Back to Journals » Journal of Pain Research » Volume 16
Subcutaneous Methylnaltrexone as Treatment for Opioid-Induced Constipation in Patients with Advanced Cancer and Noncancer Illnesses: A Post Hoc Analysis of Two Clinical Trials
Authors Shah ED , Chamberlain BH, Rhiner M, Slatkin NE , Stambler N , Israel RJ
Received 18 March 2022
Accepted for publication 22 January 2023
Published 10 February 2023 Volume 2023:16 Pages 395—406
DOI https://doi.org/10.2147/JPR.S366460
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Erica Wegrzyn
Eric D Shah,1 Bruce H Chamberlain,2 Michelle Rhiner,3 Neil E Slatkin,4,5 Nancy Stambler,6 Robert J Israel7
1Section of Gastroenterology and Hepatology, Dartmouth-Hitchcock Medical Center, Lebanon, NH, USA; 2Genesis Healthcare, Davenport, IA, USA; 3Department of Family Medicine, Loma Linda University Health, Loma Linda, CA, USA; 4School of Medicine, University of California Riverside, Riverside, CA, USA; 5Medical Affairs, Salix Pharmaceuticals, Bridgewater, NJ, USA; 6Clinical Research, Progenics Pharmaceuticals, Inc., A Subsidiary of Lantheus Holdings, Inc, New York, NY, USA; 7Clinical and Medical Affairs, Bausch Health US, LLC, Bridgewater, NJ, USA
Correspondence: Eric D Shah, Section of Gastroenterology and Hepatology, Dartmouth-Hitchcock Medical Center, 1 Medical Center Drive, Lebanon, NH, 03766, USA, Tel +1 603-650-5261, Email [email protected]
Purpose: To evaluate the efficacy and safety of subcutaneous (SC) methylnaltrexone for opioid-induced constipation (OIC) in patients with and without active cancer.
Patients and Methods: We analyzed two randomized, double-blind, placebo-controlled, Phase 3/4 trials (NCT00402038, NCT00672477). Patients received SC methylnaltrexone (study 302, 0.15 mg/kg; study 4000, 8 mg or 12 mg based on body weight) or placebo every other day for 2 weeks. Patients were stratified by cancer status. Primary efficacy endpoints included proportion of patients achieving rescue-free laxation (RFL); secondary endpoints included time to RFL, pain intensity scores, and safety/tolerability. Trial results were evaluated separately.
Results: The safety population (patients receiving ≥ 1 study drug dose) included 364 patients (study 302, n=134; study 4000, n=230). Study 302 had 78 patients with active cancer (methylnaltrexone, n=37; placebo, n=41) and 56 without cancer (methylnaltrexone, n=26; placebo, n=30); study 4000 had 152 patients with active cancer (methylnaltrexone, n=79; placebo, n=73) and 78 without cancer (methylnaltrexone, n=37; placebo, n=41). A significantly greater proportion of patients treated with methylnaltrexone achieved a laxation response within 4 hours after at least 2 of the first 4 doses versus placebo, dosed by body weight (cancer, 54.1% [methylnaltrexone] vs 7.3% [placebo], P< 0.0001; noncancer, 48.0% vs 10.0%; P< 0.005) or given as a weight-adjusted fixed dose (cancer, 59.5% vs 6.8%; noncancer, 70.3% vs 14.6%; P< 0.0001 each). With fixed-dose methylnaltrexone, average time to RFL for patients with and without cancer was < 1 hour of the first dose; with methylnaltrexone dosed by body weight, the first RFL occurred in < 4 and < 7 hours of treatment in patients with and without cancer, respectively. No significant differences were found in pain scores. SC methylnaltrexone was well tolerated at all doses in all patient cohorts.
Conclusion: SC methylnaltrexone was efficacious in inducing rapid RFL and safe among patients with and without active cancer suffering from OIC.
Keywords: methylnaltrexone, opioid-induced constipation, μ-opioid receptor antagonist, cancer, chronic pain
Introduction
Approximately 70% of patients with advanced cancer have chronic pain, which may be of moderate to severe intensity.1,2 Comparable symptom profiles, including pain prevalence, are found in patients with end-stage cancer and noncancer illnesses, thus supporting a common pathway for terminal disease symptom progression.3 Opioid-induced constipation (OIC) affects up to 60% of patients being treated for cancer-related pain and approximately 57% of patients with pain unrelated to cancer.4,5 Inadequately treated OIC frequently impacts patient quality of life and functioning, resulting in opioid dose reductions and compromised pain management.6 In addition, nausea and vomiting are commonly reported in OIC patients, which presents particular challenges in using oral laxatives or rescue treatments to adequately manage symptoms during acute episodes.7
The primary mechanism by which opioid analgesics cause OIC is through inhibition of µ-opioid receptors (MORs) in the gastrointestinal (GI) tract.8 Inhibition of peripheral MORs can lead to increased intestinal fluid absorption, decreased fluid secretion, and hindered peristalsis, leading to prolonged transit time.8–11 Standard laxative treatments can be effective in some patients but do not target the underlying inhibition of MORs in the GI tract.8,12,13 A novel class of drugs, peripherally acting MOR antagonists (PAMORAs), has been developed specifically to address these concerns by directly targeting the MORs that are directly implicated in the pathophysiology of OIC.14 Because their ability to cross the blood–brain barrier is limited, PAMORAs antagonize MORs of the enteric system without reducing pain relief of opioid analgesics within the central nervous system.14 Therefore, PAMORAs offer the opportunity to obviate the trade-off between pain management and peripheral opioid-induced side effects. Subcutaneous (SC) methylnaltrexone is approved for use in the United States in patients with OIC and chronic noncancer pain, including patients with chronic pain related to prior cancer and in adults with advanced illness or pain caused by active cancer.15 Methylnaltrexone is also available in a tablet formulation that is indicated for patients with OIC and chronic noncancer pain. Injectable methylnaltrexone dosing is either weight based or fixed, depending on the indication. Unlike other PAMORAs, which are not offered in the SC formulation, SC methylnaltrexone may be a useful choice for typical clinical situations where oral formulations may not be an option, such as when OIC is accompanied by nausea and vomiting. In addition, SC drug formulations may be particularly preferable in patients with advanced illnesses, who may have difficulty or be unable to take medications orally. Furthermore, SC methylnaltrexone has a quicker onset than oral methylnaltrexone.15
In two previously completed late-phase studies, studies 302 and 4000, SC methylnaltrexone administered by a weight-adjusted fixed dose (8 mg or 12 mg) or by body weight (0.15 mg/kg) every other day for 2 weeks was well tolerated and demonstrated robust efficacy in treating OIC without affecting central analgesia or precipitating opioid withdrawal in patients with advanced illnesses, including cancer.16,17 A 2021 study by Chamberlain et al investigated whether the efficacy and safety of methylnaltrexone differed based on cancer status. After pooling data from studies 302 and 4000 and stratifying it by cancer status (with vs without active cancer), investigators found that treatment with methylnaltrexone resulted in significantly higher proportions of patients with OIC achieving laxation responses within 4 hours of administration versus placebo, regardless of cancer status.18 Herein we present an analysis that evaluated the effectiveness of SC methylnaltrexone within a 4-hour window to assess the sustainability of this effect. To do so, we performed a post hoc analysis of studies 302 and 4000 on the efficacy and safety of SC methylnaltrexone stratified by cancer status to identify potential SC methylnaltrexone treatment differences in patients with late-stage cancer and noncancer illnesses. Unlike the Chamberlain study, the results of the two trials in this post hoc analysis are evaluated separately. We hope that providing the data individually will add to existing evidence from the pooled analysis and show the strength of effect observed with methylnaltrexone compared with placebo. Considering that a limitation of pooled studies can be that the reported effect sizes are rather small, with significance depending on large-pooled numbers of patients, we hope to provide insight into the strength of the effect in the individual studies.
Materials and Methods
Study Design
This was a post hoc analysis of two multicenter, double-blind, randomized, placebo-controlled studies (study 302, NCT00402038; study 4000, NCT00672477) in adults with advanced illness and OIC. Each study was conducted according to the ethical principles of the Declaration of Helsinki and Good Clinical Practice guidelines and approved by individual site institutional review boards. Participating sites for studies 302 and 4000 are listed in Supplementary Materials. All patients provided written informed consent before enrollment. Results from each study were previously published.16,17 In study 302, patients were randomly assigned 1:1 to receive SC injection of methylnaltrexone 0.15 mg/kg, or matched placebo, every other day for 2 weeks. Dose escalation to 0.30 mg/kg was permitted, at investigator discretion, for patients who had <3 bowel movements not associated with rescue medications or interventions by day 8. In study 4000, patients were randomly assigned 1:1 to receive SC injection of methylnaltrexone based on body weight (8 mg, 38 kg to <62 kg; 12 mg, ≥62 kg), or matched placebo, every other day for 2 weeks, up to a maximum of 7 doses. All patients who completed the treatment phase of either study could elect to enroll in a 3-month open-label extension study (study 302, NCT01367613; study 4000, NCT00672139) if they met eligibility criteria. Patients who did not enter an extension study were either contacted 30 days after the last dose for a safety follow-up (study 302) or had a follow-up visit 15 to 21 days after the last dose (study 4000).
Patients
To summarize enrollment criteria in the original clinical trials, adult (18-year-old or older) patients with a diagnosis of advanced illness, defined as those with a terminal disease, such as incurable cancer or other end-stage disease, and a life expectancy of ≥1 month were eligible for enrollment. Underlying advanced illnesses in studies 302 and 4000, respectively, included cancer (58% and 66%), cardiovascular disease (11% and 10%), pulmonary disease (10% and 12%) and neurologic disease (6% and 3%). Patients were eligible if they were on a regular regimen of opioids for control of cancer, pain/discomfort for ≥2 weeks and on a stable regimen (no dose reduction ≥50%; dosage increases and addition of another opioid permitted) for ≥3 days before the first dose of study drug. Patients were eligible if they had OIC, defined as (a) <3 laxations during the preceding week and no clinically meaningful laxation <24 hours before first dose of study drug or (b) no clinically meaningful laxation <48 hours before first dose of study drug. Patients were required to be on a stable regimen of laxatives (eg, stool softener and senna or equivalent) for ≥3 days before first dose of study drug. Patients could use rescue laxatives (eg, enema or suppository) but not <4 hours before or after study drug administration. Patients were permitted to continue baseline laxative and opioid treatment, as appropriate, during the 2-week treatment period. Patients with a history of methylnaltrexone treatment, any disease process suggestive of mechanical GI obstruction (eg, tumor adhesion), any potential non-opioid cause of bowel dysfunction that in the opinion of the investigator might have been the major contributor to the constipation, current peritoneal catheter for intraperitoneal chemotherapy or dialysis, clinically significant active diverticular disease, evidence of fecal impaction, surgically acute abdomen (study 302 only), or fecal ostomy were ineligible for enrollment. For study 4000, patients were ineligible if they received vinca alkaloids (eg, vincristine, vinblastine, vinorelbine) 4 months before screening.
Assessments
Efficacy endpoints for this post hoc analysis included proportion of patients achieving rescue-free laxation within 4 hours after first dose of study drug (study 302 coprimary endpoint); proportion of patients achieving rescue-free laxation within 4 and 24 hours after each dose; proportion of patients with a laxation response within 4 hours after at least 2 of the first 4 doses (study 302 coprimary endpoint, study 4000 primary endpoint); and time to rescue-free laxation within 4 and 24 hours of first dose. To examine if baseline functional status had an impact on response, patients were also stratified by baseline Eastern Cooperative Oncology Group (ECOG) status (≤2 or >2). Endpoints for this stratification included the proportion of patients with a laxation response within 4 hours after at least 2 of the first 4 doses.
In the original trials, patients rated their pain on an 11-point scale ranging from 0 (no pain) to 10 (worst pain imaginable), and mean changes in pain scores from baseline to first dose of study drug and day 7 were assessed. Changes in pain scores were assessed to determine whether treatment with methylnaltrexone increased pain or triggered opioid withdrawal, as would be the case with centrally acting opioid antagonists such as naloxone that compromise opioid analgesic effects, but is not expected with PAMORAs, due to their restricted ability to cross the blood-brain barrier.14,19 Safety was assessed in patients who received ≥1 dose of study drug.
Statistical Analyses
For both studies, efficacy analyses were performed on the intention-to-treat analysis population, defined as patients who received ≥1 dose of study drug. Rescue-free laxation response data were analyzed with Cochran-Mantel-Haenszel tests. P-values were based on χ2 tests. Normal significance level was 0.05, with no multiplicity adjustment. Time to rescue-free laxation within 4 and 24 hours was determined using Kaplan–Meier methods. In this post hoc analysis, outcomes were stratified by cancer status.
Results
Patients and Opioid Exposure
The post hoc analysis included an approximately equal number of patients in each treatment arm per study cohort. The safety population included 364 patients (study 302, n=134; study 4000, n=230). Study 302 had 78 patients with active cancer (methylnaltrexone, n=37; placebo, n=41) and 56 without cancer (methylnaltrexone, n=26; placebo, n=30); study 4000 had 152 patients with cancer (methylnaltrexone, n=79; placebo, n=73) and 78 without cancer (methylnaltrexone, n=37; placebo, n=41) (Figure 1).
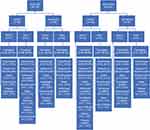 |
Figure 1 Disposition of patients by study. Abbreviations: MNTX, methylnaltrexone; PBO, placebo. |
Demographic and baseline characteristics stratified by cancer status in study 302 and study 4000 are summarized in Table 1. Across all cohorts, median daily dose opioid morphine equivalent ranged from 72.0 mg/d to 204.5 mg/d, and a higher median dose typically was observed in patients with cancer than in those without cancer. Over 98% (n=359/364) of patients were laxative-refractory at baseline. Baseline pain scores generally were higher in patients without cancer but not statistically different due to variability. In both studies, the most common concomitant opioid treatments contained morphine, codeine, or oxycodone.
 |
Table 1 Demographic and Baseline Characteristics (ITT Population) |
Efficacy
In both studies, a significantly greater percentage of patients treated with methylnaltrexone, either as a dose of 0.15 mg/kg (study 302: cancer, 51.4%, P<0.005; noncancer, 44.0%, P<0.05) or dosed according to ranges of body weight (study 4000: cancer, 69.6%; P<0.0001; noncancer, 70.3%, P<0.0001), achieved rescue-free laxation response within 4 hours of the first dose, compared with patients who received placebo (14.6%, 16.7%, 15.1%, and 22.0%, respectively; Figure 2). For both cohorts, the significant difference in rescue-free laxation response between treatment groups was maintained within 24 hours of first dose of study treatment in both studies (Figure 3). When efficacy was assessed by the proportion of patients with a laxation response within 4 hours after at least 2 of the first 4 doses, significant improvements were observed with methylnaltrexone versus placebo, regardless of cancer status (Figure 4). Similar results were also observed for this endpoint when the cohorts were further stratified by baseline ECOG status (Figure 5).
Median time to achieve first rescue-free laxation response for each cohort and treatment group in both studies 4 and 24 hours after study treatment was significantly shorter in the methylnaltrexone groups (Figure 6). At 4 hours after study treatment, patients treated with methylnaltrexone compared with placebo had a significant reduction in median time to rescue-free laxation response in both cohorts of study 302 (cancer, P<0.0005; noncancer, P<0.05) and both cohorts of study 4000 (cancer, P<0.0001; noncancer, P<0.0001). Significant reductions in median time to first rescue-free laxation response were maintained at the 24-hour postdose interval for both cohorts in both studies. In study 302, median time to first rescue-free laxation within 24 hours after first dose of either methylnaltrexone or placebo was 3.47 hours or >24 hours, respectively, in patients with cancer (P<0.005) and 6.52 hours or >24 hours, respectively, in patients without cancer (P<0.05). In study 4000, median time to first rescue-free laxation within 24 hours after first dose of either methylnaltrexone or placebo was 0.75 hour or 18.00 hours, respectively, in patients with cancer (P<0.0001) and 0.92 hour or 23.08 hours, respectively, in patients without cancer (P<0.0001).
 |
Figure 6 Time to rescue-free laxation response within 4 hours (A) and 24 hours (B) after first dose. Abbreviations: MNTX, methylnaltrexone. PBO, placebo; RFL, rescue-free laxation. |
Safety
In both studies, from baseline to day 1, there were no significant differences in pain intensity scores, including current pain and worst pain scores, between patients with and without cancer (Figure 7).
 |
Figure 7 Mean change in pain intensity from baseline to day 1 (study 302, study 4000). Abbreviations: MNTX, methylnaltrexone; PBO, placebo. Note: Error bars represent standard deviations. |
Treatment-emergent adverse events (TEAEs) that occurred in >10% of patients in any group from either study are listed in Table 2. In both studies, the most common TEAEs generally were GI related. In study 302, the most common TEAEs among patients with cancer were abdominal pain (methylnaltrexone, 24.3%; placebo, 14.6%) and vomiting (methylnaltrexone, 18.9%; placebo, 17.1%), and among patients without cancer were abdominal pain (methylnaltrexone, 7.7%; placebo, 10.0%) and flatulence (methylnaltrexone, 7.7%; placebo, 6.7%). In study 4000, abdominal pain was the most common TEAE among both patients with cancer (methylnaltrexone, 35.4%; placebo, 15.1%) and patients without cancer (methylnaltrexone, 29.7%; placebo, 19.5%).
 |
Table 2 Treatment-Emergent Adverse Events and Treatment-Emergent Serious Adverse Events Occurring in >10% of Patients in Any Group (Safety Population; Study 302, Study 4000) |
Both studies reported serious adverse events (AEs), the majority in patients with cancer. Of the patients with cancer in study 302, 6 (16.2%) treated with methylnaltrexone and 12 (29.3%) treated with placebo reported malignant neoplasm progression as the most common serious AE; of the patients with cancer in study 4000, 9 (11.4%) treated with methylnaltrexone and 13 (17.8%) treated with placebo reported disease progression as the most common serious AE. Disease progression was registered as a serious AE according to FDA definitions (eg, if the AE led to death or was life threatening, led to disability or permanent damage, or required intervention to prevent permanent damage).20
Discussion
We performed this post hoc analysis of two similarly designed late-phase randomized, placebo-controlled trials of SC methylnaltrexone in OIC patients stratified by cancer status. For patients with and without cancer in both studies, statistically significant improvement in rescue-free laxation occurred within the first 4 hours of treatment with methylnaltrexone compared with placebo. In both studies, the significant improvement in rescue-free laxation within 4 hours was maintained for 24 hours after first dose of methylnaltrexone. Repeat dosing, assessed by the proportion of patients with rescue-free laxation within 4 hours after at least 2 of the first 4 doses resulted in persistent response in both studies regardless of ECOG status at baseline. In both studies, in comparison with placebo, methylnaltrexone significantly reduced time to laxation for patients in both the active cancer and noncancer cohorts. Among patients with comparable methylnaltrexone dosing regimens, time to first rescue-free laxation was shorter for those with cancer than for those without cancer.18
In both studies, 4 hours after first dose of methylnaltrexone, there were no clinically relevant changes in pain intensity scores for advanced illness patients with and without cancer. These results are consistent with previous findings that treating OIC with methylnaltrexone does not negatively impact central opioid analgesia.9,16,17,21
In each study, baseline characteristics generally were well balanced, but there were key differences in opioid dose exposure, pain scores, and laxative use between patients with and without cancer. In both studies, median daily opioid morphine equivalent was higher in patients with cancer than in those without cancer; conversely, mean scores for pain intensity (both current and worst) generally were higher in patients without cancer than in those with cancer.
Methylnaltrexone was well tolerated in patients with advanced illnesses, which is consistent with previous reports.9,22 The majority of serious AEs occurred in patients with cancer, and the most common serious AE was malignant disease progression. However, it is important to note both studies’ lower incidence of disease progression among patients treated with methylnaltrexone versus placebo.
Until recently, there were no studies comparing SC methylnaltrexone use among patients with and without cancer; this pooled analysis of studies 302 and 4000 by Chamberlain et al is the first study to make this comparison. The results of the individual studies in this post hoc analysis support and are consistent with those of the pooled analysis,18 providing further evidence that methylnaltrexone effectively treats OIC in patients with advanced illnesses, including patients with cancer.
Furthermore, in a 2019 prospective observational study, efficacy of SC methylnaltrexone was evaluated in 23 patients with cancer and OIC per opioid subtype (oxycodone, fentanyl). Eleven patients achieved the primary endpoint of two or more laxation responses with the first four doses of methylnaltrexone, regardless of the type of opioid,23 leading investigators to recommend use of a more aggressive laxative regimen, such as SC methylnaltrexone, particularly in cases of increased opioid doses and in clinically overt OIC.
Several limitations should be considered. Both studies were of relatively short duration, with efficacy assessed after 14 days or 7 doses of study drug recognizing that opioid treatment (and subsequent OIC) are often prescribed in chronic contexts. Our study also did not account for the baseline frequency of chronic idiopathic constipation prior to opioid treatment, recognizing that chronic idiopathic constipation occurs in up to 20% of the general population.24 From an analysis perspective, caution should generally be observed for any post hoc analysis since the power calculations are made based on the original studies. For the purposes of this post hoc analysis, the primary endpoints in this study were the same as those of the original clinical trials.
Conclusions
Methylnaltrexone effectively and rapidly induced rescue-free laxation in patients with OIC occurring in active cancer and noncancer diagnoses and generally was well tolerated in all patient groups.
Abbreviations
AE, adverse event; GI, gastrointestinal; ITT, intention to treat; MOR, µ-opioid receptor; OIC, opioid-induced constipation; PAMORA, peripherally acting µ-opioid receptor antagonist; SC, subcutaneous; TEAE, treatment-emergent adverse event.
Data Sharing Statement
The datasets generated and/or analyzed during the current study are not publicly available at this time due to the proprietary nature of this information. Requests for additional information should be made to the corresponding author.
Acknowledgments
The study was funded by Salix Pharmaceuticals, a division of Bausch Health US, LLC, Bridgewater, NJ, USA, which has licensed the rights to develop and commercialize Relistor® from Progenics Pharmaceuticals, Inc., New York, NY, USA, a wholly owned subsidiary of Lantheus Holdings, Inc., North Billerica, MA, USA. Progenics Pharmaceuticals had a role in the study design, implementation of the study, and data collection. Salix Pharmaceuticals had a role in the data collection, data analysis, and the decision to publish. Technical editorial and medical writing assistance was provided under the direction of the authors by Drayton Hammond, PharmD, of Echelon Brand Communications, LLC, an OPEN Health company, Parsippany, NJ, USA. Funding for this assistance was provided by Salix Pharmaceuticals. Previous presentations: Annual Meeting of the American College of Gastroenterology, October 23–28, 2020, Virtual; American Society of Regional Anesthesia and Pain Medicine, 19th Annual Pain Medicine Meeting, November 20–22, 2020, Virtual; American Academy of Hospice and Palliative Medicine, Annual Assembly of Hospice and Palliative Care,February 17–19, 2021, Virtual.
Disclosure
Dr Shah reports travel reimbursement from Bausch Health, during the conduct of the study; personal fees from Salix, Mahana, Ardelyx, Takeda, GI Supply, and Neuraxis, outsite the submitted work. Dr Rhiner received a grant from Progenics Pharmaceuticals for study 302 and was a participating investigator in one other Progenics trial, but reports no funding received. Dr Slatkin is an employee of Salix Pharmaceuticals, a subsidiary of Bausch Health US, LLC. Dr Stambler is an employee of Progenics Pharmaceuticals, Inc., a subsidiary of Lantheus Holdings, Inc., Clinical Research, New York, NY. Dr Israel is an employee of Bausch Health US, LLC. The authors report no other conflicts of interest in this work.
References
1. van den Beuken-van Everdingen MH, Hochstenbach LM, Joosten EA, Tjan-Heijnen VC, Janssen DJ. Update on prevalence of pain in patients with cancer: systematic review and meta-analysis. J Pain Symptom Manage. 2016;51(6):1070–1090.e1079. doi:10.1016/j.jpainsymman.2015.12.340
2. Teunissen SC, Wesker W, Kruitwagen C, de Haes HC, Voest EE, de Graeff A. Symptom prevalence in patients with incurable cancer: a systematic review. J Pain Symptom Manage. 2007;34(1):94–104. doi:10.1016/j.jpainsymman.2006.10.015
3. Solano JP, Gomes B, Higginson IJ. A comparison of symptom prevalence in far advanced cancer, AIDS, heart disease, chronic obstructive pulmonary disease and renal disease. J Pain Symptom Manage. 2006;31(1):58–69. doi:10.1016/j.jpainsymman.2005.06.007
4. Glare P, Walsh D, Sheehan D. The adverse effects of morphine: a prospective survey of common symptoms during repeated dosing for chronic cancer pain. Am J Hosp Palliat Care. 2006;23(3):229–235. doi:10.1177/1049909106289068
5. Cook SF, Lanza L, Zhou X, et al. Gastrointestinal side effects in chronic opioid users: results from a population-based survey. Aliment Pharmacol Ther. 2008;27(12):1224–1232. doi:10.1111/j.1365-2036.2008.03689.x
6. Rauck RL, Hong KJ, North J. Opioid-induced constipation survey in patients with chronic noncancer pain. Pain Pract. 2017;17(3):329–335. doi:10.1111/papr.12445
7. Pappagallo M. Incidence, prevalence, and management of opioid bowel dysfunction. Am J Surg. 2001;182(suppl5A):11S–18S. doi:10.1016/S0002-9610(01)00782-6
8. Kumar L, Barker C, Emmanuel A. Opioid-induced constipation: pathophysiology, clinical consequences, and management. Gastroenterol Res Pract. 2014;2014:1–6. doi:10.1155/2014/141737
9. Slatkin N, Thomas J, Lipman AG, et al. Methylnaltrexone for treatment of opioid-induced constipation in advanced illness patients. J Support Oncol. 2009;7(1):39–46.
10. Yuan CS, Foss JF, O’Connor M, Toledano A, Roizen MF, Moss J. Methylnaltrexone prevents morphine-induced delay in oral-cecal transit time without affecting analgesia: a double-blind randomized placebo-controlled trial. Clin Pharmacol Ther. 1996;59(4):469–475. doi:10.1016/S0009-9236(96)90117-4
11. Galligan JJ, Akbarali HI. Molecular physiology of enteric opioid receptors. Am J Gastroenterol Suppl. 2014;2(1):17–21. doi:10.1038/ajgsup.2014.5
12. Wickham RJ. Managing constipation in adults with cancer. J Adv Pract Oncol. 2017;8(2):149–161.
13. Emmanuel A, Johnson M, McSkimming P, Dickerson S. Laxatives do not improve symptoms of opioid-induced constipation: results of a patient survey. Pain Med. 2017;18(10):1932–1940. doi:10.1093/pm/pnw240
14. Pergolizzi JV, Christo PJ, LeQuang JA, Magnusson P. The use of peripheral μ-opioid receptor antagonists (PAMORA) in the management of opioid-induced constipation: an update on their efficacy and safety. Drug Des Devel Ther. 2020;14:1009–1025. doi:10.2147/DDDT.S221278
15. Relistor [package insert]. Bridgewater, NJ: Salix Pharmaceuticals; 2018.
16. Bull J, Wellman CV, Israel RJ, Barrett AC, Paterson C, Forbes WP. Fixed-dose subcutaneous methylnaltrexone in patients with advanced illness and opioid-induced constipation: results of a randomized, placebo-controlled study and open-label extension. J Palliat Med. 2015;18(7):593–600. doi:10.1089/jpm.2014.0362
17. Thomas J, Karver S, Cooney GA, et al. Methylnaltrexone for opioid-induced constipation in advanced illness. N Engl J Med. 2008;358(22):2332–2343. doi:10.1056/NEJMoa0707377
18. Chamberlain BH, Rhiner M, Slatkin NE, Stambler N, Israel RJ. Subcutaneous methylnaltrexone for treatment of opioid-induced constipation in cancer versus noncancer patients: an analysis of efficacy and safety variables from two studies. J Pain Res. 2021;14:2687–2697. doi:10.2147/JPR.S312731
19. Saini HS, Alvi Z, Singh B, Elsharkawy B, Yasir M. Methylnaltrexone and naloxone for opioid-induced constipation in the critical care setting. Cureus. 2020;12(1):e6829. doi:10.7759/cureus.6829
20. What is a serious adverse event? 2016. Available from: https://www.fda.gov/safety/reporting-serious-problems-fda/what-serious-adverse-event.
21. Webster LR, Brenner DM, Barrett AC, Paterson C, Bortey E, Forbes WP. Analysis of opioid-mediated analgesia in phase III studies of methylnaltrexone for opioid-induced constipation in patients with chronic noncancer pain. J Pain Res. 2015;8:771–780. doi:10.2147/JPR.S88203
22. Michna E, Blonsky ER, Schulman S, et al. Subcutaneous methylnaltrexone for treatment of opioid-induced constipation in patients with chronic, nonmalignant pain: a randomized controlled study. J Pain. 2011;12(5):554–562. doi:10.1016/j.jpain.2010.11.008
23. Neefjes ECW, van der Wijngaart H, van der Vorst M, et al. Optimal treatment of opioid induced constipation in daily clinical practice - an observational study. BMC Palliat Care. 2019;18(1):31. doi:10.1186/s12904-019-0416-7
24. Sanchez MI, Bercik P. Epidemiology and burden of chronic constipation. Can J Gastroenterol. 2011;25(Suppl B):11b–15b. doi:10.1155/2011/974573
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




