Back to Journals » Breast Cancer: Targets and Therapy » Volume 15
Survival Outcomes and Efficacy of Platinum in Early Breast Cancer Patients with Germline BRCA1 or BRCA2 Mutation: A Multicenter Retrospective Cohort Study
Authors Chen X, Qian X, Xiao M, Zhang P
Received 15 June 2023
Accepted for publication 16 August 2023
Published 4 September 2023 Volume 2023:15 Pages 671—682
DOI https://doi.org/10.2147/BCTT.S423330
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Pranela Rameshwar
Xi Chen, Xiaoyan Qian, Min Xiao, Pin Zhang
Department of Medical Oncology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, People’s Republic of China
Correspondence: Pin Zhang, Department of Medical Oncology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, People’s Republic of China, Tel +861087788200, Email [email protected]
Purpose: The study aimed to compare the survival outcomes and efficacy of platinum in early breast cancer patients with BRCA1 and BRCA2 mutations.
Methods: Patients diagnosed with stage I–III breast cancer and carrying germline pathogenic/likely pathogenic BRCA mutations in three medical institutions in China from April 2016 to January 2021 were retrospectively analyzed. Data on clinical and pathological characteristics, treatment information, pathogenic variants of BRCA, and survival outcomes were collected for all eligible patients.
Outcomes: One hundred and sixty-nine patients with BRCA mutations were enrolled, including BRCA1 mutation (53.3%, n = 90) and BRCA2 mutation (46.7%, n = 79). The median age was 39 years, and most patients (68.1%, n = 115) were stage I–II. Patients with BRCA1 mutations were characterized by histological grade III (55.6%) and higher Ki-67 index (Ki-67 ≥ 30%, 78.9%) compared with patients with BRCA2 mutations (27.8%, 58.2%). BRCA1 mutation patients accounted for a significantly higher proportion of triple negative breast cancer than BRCA2 mutation patients (71.1% vs 19.0%, P < 0.0001). A total of 142 (84.0%) patients received neo/adjuvant chemotherapy, including anthracycline and/or taxane-based regimens (55.6%) or platinum-based regimens (27.2%). Median follow-up was 33.2 months. Three-year DFS (disease-free survival) and DRFS (distant recurrence-free survival) had no significant differences between patients with BRCA1 and BRCA2 mutations (82.0% vs 85.4%, P = 0.35; 94.3% vs 94.6%, P = 0.39). The 3-year DFS rate in BRCA1 mutation cohort of patients received platinum regimen was significantly higher than patients received non-platinum regimen (96.0% vs 75.2%, P = 0.01). No differences between DFS and DRFS were observed in patients with BRCA2 mutation received platinum regimen and non-platinum regimen.
Conclusion: Similar survival outcomes were observed in early breast cancer patients with BRCA1 and BRCA2 mutation, though they had different biological characteristics. Patients with BRCA1 mutations are more benefit from platinum-regimen. The value of platinum-regimen for early breast cancer patients with BRCA1 and BRCA2 needs to be verified further.
Plain Language Summary: BRCA pathogenic mutation has been the principal genetic cause of breast cancer. Since the majority of studies have focused on the clinical manifestation and survival of patients with BRCA mutations and the wild type, few studies gave a concern about the survival outcome of breast cancer patients with BRCA1 and BRCA2 mutation. We aimed to compare the survival outcomes and efficacy of platinum between early breast cancer patients with BRCA1 and BRCA2. Our results showed that patients with BRCA1 and BRCA2 mutations had different biological characteristics, but similar survival outcomes in the early stages. Patients with BRCA1 mutations are more sensitive to platinum therapy in neoadjuvant settings.
Keywords: breast cancer, BRCA mutation, survival outcome, chemotherapy, platinum
Introduction
Breast cancer is one of the most common cancers and leading cause of cancer death in women worldwide.1 The prognosis for patients with breast cancer varies greatly and is highly dependent on the inherent tumor biology and the stage of malignant progression at diagnosis. Numerous studies have been developed to find potential biomarkers for breast cancer survival and progression.2,3 BRCA1 and BRCA2 are the two most important breast cancer susceptibility genes. A substantial proportion of hereditary breast cancers can be attributed to BRCA mutation.4 BRCA pathogenic mutations can trigger the loss of BRCA1 and BRCA2 protein function, causing homologous recombination deficiency and ultimately resulting in tumorigenesis.5 According to a previous study, pathogenic mutations in BRCA genes confer a high risk of breast cancer and contralateral breast cancer.6 The cumulative risk of breast cancer until the age of 70 is 37.9% in BRCA1 carriers and 36.5% in BRCA2 carriers in China, respectively.7 The 10-year cumulative risk of contralateral breast cancer was estimated to be 15.5% for BRCA1 carriers and 17.5% for BRCA2 carriers.8
Since BRCA mutation was at a high prevalence among breast cancer patients, numerous studies evaluated the spectrum and prevalence of BRCA in different races.9–11 As reported, the frequency of BRCA mutations in unselected breast cancer patients is approximately 5% in China,12,13 the ratio would be higher in selected patients.14–16 The number of reports on BRCA mutations in breast cancer has been increasing, and the majority of studies to date has focused on the clinical manifestation and survival of patients with BRCA mutations and the wild type.17–21
It has been reported that breast cancer patients with BRCA mutations have significant and distinct clinicopathological features, with a tendency for triple-negative in BRCA1 and hormone receptor-positive in BRCA2.22,23 As we all know, triple-negative subtype is a kind of breast cancer with poor prognosis which lacked endocrine therapy and targeted therapy.24 The luminal subtype is hormone sensitive and has a relatively good prognosis.25 Therefore, the prognosis of patients with BRCA2 should be better than that of BRCA1. However, few studies gave a concern on the survival outcomes of breast cancer patients with BRCA1 and BRCA2 mutations.
Theoretically, BRCA mutant cells are sensitive to DNA-damaging agents such as platinum agents.26 Carboplatin has been proven to be effective in metastatic TNBC with germline BRCA mutations.27 Several studies have demonstrated that patients with BRCA mutations achieved higher pCR rates from platinum neoadjuvant.28 There is a growing interest in the exploration of platinum-based regimen efficacy in early breast cancer patients with BRCA mutations.
We conducted this multi-center study in an attempt to compare the survival outcomes and efficacy of platinum between early breast cancer patients with BRCA1 and BRCA2 mutations in China.
Materials and Methods
Patients
Patients were collected from three medical institutions in China: Cancer Hospital, the Chinese Academy of Medical Sciences, Huanxing Cancer Hospital, and Sanhuan Cancer Hospital. They need to meet the following inclusion criteria: 1) females aged >18 years with pathologically diagnosed invasive breast cancer; 2) harboring germline pathogenic/likely pathogenic BRCA mutation; 3) early breast cancer (clinical or pathologic stage I–III); and 4) pathological, surgical, and treatment information, and follow-up data were available. Patients were excluded if any of the following conditions were present: 1) recurrent or metastatic breast cancer; 2) harboring benign/likely benign/variant of unknown significance BRCA mutation. 3) noninvasive breast cancer; 4) no survival follow-up information.
Clinical and pathological data, including age, family history of cancer, histopathology, tumor size, nodal status, breast cancer stage, and estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER-2) status, were extracted for this study.
BRCA Mutation Analysis
Genomic DNA was extracted from peripheral blood or saliva. Germline variants were analyzed using a multiplex amplicon-based library preparation system and targeted a panel covering the coding regions and consensus splice sites of BRCA for sequencing using an Illumina HiSeq 4000 Platform. The clinical significance (benign/likely benign/variant of unknown significance (VUS)/likely pathogenic/pathogenic) of each variant was annotated according to the ACMG/AMP guidelines. The mutation curation was also conducted by two experienced medical geneticists independently blind to the imaging interpretation.
Survival Definitions
DFS was defined as the time from surgery to the first appearance of one of the following invasive events: locoregional recurrence, distant metastasis, new contralateral or ipsilateral breast cancer, second primary malignancy or death from any cause. The DRFS was calculated as the time from surgery to the first occurrence of invasive breast cancer recurrence at a distant site. Overall survival data are still mature, with no death events in the cohort.
Statistical Analysis
Statistical analysis was conducted using SPSS version 26.0 for Windows (IBM SPSS Inc., Armonk, NY, USA). The chi-square test or Fisher’s exact test was used to compare categorical variables, while a t-test was performed to compare continuous variables.
Survival outcomes, including DFS and DRFS, were estimated using the Kaplan–Meier (K-M) method. Z-test was used to compare survival rates at fixed time points. Correlates of DFS and DRFS were explored with univariate and multivariate analyses via the factors with univariate P < 0.2 were included in the multivariate models. All tests were a two-sided test, and P <0.05 was considered statistically significant.
Results
From April 2016 to January 2021, 824 breast cancer patients who received BRCA testing from three centers were screened, and 169 patients with pathogenic or likely pathogenic BRCA variants were included: 90 with BRCA1 and 79 with BRCA2 (1 patient with both BRCA1 and BRCA2 mutations was classified into the BRCA1 group). The flowchart is shown in Figure S1.
Patients Characteristics
Clinicopathological characteristics of the patients are presented in Table 1. The median age at diagnosis was 39 years (range, 22–67). The majority of the patients were premenopausal (79.9%) and had a family cancer history (72.2%), especially breast cancer and ovarian cancer history. Half of the patients (n = 85, 50.3%) were lymph node-negative. Tumor size of T1 was reported in 78 (46.2%) of all the patients. Only five patients (3%) were HER-2 positive.
 |
Table 1 Baseline Characteristics of Patients |
Patients with BRCA1 mutations tended to be younger than those with BRCA2 mutations (median age, 38 years vs 40 years, P = 0.014). Compared to BRCA2 mutations, patients with BRCA1 mutations had a higher histological grade and ki-67 index (P < 0.0001). The BRCA1 mutation group had a significantly higher proportion of TNBC subtypes than BRCA2 (71.1% vs 19.0%, P < 0.0001). And there were more luminal subtype patients in the BRCA2 mutation group (73.4% vs 23.3%, P < 0.0001). No significant differences were observed in tumor size, lymph node status, tumor stage, histological type, or breast surgery between the two groups.
Treatment
A total of 142 (84.0%) patients received neoadjuvant (23.2%, n = 33) or adjuvant chemotherapy (76.8%, n = 109). The remaining 27 patients did not receive chemotherapy and 12 patients of them received endocrine therapy only. Sixty-eight (47.9%) patients were treated with standard anthracycline-and-taxane-based regimen (AC-T or AT). Seventeen (12.0%) patients received taxane-based or anthracycline-based regimens(TC or AC) (Figure 1). Of note, there were 46 patients (27.2%) treated with platinum-containing regimens. Twenty-two patients of them were treated with taxane plus carboplatin in an adjuvant setting as part of a randomized, Phase II trial. There was no obvious difference in the regimen of chemotherapy between the BRCA1 and BRCA2 groups, except that the proportion of patients receiving platinum-based regimens was higher in BRCA1 group than BRCA2 group (31.1% vs 22.8%). Most of patients with BRCA2 mutations (70.9%, n = 56) received endocrine therapy. All HER-2 positive patients in this cohort were treated with trastuzumab.
Mutation Sites of BRCA
In total, 127 mutation sites were detected in our study, the majority of mutation type was frameshift mutations (58.6%) and nonsense mutations (23.7%), leading to the truncation of the corresponding proteins. About 19.7% mutation sites of the total have not been reported in the Clinvar database. Exons 11 and 24 were frequently mutated in BRCA1, accounting for 43% and 19% of the BRCA1, respectively, and the frequently mutated exons in BRCA2 are exons 10 and 11, accounting for 15% and 44%. All mutation sites were visualized using the Mutation Mapper software in Supplemental Material (Table S1 and Figure S2). It is worth noting that BRCA1c.5470_5477del mutations were detected in 11 patients, which was significantly higher than others, and this mutation is currently only reported in Asian populations.
Survival and Prognostic Factors
At data cutoff 28 July 2021, the median follow-up time was 33.2 months (interquartile range, 20.8–84.9 months). A total of 56 events of DFS events occurred, and 46.4% (26/56) of the events were contralateral invasive breast cancer, followed by locoregional recurrence (21.4%, 12/56) and distant metastasis (17.8%, 10/56) (Table S2). No apparent difference in the type of DFS events was observed between the BRCA1 and BRCA2 groups. The 3-year DFS and DRFS rates were 83.3% (95% confidence interval [CI], 76.4–90.2%), 94.4% (95% CI, 90.3–98.5%), respectively.
There were no significant differences in survival outcomes between patients with BRCA1 and BRCA2 mutations. The 3-year estimated DFS rate was 82.0% (95% CI, 72.2–91.8%) in patients with BRCA1 mutations compared to 85.4% (95% CI, 76.4–94.4%) in patients with BRCA2 mutations (Z= 0.50, P = 0.35). A similar result was observed for the 3-year DRFS rate (94.3% [95% CI, 88.8–99.8%] vs 94.6% [95% CI, 88.5–100.0%]; Z= 0.01, P = 0.39) (Figure 2).
 |
Figure 2 Kaplan–Meier curves in patients with BRCA mutation according to BRCA1 or BRCA2 group for disease-free survival (A) and distant recurrence-free survival (B). |
Univariate and multivariate analyses of DFS were performed separately for the BRCA1 and BRCA2 patients (Table 2 and 3). In the BRCA1 group, receipt of adjuvant chemotherapy was associated with better DFS (HR, 0.19; 95% CI, 0.045–0.793; P = 0.023) after adjustment. Meanwhile, patients with BRCA2 mutations younger than 35 years old had a higher risk of recurrence compared with patients aged 35–50 years old (HR, 0.30; 95% CI, 0.002–0.393; P = 0.007).
 |
Table 2 Univariate Analyses and Multivariate Analysis of Disease-Free Survival in BRCA1 Group |
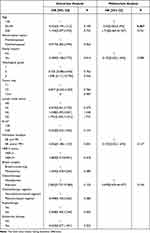 |
Table 3 Univariate Analyses and Multivariate Analysis of Disease-Free Survival in BRCA2 Group |
Platinum Treatment and Survival
We further explored the benefits of a platinum regimen in the BRCA1 and BRCA2 groups. Figure 3 shows that the 3-year DFS rate of patients received platinum regimen was significantly better than that of patients received non-platinum regimen in the BRCA1 mutation group [96.0% (95% CI, 88.0–100.0%) vs 75.2% (95% CI, 61.7–88.7%), Z = 2.63, P = 0.01]. The 3-year DFS rate was 90.7% (95% CI, 80.7–100%) among patients received non-platinum regimen versus 100.0% among patients received platinum regimen in BRCA2 mutation group (Z = 1.82, P = 0.076). There were no significant differences in the DRFS outcomes among patients received platinum regimen and non-platinum regimen in BRCA1 and BRCA2 groups. The 3-year DRFS rates of patients who received platinum regimen and non-platinum regimens were estimated to be 96.0% (88.4%-100.0) and 93.3% (86.1–100%) of BRCA1 mutation group, 100% and 93.7% (85.3%-100) of BRCA2 mutation group (Figure 3).
Discussion
Our study provided the clinical and pathological characteristics as well as survival outcomes of early breast cancer patients with BRCA mutations. It suggested that patients with BRCA1 mutations have more invasive biological characteristics than BRCA2, but there were no significant differences in survival outcomes between them. Patients with BRCA1 mutations tended to benefit more from the platinum regimen than those with BRCA2 mutations.
As reported previously, patients with BRCA1 mutations tend to be hormone receptor-negative, whereas patients with BRCA2 mutations tend to be hormone receptor-positive.22 In our cohort, the proportion of TNBC in BRCA1 mutation breast cancer was as high as 71%, which is consistent with the previous reports in Chinese.29,30 Luminal breast cancer is the majority subtype of BRCA2 breast cancer, accounting for 73.4%. Similar findings have been found in other studies.18,31,32 Compared to patients with BRCA2 mutations, patients with BRCA1 mutations also have other features, like higher histological grade Ki-67 index and negative expression of HER-2. Generally, the biological characteristics of patients with BRCA1 mutations are more invasive.
Our study provided survival outcomes for early breast cancer patients with BRCA mutations, with 3-year DFS and DRFS rates of 83.3% and 94.4%, respectively. The OlympiA Phase III trial33 enrolled early breast cancer patients with BRCA mutations and high-risk clinicopathological factors, who had received local treatment and neoadjuvant or adjuvant chemotherapy. Patients were randomly to receive 1 year of olaparib or placebo. It demonstrated that olaparib was superior to placebo with an improvement in 3-year DFS and DDFS (DFS 85.9% vs 77.1%, DDFS 87.5% vs 80.4%). None of the patients in our cohort received olaparib. Compared to the control group of OlympiA trial, the survival rates in our study were numerically higher, which could be explained by the higher risk of recurrence of patients enrolled in OlympiA study and patients of our cohort were in more earlier stages (T1 46.2%, N0 50.3%).
There is a scarcity of study to investigate the survival outcome between BRCA1 and BRCA2. In our cohort, there was no significant difference in DFS and DRFS between the two groups, which was contrast to our expectations. Lambertini et al also found that the type of BRCA gene does not appear to have prognostic value, with no observed differences in DRFI and OS between BRCA1 and BRCA2.31 As for the reason, there has been reported that BRCA-mutated patients with triple-negative breast cancer may have a trend for a survival advantage,34 it may be one of the hypotheses. On the other hand, Vocka et al reported that patients with hormone receptor-positive breast cancer who carry BRCA mutation showed a higher risk of distant relapses and worse prognosis.35 Thus, more studies are needed to explore the mechanisms and prognostic differences in patients with BRCA1 and BRCA2.
Theoretically, BRCA mutant cells are sensitive to DNA-damaging agents such as platinum agents.26 Carboplatin has been proven to be effective in metastatic TNBC with germline BRCA mutations.27 Up to now, the chemotherapy for early breast cancer patients with BRCA mutations has remained a matter of debate since the effect of platinum agents in neoadjuvant and adjuvant settings remains unclear. Several studies have demonstrated that patients with BRCA mutation achieved higher pCR rates in platinum neoadjuvant.28,36 Few studies have explored the therapeutic sensitivity differences between BRCA1 and BRCA2 of the treatment of platinum regimens in early breast cancer. Our results indicate that early breast cancer patients with BRCA1 mutations received platinum-containing regimens exhibited a better DFS than non-platinum regimens with statistical significance while patients with BRCA2 mutations are not. Therefore, patients with BRCA1 mutations may be more sensitive to platinum-based therapies. It may be largely attributable to the fact that most patients with BRCA1 mutations are triple negative. The results should be treated with caution because the number of patients with BRCA2 mutations receiving platinum-containing regimens is small. The value of platinum agents for early breast cancer patients with BRCA1 and BRCA2 mutations in adjuvant or neoadjuvant settings needs to be verified by randomized clinical trials.
The results of our study should be considered with its limitations. First, it was a retrospective study with a limited sample size and the comparison of survival endpoints may be underpowered statistically. Second, all study participants were from medical center in China. Therefore, the generalizability of the study findings to other populations is limited. When interpreting the results of our study, we should be conscious of the heterogeneity in patient characteristics and studies that include a diverse population are still needed. As the median follow-up time was 3 years, patients’ survival outcome needed further follow-up.
Conclusions
In conclusion, patients with BRCA1 mutations showed more invasive biological characteristics than those with BRCA2 mutations; however, there was no significant difference in survival outcomes between them. Patients with BRCA1 mutations are more benefit from platinum-regimens. It is necessary to further explore the influence of the biological behavior of BRCA1 and BRCA2 mutations in breast cancer in order to provide precise clinical management and treatment of BRCA1 and BRCA2 mutations in breast cancer.
Institutional Review Board Statement
The retrospective study was reviewed and approved by the Institutional Review Board of the National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences, and Peking Union Medical College (23/185-3927). All procedures were carried out in accordance with the Declaration of Helsinki.
Data Sharing Statement
The data presented in this study are available upon request from the corresponding author.
Informed Consent Statement
Informed consent has been obtained from the study participants prior to study commencement.
Acknowledgments
For their support and research assistance, I would like to thank the following professors who have contributed substantially to the completion of this study:
Prof. Qing Li, Prof. Qiao Li, Prof. Jiayu Wang, Prof. Ying Fan, Prof. Yang Luo, Prof. Peng Yuan, Prof. Fei Ma, Prof. Binghe Xu. They made a significant contribution to the work reported. The authors wish to thank all study participants who participated in this work.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research received no external funding.
Disclosure
The authors declare no potential conflicts of interest.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Zhou L, Rueda M, Alkhateeb A. Classification of breast cancer Nottingham prognostic index using high-dimensional embedding and residual neural network. Cancers. 2022;14(4):934. doi:10.3390/cancers14040934
3. Mercatelli D, Formaggio F, Caprini M, et al. Detection of subtype-specific breast cancer surface protein biomarkers via a novel transcriptomics approach. Biosci Rep. 2021;41(12). doi:10.1042/BSR20212218
4. Nielsen FC, van Overeem HT, Sorensen CS. Hereditary breast and ovarian cancer: new genes in confined pathways. Nat Rev Cancer. 2016;16(9):599–612. doi:10.1038/nrc.2016.72
5. Lord CJ, Ashworth A. BRCAness revisited. Nat Rev Cancer. 2016;16(2):110–120. doi:10.1038/nrc.2015.21
6. Graeser MK, Engel C, Rhiem K, et al. Contralateral breast cancer risk in BRCA1 and BRCA2 mutation carriers. J Clin Oncol. 2009;27(35):5887–5892. doi:10.1200/JCO.2008.19.9430
7. Yao L, Sun J, Zhang J, et al. Breast cancer risk in Chinese women with BRCA1 or BRCA2 mutations. Breast Cancer Res Treat. 2016;156(3):441–445. doi:10.1007/s10549-016-3766-3
8. Su L, Xu Y, Ouyang T, et al. Contralateral breast cancer risk in BRCA1 and BRCA2 mutation carriers in a large cohort of unselected Chinese breast cancer patients. Int J Cancer. 2020;146(12):3335–3342. doi:10.1002/ijc.32918
9. Metcalfe KA, Poll A, Royer R, et al. Screening for founder mutations in BRCA1 and BRCA2 in unselected Jewish women. J Clin Oncol. 2010;28(3):387–391. doi:10.1200/JCO.2009.25.0712
10. Foglietta J, Ludovini V, Bianconi F, et al. Prevalence and spectrum of BRCA germline variants in central Italian high risk or familial breast/ovarian cancer patients: a monocentric study. Genes. 2020;11(8):925. doi:10.3390/genes11080925
11. Subramaniyan V, Fuloria S, Gupta G, et al. A review on epidermal growth factor receptor’s role in breast and non-small cell lung cancer. Chem Biol Interact. 2022;351:109735. doi:10.1016/j.cbi.2021.109735
12. Sun J, Meng H, Yao L, et al. Germline mutations in cancer susceptibility genes in a large series of unselected breast cancer patients. Clin Cancer Res. 2017;23(20):6113–6119. doi:10.1158/1078-0432.CCR-16-3227
13. Gao X, Nan X, Liu Y, et al. Comprehensive profiling of BRCA1 and BRCA2 variants in breast and ovarian cancer in Chinese patients. Hum Mutat. 2020;41(3):696–708. doi:10.1002/humu.23965
14. Lang GT, Shi JX, Hu X, et al. The spectrum of BRCA mutations and characteristics of BRCA-associated breast cancers in China: screening of 2991 patients and 1043 controls by next-generation sequencing. Int J Cancer. 2017;141(1):129–142. doi:10.1002/ijc.30692
15. Deng M, Chen HH, Zhu X, et al. Prevalence and clinical outcomes of germline mutations in BRCA1/2 and PALB2 genes in 2769 unselected breast cancer patients in China. Int J Cancer. 2019;145(6):1517–1528. doi:10.1002/ijc.32184
16. Liu Y, Wang H, Wang X, et al. Prevalence and reclassification of BRCA1 and BRCA2 variants in a large, unselected Chinese Han breast cancer cohort. J Hematol Oncol. 2021;14(1):18. doi:10.1186/s13045-020-01010-0
17. Atchley DP, Albarracin CT, Lopez A, et al. Clinical and pathologic characteristics of patients with BRCA-positive and BRCA-negative breast cancer. J Clin Oncol. 2008;26(26):4282–4288. doi:10.1200/JCO.2008.16.6231
18. Incorvaia L, Fanale D, Bono M, et al. BRCA1/2 pathogenic variants in triple-negative versus luminal-like breast cancers: genotype–phenotype correlation in a cohort of 531 patients. Ther Adv Med Oncol. 2020;12:386359036. doi:10.1177/1758835920975326
19. Copson ER, Maishman TC, Tapper WJ, et al. Germline BRCA mutation and outcome in young-onset breast cancer (POSH): a prospective cohort study. Lancet Oncol. 2018;19(2):169–180. doi:10.1016/S1470-2045(17)30891-4
20. Liu M, Xie F, Liu M, et al. Association between BRCA mutational status and survival in patients with breast cancer: a systematic review and meta-analysis. Breast Cancer Res Treat. 2021;186(3):591–605. doi:10.1007/s10549-021-06104-y
21. Song Y, Barry WT, Seah DS, et al. Patterns of recurrence and metastasis in BRCA1/BRCA2 -associated breast cancers. Cancer. 2020;126(2):271–280. doi:10.1002/cncr.32540
22. Guzman-Arocho YD, Rosenberg SM, Garber JE, et al. Clinicopathological features and BRCA1 and BRCA2 mutation status in a prospective cohort of young women with breast cancer. Br J Cancer. 2022;126(2):302–309. doi:10.1038/s41416-021-01597-2
23. Schmidt MK, van den Broek AJ, Tollenaar RA, et al. Breast cancer survival of BRCA1/BRCA2 mutation carriers in a hospital-based cohort of young women. J Natl Cancer Inst. 2017;109(8). doi:10.1093/jnci/djw329
24. Bianchini G, Balko JM, Mayer IA, et al. Triple-negative breast cancer: challenges and opportunities of a heterogeneous disease. Nat Rev Clin Oncol. 2016;13(11):674–690.
25. Sebastian W, Forchette L, Donoughe K, et al. Genetics, treatment, and new technologies of hormone receptor-positive breast cancer. Cancers. 2023;15(4). doi:10.3390/cancers15041303
26. Torrisi R, Zuradelli M, Agostinetto E, et al. Platinum salts in the treatment of BRCA-associated breast cancer: a true targeted chemotherapy? Crit Rev Oncol Hematol. 2019;135:66–75. doi:10.1016/j.critrevonc.2019.01.016
27. Tutt A, Tovey H, Cheang M, et al. Carboplatin in BRCA1/2-mutated and triple-negative breast cancer BRCAness subgroups: the TNT Trial. Nat Med. 2018;24(5):628–637. doi:10.1038/s41591-018-0009-7
28. Byrski T, Huzarski T, Dent R, et al. Pathologic complete response to neoadjuvant cisplatin in BRCA1-positive breast cancer patients. Breast Cancer Res Treat. 2014;147(2):401–405. doi:10.1007/s10549-014-3100-x
29. Zhang J, Sun J, Chen J, et al. Comprehensive analysis of BRCA1 and BRCA2 germline mutations in a large cohort of 5931 Chinese women with breast cancer. Breast Cancer Res Treat. 2016;158(3):455–462. doi:10.1007/s10549-016-3902-0
30. Zhang J, Pei R, Pang Z, et al. Prevalence and characterization of BRCA1 and BRCA2 germline mutations in Chinese women with familial breast cancer. Breast Cancer Res Treat. 2012;132(2):421–428. doi:10.1007/s10549-011-1596-x
31. Lambertini M, Ceppi M, Hamy AS, et al. Clinical behavior and outcomes of breast cancer in young women with germline BRCA pathogenic variants. NPJ Breast Cancer. 2021;7(1):16. doi:10.1038/s41523-021-00224-w
32. Yap KM, Sekar M, Fuloria S, et al. Drug delivery of natural products through nanocarriers for effective breast cancer therapy: a comprehensive review of literature. Int J Nanomedicine. 2021;16:7891–7941. doi:10.2147/IJN.S328135
33. Hoshi A, Bando H, Sekine I. Adjuvant olaparib in BRCA-mutated breast cancer. N Engl J Med. 2021;385(15):1439–1440. doi:10.1056/NEJMc2112373
34. Ye F, He M, Huang L, et al. Insights into the impacts of BRCA mutations on clinicopathology and management of early-onset triple-negative breast cancer. Front Oncol. 2020;10:574813. doi:10.3389/fonc.2020.574813
35. Vocka M, Zimovjanova M, Bielcikova Z, et al. Estrogen receptor status oppositely modifies breast cancer prognosis in BRCA1/BRCA2 mutation carriers versus non-carriers. Cancers. 2019;11(6):738. doi:10.3390/cancers11060738
36. Pavese F, Capoluongo ED, Muratore M, et al. BRCA mutation status in triple-negative breast cancer patients treated with neoadjuvant chemotherapy: a pivotal role for treatment decision-making. Cancers. 2022;14(19):4571. doi:10.3390/cancers14194571
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


