;11(3):837–846. doi:10.1046/j.1460-9568.1999.00491.x
60. Ramer MS, Bisby MA. Differences in sympathetic innervation of mouse DRG following proximal or distal nerve lesions. Exp Neurol. 1998;152(2):197–207. doi:10.1006/exnr.1998.6855
61. Gloster A, Diamond J. Sympathetic nerves in adult rats regenerate normally and restore pilomotor function during an anti-NGF treatment that prevents their collateral sprouting. J Comp Neurol. 1992;326(3):363–374. doi:10.1002/cne.903260305
62. Dong C, Xie Z, Fan J, Xie Y. Ectopic discharges trigger sympathetic sprouting in rat dorsal root ganglia following peripheral nerve injury. Sci China C Life Sci. 2002;45(2):191–200. doi:10.1360/02yc9022
63. Strong JA, Strong JA. Recent evidence for activity-dependent initiation of sympathetic sprouting and neuropathic pain. Sheng Li Xue Bao. 2008;60(5):617–627.
64. Liem L, van Dongen E, Huygen FJ, Staats P, Kramer J. The dorsal root ganglion as a therapeutic target for chronic pain. Reg Anesth Pain Med. 2016;41(4):511–519. doi:10.1097/aap.0000000000000408
65. Chung K, Yoon YW, Chung JM. Sprouting sympathetic fibers form synaptic varicosities in the dorsal root ganglion of the rat with neuropathic injury. Brain Res. 1997;751(2):275–280. doi:10.1016/S0006-8993(96)01408-4
66. Shinder V, Govrinlippmann R, Cohen S, et al. Structural basis of sympathetic-sensory coupling in rat and human dorsal root ganglia following peripheral nerve injury. J Neurocytol. 1999;28(9):743–761. doi:10.1023/A:1007090105840
67. Devor M, Jänig W, Michaelis M. Modulation of activity in dorsal root ganglion neurons by sympathetic activation in nerve-injured rats. J Neurophysiol. 1994;71(1):38. doi:10.1152/jn.1994.71.1.38
68. Sawynok J, Xue JL. Adenosine in the spinal cord and periphery: release and regulation of pain. Prog Neurobiol. 2003;69(5):313–340. doi:10.1016/s0301-0082(03)00050-9
69. Ogon I, Takebayashi T, Miyakawa T, et al. Suppression of sympathetic nerve sprouting by local administration of an α-antagonist around the dorsal root ganglion in a lumbar radiculopathy model. Spine. 2018;43(6):E321–e326. doi:10.1097/brs.0000000000002333
70. Ogon I, Takebayashi T, Iwase T, et al. Sympathectomy and sympathetic blockade reduce pain behavior via alpha-2 adrenoceptor of the dorsal root ganglion neurons in a lumbar radiculopathy model. Spine. 2015;40(24):E1269–1275. doi:10.1097/brs.0000000000001050
71. Kingery WS, Guo TZ, Davies MF, Limbird L, Maze M. The alpha(2A) adrenoceptor and the sympathetic postganglionic neuron contribute to the development of neuropathic heat hyperalgesia in mice. Pain. 2000;85(3):345–358. doi:10.1016/S0304-3959(99)00286-9
72. Summers RJ, McMartin LR. Adrenoceptors and their second messenger systems. J Neurochem. 1993;60(1):10–23. doi:10.1111/j.1471-4159.1993.tb05817.x
73. Shi TS, Winzer-Serhan U, Leslie F, Hokfelt T. Distribution and regulation of alpha(2)-adrenoceptors in rat dorsal root ganglia. Pain. 2000;84(2–3):319–330. doi:10.1016/S0304-3959(99)00224-9
74. Wang YL, Su YS, He W, Jing XH. Electroacupuncture relieved visceral and referred hindpaw hypersensitivity in colitis rats by inhibiting tyrosine hydroxylase expression in the sixth lumbar dorsal root ganglia. Neuropeptides. 2019;77:101957. doi:10.1016/j.npep.2019.101957
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.