Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Successful Treatment of Psoriasis Combined with Bullous Pemphigoid with Dupilumab: A Case Report
Authors Liu JH, Gao Q , Ma WY , Cheng ZL, Luo NN , Hao PS
Received 30 March 2023
Accepted for publication 31 May 2023
Published 20 June 2023 Volume 2023:16 Pages 1583—1587
DOI https://doi.org/10.2147/CCID.S415019
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jeffrey Weinberg
Jing-Hua Liu,1,2 Qian Gao,1 Wen-Yi Ma,1 Zi-Lin Cheng,1 Na-Na Luo,1 Ping-Sheng Hao1
1Department of Dermatology, Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, Sichuan, People’s Republic of China; 2Dalian Dermatosis Hospital, Dalian, Liaoning, People’s Republic of China
Correspondence: Ping-Sheng Hao, Hospital of Chengdu University of Traditional Chinese Medicine, No. 39 Shi-er-qiao Road, Chengdu, Sichuan Province, People’s Republic of China, Tel +86-13881965024, Fax +86-28-87732407, Email [email protected]
Abstract: Psoriasis is an immune-mediated chronic inflammatory disease that can be combined with complications such as diabetes, cardiovascular disease, obesity, and kidney disease. The comorbidity of psoriasis with autoimmune bullous diseases (AIBD) has been reported previously in several cases, the most frequent of which is bullous pemphigoid (BP). The underlying mechanisms of psoriasis with BP are not clear and there are no uniform treatment criteria. Based on previous case reports, the coexistence of psoriasis and BP may be related to inflammatory activity, medications, phototherapy, and infection. We report a case of a psoriasis patient who developed BP after taking Chinese herbal compounds and was successfully treated with dupilumab, which is the first reported case of applying dupilumab to treat psoriasis with BP comorbidities.
Keywords: autoimmune bullous diseases, dupilumab, biologics, comorbidity, Chinese herbal compounds
Introduction
Both psoriasis and BP are serious dermatologic conditions and their comorbidity can pose a critical risk to patients’ health. Clinicians must recognize this condition and provide appropriate treatment. Both psoriasis and autoimmune bullous diseases (AIBD) target the epidermis, and psoriasis and BP are believed to have a bidirectional association.1 However, the cause and mechanism of psoriasis and BP coexistence are still unknown, and there are no specific treatment plans available. Some drugs have been reported in the literature as treatments for the combination of psoriasis and BP. Still, we report for the first time the application of dupilumab for this disorder. In this case, we report a patient with a 20-year history of psoriasis and gout with an abnormal renal function who developed blisters all over his body that progressively worsened after taking herbal medicine for psoriasis for four days. Eventually, the administration of dupilumab relieved the lesions of psoriasis and BP.
Case Report
A 73-year-old man was admitted with vesicles and blisters that had been present for 20 days. He had been suffering from plaque psoriasis for 20 years but had not received any formal treatment. Four days after taking herbal medicine for psoriasis, a small number of blisters appeared on his trunk, but he did not seek medical attention and continued to take the medicine. As a result, the lesions worsened, and oral mucosal damage appeared. He had a 20-year history of gout but no other chronic diseases such as hypertension or diabetes mellitus.
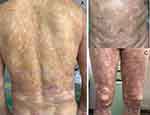
Physical examination showed swelling erythema, infiltrative plaques, and extensive tense blisters on his trunk, extremities and head. Two small ulcers were seen on his oral mucosa. Scattered basal flushing erosions and crusts on the trunk and extremities. Nikolsky’s sign was not observed. The Psoriasis Area and Severity Index (PASI) score was 8.9 and the Bullous Pemphigoid Disease Area Index (BPDAI) is 62 (Figure 1). Laboratory analysis revealed increased levels of BP180 (200RU/mL), BP230 (141.51 RU/mL), white blood cell count (12.11×10^9/L), eosinophils (2.87×10^9/L), C-reactive protein (78.18 mg/L), erythrocyte sedimentation rate (20mm/h), creatinine (124.11 μmol/L) and immunoglobulin E (IgE) level (710.6IU/mL). Skin biopsies obtained from the vesicular lesion revealed subepidermal blisters accompanied by a small quantity of inflammatory cell infiltration. Furthermore, a minor infiltration of lymphocytes and eosinophils was detected surrounding the superficial dermal vessels (Figure 2A). Direct immunofluorescence (IF) showed that immunoglobulin G (IgG) and C3 were linearly deposited at the basement membrane zone (BMZ) (Figure 2B). The patient was diagnosed with bullous pemphigoid (BP), psoriasis vulgaris (PV), and gout.
Considering that systemic steroids may contribute to psoriasis flares when the dose is reduced or withdrawn. Immunosuppressants are not appropriate because of the abnormal renal function of the patient. From the point of view of patient safety, we chose dupilumab. Treatment with dupilumab 600 mg, doxycycline 0.2 g daily, and potent topical steroids was initiated. After ten days, although lesions of both PV and BP had improved, more than ten blisters still appeared daily, and dupilumab 300mg was given again to manage the condition. Following a 16-day treatment, the patient’s PV and BP lesions significantly diminished. The post-treatment PASI score was 2.2 (reached PASI 75) and the BPDAI score was 2 (96% improvement) (Figure 3). However, due to financial constraints, the application of dupilumab was discontinued, and he was treated mainly with topical corticosteroids and vitamin D3 analogs. No lesions of psoriasis or BP appeared during the six-month follow-up period.
Discussion
The pathogenesis of psoriasis and BP exhibit a correlation, as a study showed that patients with psoriasis faced a 3.05 times higher risk of developing BP compared to those without psoriasis, and more than one-third of BP was diagnosed within a year of psoriasis diagnosis in patients with psoriasis combined with BP.2 Conversely, the incidence of psoriasis in patients with BP is 2.5 times higher than in those without BP.3 An Israeli study showed that male gender, smoking, and hypertension are risk factors for BP combined with psoriasis compared to BP patients alone.1 BP patients with coexistent psoriasis exhibit a less severe erosive phenotype and lower levels of pathogenic autoantibodies, and they tend to present at a younger age.4 In this case, however, the patient had blisters and erosions all over his body, accompanied by oral mucosal ulcers.
Previous reports have been conducted on the etiologies of BP in patients with psoriasis. Iskandarli et al5 reported a case of BP after a sudden interruption of treatment in pustular psoriasis and suggested that the presence of BP is a sign of active inflammation in psoriasis. BP is often triggered by anti-psoriatic drugs, such as cyclosporine,6 etanercept,7 adalimumab,8 stelara,9 and secukinumab.10 In addition, Minakawa et al11 reported a case of dipeptidyl peptidase-4 inhibitor-induced anti-laminin γ1-like pemphigoid in a diabetic patient with psoriasis, and Saraceno et al12 reported BP occurs following the use of losartan for hypertension in a psoriasis patient, but the patient received UVB therapy before the onset of bullae. The development of BP is associated with an underlying genetic susceptibility, and drugs are thought to be triggers for individuals with underlying genetic qualities, leading to an enhanced immune reaction or altered antigenic properties of the epidermal BMZ. These functional groups in these drugs can structurally modify molecules to either act as semi-antigens or disrupt BMZ integrity and expose epitope antigens, producing autoantibodies.13,14 In this case, however, the herbal compound was the causative agent of BP, but its ingredients were complex and the specific pathogenic mechanism was unclear.
Methotrexate was the most commonly used treatment for psoriasis combined with BP in previous reports, followed by cyclosporine, and, in most cases, combined with topical steroids.14 Systemic steroids were not applied in this case, considering the exacerbation of psoriasis caused by the withdrawal or reduction of steroids. As the patient had abnormal renal function with high levels of eosinophils and high IgE levels, a combination of these conditions resulted in the administration of dupilumab. Although many reports suggested that biologics could trigger BP, there were many cases of successful treatment by biologics, such as etanercept,15,16 ustekinumab,17 secukinumab,18 ixekizumab,19 and rituximab.12,20 However, the application of dupilumab for the treatment of the coexistence of psoriasis and BP has not been reported in previous reports. In our case, the lesions of both psoriasis and BP improved significantly after treatment with dupilumab, indicating that dupilumab is effective in treating psoriasis comorbid with BP.
Dupilumab is a fully human monoclonal antibody targeting IL-4Rα, effectively treating type 2 inflammation-driven atopic diseases by inhibiting the IL-4 and IL-13 signaling pathways.21 A growing number of studies have identified Th2-related cytokines and chemokines involved in the development of BP.22–24 Clinical reports have confirmed the efficacy and safety of dupilumab in treating BP, establishing it as a potentially superior option for refractory cases.25–27 One study23 demonstrated that dupilumab, in addition to suppressing the type 2 inflammatory pathway in patients with atopic dermatitis (AD), also downregulated the expression of Th17 and Th22 inflammatory pathway-related molecules, including IL-17A, CXCL1, and IL-22. This may partially explain why dupilumab is effective in treating psoriasis. However, we believe that the successful treatment of psoriatic lesions with dupilumab in this case is related to the specific immune environment that co-exists with psoriasis and BP. The efficacy may not be as prominent if treating psoriasis alone.
In real-world scenarios, the comorbidity of psoriasis and BP is accompanied by other complexities that can be a challenge for drug selection. This requires a more in-depth study of the mechanisms underlying the bidirectional association of psoriasis and BP to develop a scientific treatment program.
Consent Statement
Informed consent was obtained from the patient for the publication of clinical information and related images. Institutional approval was not required to publish the case details.
Funding
Hospital of Chengdu University of TCM Scientific Research Capacity Enhancement “Hundred Talents Program” (Grant No.20B04).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Kridin K, Ludwig RJ, Schonmann Y, Damiani G, Cohen AD. The bidirectional association between bullous pemphigoid and psoriasis: a population-based cohort study. Front Med. 2020;7:511. doi:10.3389/fmed.2020.00511
2. Ho YH, Hu HY, Chang YT, Li CP, Wu CY. Psoriasis is associated with increased risk of bullous pemphigoid: a nationwide population-based cohort study in Taiwan. J Dermatol. 2019;46(7):604–609. doi:10.1111/1346-8138.14902
3. Phan K, Goyal S, Murrell DF. Association between bullous pemphigoid and psoriasis: systematic review and meta-analysis of case-control studies. Australas J Dermatol. 2019;60(1):23–28. doi:10.1111/ajd.12899
4. Ständer S, Schmidt E, Zillikens D, Thaci D, Ludwig RJ, Kridin K. Patients with bullous pemphigoid and comorbid psoriasis present with less blisters and lower serum levels of anti-BP180 autoantibodies. J Eur Acad Dermatol Venereol. 2021;35(4):981–987. doi:10.1111/jdv.17013
5. Iskandarli M, Gerceker Turk B, Yaman B, Ozturk G. Pemphigoid diseases as a sign of active psoriasis: a case report and brief review. Dermatology. 2015;231(4):319–321. doi:10.1159/000435912
6. Svigos K, Fried L, Yin L, Brinster N, Lo Sicco K, Adotama P. A new eruption of bullous pemphigoid within psoriatic plaques following cyclosporine withdrawal. JAAD Case Rep. 2021;8:23–25. doi:10.1016/j.jdcr.2020.12.001
7. Wilmer EN, Becker N, Chen A, Kroumpouzos G. Etanercept-induced generalization of chronic, localized, anogenital bullous pemphigoid in a psoriatic patient. JAAD Case Rep. 2016;2(1):25–27. doi:10.1016/j.jdcr.2015.12.006
8. Sugaya M, Ishii M, Takahashi-Shishido N, Ichimura Y, Morimura S. Case of bullous pemphigoid under treatment with Adalimumab for hidradenitis suppurativa. J Dermatol. 2021;48(4):e163–e164. doi:10.1111/1346-8138.15770
9. Onsun N, Sallahoglu K, Dizman D, Su O, Tosuner Z. Bullous pemphigoid during ustekinumab therapy in a psoriatic patient. Eur J Dermatol. 2017;27(1):81–82. doi:10.1684/ejd.2016.2888
10. Ho P-H, Tsai T-F. Development of bullous pemphigoid during secukinumab treatment for psoriasis. J Dermatol. 2017;44(9):e220–e221. doi:10.1111/1346-8138.13909
11. Minakawa S, Matsuzaki Y, Hashimoto T, et al. Dipeptidyl peptidase-4 inhibitor-associated anti-laminin-γ1 (p200) pemphigoid in a patient with psoriasis vulgaris. J Dermatol. 2020;47(1):e25–e26. doi:10.1111/1346-8138.15126
12. Saraceno R, Citarella L, Spallone G, Chimenti S. A biological approach in a patient with psoriasis and bullous pemphigoid associated with losartan therapy. Clin Exp Dermatol. 2008;33(2):154–155. doi:10.1111/j.1365-2230.2007.02603.x
13. Ruocco V, Sacerdoti G. Pemphigus and bullous pemphigoid due to drugs. Int J Dermatol. 1991;30(5):307–312. doi:10.1111/j.1365-4362.1991.tb03867.x
14. Maronese CA, Cassano N, Genovese G, Foti C, Vena GA, Marzano AV. The intriguing links between psoriasis and bullous pemphigoid. J Clin Med. 2022;12(1):65.
15. Nin M, Tokunaga D, Ishii N, Komai A, Hashimoto T, Katoh N. Case of coexisting psoriatic arthritis and bullous pemphigoid improved by etanercept. J Dermatol. 2013;40(1):55–56. doi:10.1111/j.1346-8138.2012.01659.x
16. Cusano F, Iannazzone SS, Riccio G, Piccirillo F. Coexisting bullous pemphigoid and psoriasis successfully treated with etanercept. Eur J Dermatol. 2010;20(4):520. doi:10.1684/ejd.2010.0970
17. Loget J, Plee J, Antonicelli F, Bernard P. A successful treatment with ustekinumab in a case of relapsing bullous pemphigoid associated with psoriasis. J Eur Acad Dermatol Venereol. 2017;31(5):e228–e230. doi:10.1111/jdv.14002
18. Yun JS, Scardamaglia L, Tan CG, McCormack CJ. Successful secukinumab treatment of active bullous pemphigoid and chronic severe psoriasis: a case report. Australas J Dermatol. 2022;63(2):e155–e158. doi:10.1111/ajd.13803
19. Xiao Y, Gu Y, Xia D, Zhou X, Li W. Ixekizumab successfully treated refractory psoriasis concurrent bullous pemphigoid. J Dermatol. 2023;50(2):e76–e78. doi:10.1111/1346-8138.16559
20. Wang TS, Tsai TF. Remission of bullous pemphigoid after rituximab treatment in a psoriasis patient on regular low-dose methotrexate. Acta Derm Venereol. 2014;94(1):108–109. doi:10.2340/00015555-1619
21. Beck LA, Thaçi D, Hamilton JD, et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N Engl J Med. 2014;371(2):130–139. doi:10.1056/NEJMoa1314768
22. Gounni Abdelilah S, Wellemans V, Agouli M, et al. Increased expression of Th2-associated chemokines in bullous pemphigoid disease. Role of eosinophils in the production and release of these chemokines. Clin Immunol. 2006;120(2):220–231. doi:10.1016/j.clim.2006.03.014
23. Guttman-Yassky E, Bissonnette R, Ungar B, et al. Dupilumab progressively improves systemic and cutaneous abnormalities in patients with atopic dermatitis. J Allergy Clin Immunol. 2019;143(1):155–172. doi:10.1016/j.jaci.2018.08.022
24. Wang Y, Mao X, Liu Y, Yang Y, Jin H, Li L. IL-13 genetic susceptibility to bullous pemphigoid: a potential target for treatment and a prognostic marker. Front Immunol. 2022;13:824110. doi:10.3389/fimmu.2022.824110
25. Abdat R, Waldman RA, de Bedout V, et al. Dupilumab as a novel therapy for bullous pemphigoid: a multicenter case series. J Am Acad Dermatol. 2020;83(1):46–52. doi:10.1016/j.jaad.2020.01.089
26. Liang J, Abulikemu K, Maolidan A, et al. Nine cases of refractory bullous pemphigoid treated with dupilumab and literature review. Int Immunopharmacol. 2023;116:109788. doi:10.1016/j.intimp.2023.109788
27. Wang M, Wang J, Shi B. Case report: dupilumab for the treatment of bullous pemphigoid. Dermatol Ther. 2022;35(7):e15541. doi:10.1111/dth.15541
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.