Back to Journals » Biologics: Targets and Therapy » Volume 16
Successful Treatment of an Adult with Atopic Dermatitis and Lamellar Ichthyosis Using Dupilumab
Authors Binkhonain FK , Aldokhayel S, BinJadeed H , Madani A
Received 2 March 2022
Accepted for publication 4 May 2022
Published 23 June 2022 Volume 2022:16 Pages 85—88
DOI https://doi.org/10.2147/BTT.S362391
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Shein-Chung Chow
Faisal K Binkhonain, Sara Aldokhayel, Hessah BinJadeed, Abdulaziz Madani
Dermatology Department, College of Medicine, King Saud University, Riyadh, Saudi Arabia
Correspondence: Abdulaziz Madani, Dermatology Department, King Saud University, Riyadh, 7805, Saudi Arabia, P.O. Box: 11472, Tel +966505259001, Email [email protected]
Abstract: Lamellar ichthyosis (LI) is a rare autosomal cornification disorder, with most cases due to a mutation in the transglutaminase-1 (TGM1) gene on chromosome 14. Patients with LI usually present with a collodion membrane and mild erythroderma at birth, with the collodion membranes shedding within the first weeks of life and being replaced by a generalized scale. Typically, LI is managed with oral retinoids, emollients, and keratolytic agents, eg, lactic acid. We report an LI case associated with atopic dermatitis and asthma that showed a marked improvement with dupilumab treatment. This finding is highly significant as it may represent a breakthrough in the treatment of LI, thus more research is needed to investigate the potential benefits of dupilumab for the treatment of ichthyosis, such as the effects observed in our patient.
Keywords: dupilumab, biologics, lamellar ichthyosis
Introduction
Lamellar ichthyosis (LI) is a rare autosomal recessive cornification disorder with a genetically variable pattern of inheritance. A mutation in the transglutaminase-1 (TGM1) gene on chromosome 14q11 has been observed in 50% of LI patients, with only 5% of LI patients having a mutation in the CYP4F22 gene located on chromosome 19p13.12. Other gene defects linked with LI are CERS3, PNPLA1, and ABCA12. Clinically, patients with LI have an encased collodion membrane and underlying erythroderma at birth. These collodion membranes are typically shed within the first months of life and transformed into generalized large scales. Other signs and symptoms of this disease include eclabium, ectropion, and scarring alopecia, mainly on the scalp periphery due to the tautness of the facial skin. In addition, the accumulation of scales over the sweat ducts can lead to obstruction causing heat intolerance.1 Recently, several associated comorbidities have also been reported including atopic dermatitis, rickets, chronic irreversible pulpitis, pseudoainhum of the toes, and nuclear cataract.2–5 The use of oral retinoids, emollients, and keratolytic agents eg, lactic acid are the main treatment methods for LI.6 This article reports the successful treatment of an adult with LI and atopic dermatitis using Dupilumab.
Case Presentation
A 22-year-old male presented to our dermatology department three years ago complaining of severe pruritus, dryness, and scaling since birth. The skin lesions started to appear on the extensor surface of the lower and upper limbs, slowly progressing to involve the whole body including the trunk, scalp, forehead, and cheeks. His condition was associated with atopic dermatitis and asthma. Furthermore, the patient had a history of decreased vision and bilateral macular atrophy since childhood, and he was subsequently diagnosed with Stargardt's syndrome. He also reported a history and family history of a left corneal ulcer due to severe dryness. An uncle on his father’s side had LI since birth, and all of his siblings have atopic dermatitis not associated with LI. A skin examination revealed diffuse thick brown, plate-shaped scales throughout the body (Figure 1), with a large hyperpigmented lichenified plaque over the antecubital fossa (Figure 2).
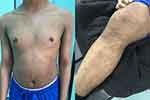 |
Figure 1 Diffuse thick brown plate-shaped scales on the extensor surface of the right lower limb, bilateral upper limbs, and anterior trunk. |
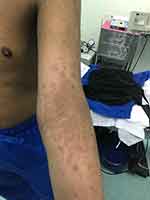 |
Figure 2 Lichenified hyperpigmented plaque over the antecubital fossa. |
The diagnosis of LI was made clinically and confirmed by histopathological features and whole-exome sequencing which identified a mutation in the CYP4F22 gene. The patient was initially started on 10 mg acitretin for three months to treat LI, which was stopped due to the worsening atopic dermatitis. He was prescribed 10 mg methotrexate for four months to treat atopic dermatitis which resulted in minimal improvement and was stopped due to severe gastrointestinal upset. Subsequently, the patient received 300 mg dupilumab twice a month for the worsening atopic dermatitis, with marked clinical improvements in the LI, atopic dermatitis, and asthma three months later.
Discussion
LI is a chronic disease that is usually diagnosed at birth and continues for a long time. Newborns are often covered by a collodion membrane that peels off during the first days of life. Once the membrane has shed, it is replaced by a widespread scaling with varying cutaneous redness. The classic form of LI is marked by dusky colored plate-like scales that form a mosaic or bark-like pattern with no significant erythroderma. In the advanced form of the disease, severe tautness of the facial skin can result in other clinical findings such as ectropion, eclabium, scarring alopecia affecting the scalp and the eyebrows, and palmoplantar hyperkeratosis. Furthermore, the accumulation of scales on the sweat ducts and the external ear can lead to heat intolerance and hearing defects, respectively.7 In addition, the disease might be associated with endocrine, immunological, and central nervous system diseases.8
LI is autosomal recessive with an estimated prevalence of 1 in 100,0009 and is generally a very heterogeneous disorder. Several gene mutations have been linked with LI, including TGM1, CYP4F22, CERS3, PNPLA1, and ABCA12. TGM1 gene defects compromise most LI cases, whereas the CYP4F22 gene defects represent only 2% to 6% of LI cases. TGM1 gene encodes one of the transglutaminase enzymes located in the epidermis that has a major role in the development of the keratinized envelope, thus, the cornified envelope is missing in a patient with the TGM mutation. CYP4F22 is found on chromosome 19p13.12 and is a member of the heme-thiolate cytochrome P450 subfamily 4 (CYP4) and is essential in lipid metabolism. CYP4F22 encodes ω-hydroxyls in the epidermis which are important in the formation of acyl ceramides (acylCer) vital for normal skin barrier function. Furthermore, other genes associated with LI include ALOX12B, ALOXE3, NIPAL4, CYP4F22, and ABCA12.10
In our case, the patient developed LI with atopic dermatitis simultaneously at birth, later developing Stargardt's disease and asthma. Several types of ichthyoses can be diagnosed clinically but the clinical diagnosis may be challenging in some patients due to the various heterogeneities. Most forms of ichthyosis have no evident histopathological features but the presence of a mutation in TGM1 can confirm the diagnosis. Delaying skin biopsy until clear phenotypes develop is preferred.7,11
In general, ichthyosis is treated with topical agents and oral medications, consisting of hydration, keratolytics, and modulators of keratinocyte differentiation. Hydration is achieved with creams, ointment, and a low concentration of salts, urea, or glycerol. Other vital topical agents for desquamation include keratolytic agents such as alpha-hydroxy acids (lactic acid, glycolic acid), salicylic acid, N-acetylcysteine, propylene glycol, and high-dose urea. Furthermore, retinoids (tretinoin, adapalene, tazarotene) and calcipotriol are effective in controlling epidermal proliferation. Regarding oral medications, oral retinoids are the cornerstone treatment for severe systemic LI. In such cases, the keratolytic effects of oral retinoids not only permit shedding but also prevent further hyperproliferation.12 In our patient, although acitretin provided a good therapeutic effect for LI, it was discontinued due to worsening atopic dermatitis. Dupilumab was prescribed for three months to treat the worsening atopic dermatitis and markedly improved the LI (Figure 3), atopic dermatitis, and asthma.
 |
Figure 3 Marked improvement of both lamellar ichthyosis and atopic dermatitis after three months of dupilumab treatment. |
Dupilumab is a monoclonal antibody that inhibits both interleukin-4 (IL-4) and IL-13 signaling. It can be potentially used for the management of a wide spectrum of dermatological, respiratory, and gastrointestinal disorders, for example, prurigo nodularis, nummular eczema, allergic contact dermatitis, chronic hand eczema, spontaneous chronic urticaria, bullous pemphigoid, alopecia areata, Netherton syndrome, allergic bronchopulmonary aspergillosis, chronic eosinophilic pneumonia, allergic rhinitis, eosinophilic gastrointestinal disorders, particularly eosinophilic esophagitis, and food allergies.13 Dupilumab is useful for the treatment of atopic dermatitis mainly due to its role in helper (Th)2-mediated immunity. Furthermore, it also decreased atopic dermatitis-induced molecular cutaneous changes, which results in a marked improvement in atopic dermatitis clinical presentation.
Conclusion
Three-month treatment with dupilumab improved a case of lamellar ichthyosis associated with atopic dermatitis and asthma.
Abbreviations
LI, Lamellar Ichthyosis; TGM1, Transglutaminase-1; acylCer, Acyl ceramides.
Informed Consent for Publication
Informed consent for publication was obtained from the study participants prior to study commencement. Institutional approval was not required to publish the case details.
Acknowledgements
We also show our gratitude to Dr. Fahad Alsaif, Professor of Dermatology at King Saud University for identifying the case and providing appropriate care. It was his recommendation to start treatment with dupilumab, which is the cornerstone of this case report. His expertise and guidance are highly appreciated.
Funding
This article has no funding source.
Disclosure
The authors have no conflicts of interest to declare in this work.
References
1. Youssefian L, Vahidnezhad H, Saeidian AH, et al. Autosomal recessive congenital ichthyosis: genomic landscape and phenotypic spectrum in a cohort of 125 consanguineous families. Hum Mutat. 2019;40(3):288–298. doi:10.1002/humu.23695
2. Schmuth M, Blunder S, Dubrac S, Gruber R, Moosbrugger-Martinz V. Epidermal barrier in hereditary ichthyoses, atopic dermatitis, and psoriasis. J der Deutschen Dermatologischen Gesellschaft. 2015;13:1119–1123. doi:10.1111/ddg.12827
3. Ali R, Aman S, Nadeem M. Lamellar ichthyosis with rickets. Pak J Med Sci. 2013;29(2):660–662. doi:10.12669/pjms.292.3298
4. Ena P, Pinna A. Lamellar ichthyosis associated with pseudoainhum of the toes and eye changes. Clin Exp Dermatol. 2003;28(5):493–495. doi:10.1046/j.1365-2230.2003.01335
5. Pranitha V. Lamellar ichthyosis: a case Report. J Clin Diagn Res. 2014;8(11):ZD01–ZD2. doi:10.7860/JCDR/2014/9201.5108
6. Gånemo A, Virtanen M, Vahlquist A. Improved topical treatment of lamellar ichthyosis: a double-blind study of four different cream formulations. Br J Dermatol. 1999;141(6):1027–1032. doi:10.1046/j.1365-2133.1999.03200.x
7. Gulasi S. Congenital ichthyosis: a case treated successfully with Acitretin. Iran J Pediatr. 2016;26(5):e2442. doi:10.5812/ijp.2442
8. Osório F, Leão M, Azevedo F, Magina S. Lamellar ichthyosis due to ALOX12B mutation. Actas Dermosifiliogr. 2013;104(5):443–444. doi:10.1016/j.ad.2012.07.011
9. Vahlquist A, Gånemo A, Virtanen M. Congenital ichthyosis: an overview of current and emerging therapies. Acta Derm Venereol. 2008;88(1):4–14. doi:10.2340/00015555-0415
10. Esperón-Moldes U, Ginarte-Val M, Rodríguez-Pazos L, et al. Novel CYP4F22 mutations associated with autosomal recessive congenital ichthyosis (ARCI). Study of the CYP4F22 c.1303C>T founder mutation. PLoS One. 2020;15(2):e0229025. doi:10.1371/journal.pone.0229025
11. DiGiovanna JJ, Robinson-Bostom L. Ichthyosis: etiology, diagnosis, and management. Am J Clin Dermatol. 2003;4(2):81–95. doi:10.2165/00128071-200304020-00002
12. Limmer AL, Nwannunu CE, Patel RR, Mui UN, Tyring SK. Management of ichthyosis: a brief review. Skin Therapy Lett. 2020;25(1):5–7.
13. Napolitano M, Di Guida A, Nocerino M, Fabbrocini G, Patruno C. The emerging role of dupilumab in dermatological indications. Expert Opin Biol Ther. 2021;21(11):1461–1471. doi:10.1080/14712598.2021.1907341
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Characterization of Severe Uncontrolled Asthma in Japan: Analysis of Baseline Data from the PROSPECT Study
Koya T, Asai K, Iwanaga T, Hara Y, Takahashi M, Makita N, Hayashi N, Tashiro N, Tohda Y
Journal of Asthma and Allergy 2023, 16:597-609
Published Date: 2 June 2023
Successful Treatment of Psoriasis Combined with Bullous Pemphigoid with Dupilumab: A Case Report
Liu JH, Gao Q, Ma WY, Cheng ZL, Luo NN, Hao PS
Clinical, Cosmetic and Investigational Dermatology 2023, 16:1583-1587
Published Date: 20 June 2023
