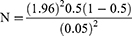Back to Journals » Veterinary Medicine: Research and Reports » Volume 12
Study on Prevalence of Hard Ticks and Their Associated Risk Factors in Small Ruminants of Boloso Sore Districts of Wolaita Zone, Southern Ethiopia
Authors Mathewos M , Welamu W , Fesseha H , Aliye S, Endale H
Received 6 September 2021
Accepted for publication 27 October 2021
Published 12 November 2021 Volume 2021:12 Pages 293—301
DOI https://doi.org/10.2147/VMRR.S336467
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Young Lyoo
Mesfin Mathewos, Wengelu Welamu, Haben Fesseha, Saliman Aliye, Habtamu Endale
School of Veterinary Medicine, Wolaita Sodo University, Wolaita Sodo, Ethiopia
Correspondence: Mesfin Mathewos Email [email protected]
Background: Ticks and tick-borne infections are the most serious health threats to small ruminants in Ethiopia, resulting in huge economic losses.
Methods: A cross-sectional study using a simple random sampling technique was conducted to determine the prevalence and potential risk factors of hard ticks in small ruminants of the Boloso Sore district of Wolaita Zone. Ticks were identified to species level under a stereomicroscope using morphological identification keys.
Results: From a total of 400 examined animals, 68.75% (275/400) of them were infested with hard ticks with the respective prevalence of 69.09% (152/220) in goats and 68.33% (123/180) in sheep. A total of 1192 (552 males and 640 females) adult ixodid ticks that belong to a total of four species, which were grouped under three genera: Amblyomma, Hyalomma, and Rhipicephalus, and one subgenus; Boophilus were collected from the head, ear, under tail and legs of goats and sheep. In this study, Amblyomma variegatum 44.97% (536/1192) was found to be the most abundant tick species followed by Boophilus decoloratus 30.79% (367/1192), Rhipicephalus pulchellus 20.47% (244/1192), and Hyalomma truncatum 3.77% (45/1192). The sex, age, and body condition score of animals with a high prevalence of hard ticks showed statistically significant differences (p< 0.05). Male ticks dominated females in all cases except for Boophilus decoloratus. There was no statistically significant relationship (p > 0.05) between tick infestation and animal origin or species.
Conclusion: Ticks were the most significant production and health constraints for small ruminants in the study region. Therefore, the increasing threat of ticks of small ruminants warrants urgent strategic application of acaricides and the creation of awareness among livestock owners to prevent and control tick infestation.
Keywords: ixodid ticks, prevalence, small ruminants, Boloso Sore, Ssouthern Ethiopia
Introduction
Ethiopia has Africa’s largest livestock population, with 57.83 million cattle, 28 million sheep, 28.6 million goats, 1.23 million camels, 60.5 million poultry, 2.1 million horses, 0.4 million mules, and 7.88 million donkeys.1 There are 2.4 million sheep and 2.2 million goats in southern Ethiopia, which are key sources of income for the agricultural community.2 Livestock generates 12–15% of total export revenues, through the export of live animals, meat, hides, and skins.3,4
Small ruminants contribute significantly to food production in the country, accounting for 35% of meat consumption and 14% of milk consumption. Small ruminants contribute 40% of cash revenue and 19% of household meat consumption in the central highlands, where a mixed crop-livestock production system is practiced. Sheep and goats are considered investments and insurance because of their high productiveness, short gestation intervals, and ability to adapt to harsh environments. They also provide income to purchase food during crop failure seasons and to meet seasonal purchases such as improved seed, fertilizer, and medicine for rural households.5
Cattle hide is being used at a rate of 48%, goat skin at 75%, and sheep skin at 97% with the expected off-take rate of 33%, 35%, and 7% for sheep, goat, and cattle, respectively. Ethiopia exports a diverse range of processed and semi-processed sheep and goat skins, accounting for 12–16% of total value exports.5,6 Unfortunately, in recent years, this rank has been demoted to the fifth level, due to the rejection and downgrading of hides, as well as skin problems caused by parasite infestation.7
Ticks are blood-sucking external parasites that feed on the blood of vertebrates, especially mammals and birds.8 They are directly or indirectly responsible for significant financial losses in Ethiopia’s livestock sector, which accounts for 75% of all animal exports. The rejection and degradation of hides and skins in Ethiopia resulted in a conservative estimate of 1 million birr loss each year.9,10 Ticks spend most of their lives away from their hosts, hiding in the ground and plants or their hosts’ nests.11,12 Ticks are classified into three families: Ixodidae (hard ticks), which has 702 species, Argasidae (soft ticks), which has 193 species, and Nuttalliellidae, which has a single species. There are four stages in the life cycle of the ixodid tick: eggs, six-legged larva, eight-legged nymph, and adult. In accordance with the number of hosts necessary to complete their lifecycle, they are divided into one-host, two-host, and three-host life cycles.13,14
In Ethiopia, ticks have been the subject of a lot of research as compared to other key skin disorders that affect animals. The main genera of ticks found in Ethiopia are Amblyomma, Rhipicephalus, Hyalomma and Haemaphysalis, and a subgenus Rhipicephalus (Boophilus). Previous studies documented the presence of more than 60 species of ticks in the country including genus Amblyomma (8 spp.), subgenus Boophilus (2 spp.), Haemaphysalis (4 spp.), Hyalomma (9 spp.), Rhipicephalus (15 spp.), Ixodes (1 spp.), Argas (1 spp.) and Ornithodorus (2 spp.) which are reported to have great veterinary and medical importance in Ethiopia. Among these, A. variegatum and Rh. (Boophilus) decoloratus are the most important and widely distributed.15,16 A. coherence, A. gemma, A. lepidium, H. marginatum rufipes, H. truncatum, and R. evertsi are also commonly found in Ethiopia.17
Even though different studies were done in several parts of the country including southern Ethiopia, no study was conducted on the prevalence and associated risk factors of hard ticks of small ruminants in the selected districts of the Wolaita zone due to little emphasis given to ectoparasites of small ruminants. As a result, the goal of this study was to evaluate the risk factors and determine the prevalence of Ixodid ticks in small ruminants in selected districts of Boloso Sore, southern Ethiopia.
Materials and Methods
Study Area
Boloso Sore is located 380 kilometers southwest of Ethiopia’s capital city, Addis Ababa, in the Wolaita Zone of the southern Nations, Nationalities, and Peoples’ Region. It is bordered on the west by Boloso Bombe, on the north by Kembata Tembaro, on the south by Sodo Zuria and Damot Sore, and on the east by Damota Gale. Based on the climate, 80% of the population was live in Woina Dega (mid-altitude), the rest was live in Kola (Lowland), and a negligible percentage was live in Dega (Highland). The site is situated at 7°05′ N 37°40′ E/7.083° N 37.667° E and has an elevation of 1350–2380m above sea level. A short-wet season runs from March to May, and a long rainy season runs from June to September, with an average annual rainfall of 1300mm and an average daily temperature of 20.4°C. The livestock population of Boloso Sore district was estimated to be 84,391 cattle, 57,331 ovines, 8396 caprines, 7321 equines, and 91,375 poultry, according to the Wolaita Zone Livestock and Fishery Resources office’s 2016 report.18
Study Animals
The study animals were local goats and sheep that were brought to Areka wereda (Boloso Sore) veterinary clinics and animal health posts of Areka Ketema, Doubo, Dolla, Gurumo Koyesha, and Gara Godo. It is comprised of both sexes, of all ages, and from intensive, semi-intensive, and extensive farming systems. The animals’ ages were estimated by information collected from their owners and by examining the dentition patterns of the animals. According to the classification approach of Gatenby,19 the small ruminants were classified as young (0 to 1 year), adult (2 to 4 years), and old (above 4 years old animal). The body condition score was determined according to20 and classified into three groups as poor, medium, and good.
Study Design
A cross-sectional study was employed to assess the associated risk factors and determine the prevalence of Ixodidae ticks of small animals from September 2020 to June 2021 in Boloso sore districts. During data collection, the origin of the animal, species, sex, age, body condition score of the research animals, and tick predilection sites were recorded to determine the risk variables associated with the occurrence of tick infestation.
Sampling Technique and Sample Size Determination
Small ruminants were chosen from five kebeles in the Boloso sore areas using a simple random sampling technique. Because no previous research had been conducted on the prevalence and risk factors of Ixodidae ticks in small ruminants in the study area, the current study used a 50% expected prevalence, a 95% confidence level, and a 5% absolute precision. The overall number of animals to be included in the study was calculated using a formula of Thrusfield21:
where Pexp = expected prevalence (0.5), Zα =significance level (1.96), p = prevalence (50%), N = required sample size and d = desired absolute precision (0.05).
Accordingly, the sample size of 384 animals was determined. But 400 small animals were sampled to increase precision from five kebeles; 100 from Doubo (54 goats and 46 sheep), 60 from Dolla (34 goats and 26 sheep), 69 from Gurumo Koysha (38 goats and 31 sheep), 90 from Areka Ketema (49 goats and 41 sheep) and 81 from Gara Godo (45 goats and 36 sheep).
Study Method
Collection and Identification of Ticks
All visible adult ticks were removed with forceps and gloved handpicking while rotating horizontally to avoid damage to the tick mouthparts and harm to the host, and the tick was then preserved in a 70% ethanol. The bottle was tagged with the date, location, gender, age, and body site before being sent to the Wolaita Sodo University Veterinary Laboratory for parasitological examination. Ticks from each container were placed on Petri dishes and inspected under a stereomicroscope with tick identification keys to determine the species as described by Taylor et al and Walker,22,23 and which was identified by the color, size, and shape of their mouthparts, as well as the scutum, anal groove, festoon, punctuation, and legs.24
Data Management and Analysis
The data was coded and placed into a Microsoft Excel spreadsheet, which was then analyzed using STATA version 20. The number of tick-infested animals divided by the total number of tick-infested animals in each study area was used to assess the tick prevalence in small ruminants in each study area. The Pearson chi-square (χ2) test was used to determine the degree of connection with the major risk factors. Also, multivariate logistic regression analyses were used to examine the relationship between tick prevalence (0 = negative, 1 = positive) and categorical independent factors. A significant association was defined at a p-value less than 0.05.
Results
Prevalence of Hard Ticks in Small Ruminants
In the present study, a total of 275 (68.75%) out of 400 small ruminants (220 goats and 180 sheep) were found to be infested with at least one and/or different species of ticks. Infestation rates were found to be higher in goats at 69.09% (152/220) than in sheep at 68.33% (123/180). A statistically significant difference (p< 0.05) was observed between the sex, age groups, and body conditions, and tick infestation except for species of study animals with the occurence of ixodid tick (Table 1).
 |
Table 1 Association of Tick Infestation with Various Risk Factors |
The odds of old small ruminants infested by hard ticks were 4.27 (CI, 2.014–9.053) times higher than young small ruminants, while adults were held constant. The odds of female small ruminants infested by hard ticks were 2.54 (CI, 1.639–3.926) times higher than male. Moreover, the odds of small ruminants with poor body conditions infested by hard ticks were 8.41 (CI, 0.616–1.904) times higher than that of medium body conditioned animals, while good body conditioned small ruminants were held constant (Table 2).
 |
Table 2 Multivariate Logistic Regression Analysis of Risk Factors with Tick Prevalence in the Study Area |
Species Wise Prevalence of Hard Tick in Small Ruminants
The current investigation revealed that four tick species (three tick genera and one sub-genera) were identified based on different identification keys of ticks. Taking into account the relative abundance of each tick, Amblyomma variegatum (44.97%) was the most abundant tick species followed by Boophilus decoloratus (30.79%), Rhipicephalus pulchellus (20.47%), and Hyalomma truncatum (3.77%) in both goats and sheep. Both sheep and goats were infested with these identified tick genus and species during the study period. Factors such as sex, age, and body conditions score have showed a statistically significant differences (p< 0.05) with the prevalence of hard ticks (Table 3).
 |
Table 3 Association of Tick Infestation with Potential Risk Factors in Small Ruminants |
Sex Ratio of Tick Species
A total of 1192 ticks were gathered from 152 of 220 goats and 123 of 180 sheep in this investigation. The tick count in males of the genus Amblyomma, Rhipicephalus, and Hyalomma species was higher in female. However, females of the subgenus Rh. (Bo.) decoloratus were collected in greater numbers than males. A. variegatum, Rh. (Bo.) decoloratus, Rh. pulchellus and H. truncatum had male to female ratios of 1.47:1, 0.3:1, 1.05:1, and 1.04:1, respectively (Table 4).
 |
Table 4 Total Adult Tick Species, the Sex Ratio in Small Ruminants of the Study Area |
Tick Species Attachment Sites
The present study disclosed that the preferred attachment sites of ixodid ticks infesting small ruminants vary among different genera of ticks. This study revealed that A. variegatum has got greater preference for head, tail, ear, and leg part of small ruminants in descending order of tick attachment, whereas H. truncatum showed a greater preference for ear, tail, and leg. Likewise, Rh. pulchellus has got greater preferences of attachment on the head, ears, and tail regions. Rh. (Bo.) decoloratus appears to have a stronger predilection for attachment sites on small ruminants’ heads, ears, and tails (Table 5).
 |
Table 5 Tick Species’ Attachment Preferences on Small Ruminants of the Study Area |
Discussion
In this study period, a total of 400 sheep and goats were examined and a total of 1192 visible adult ticks were collected from different parts of the body, ie, 152 of 220 goats and 123 of 180 sheep. Four types of tick genera, including Amblyomma (44.97%), Boophilus (30.79%), Rhipicephalus (20.47%), and Hyalomma (3.77%) were identified from small ruminants of the study area. Consequently, this study indicated that tick infestation is still common in the study site, and ticks are the most prevalent external parasites of small ruminants.
In Ethiopia, the range and number of tick species infesting small ruminants varies by region. The finding of the current prevalence of tick was 68.75% in the study area and this was comparable with the previous investigation conducted by Ahmed et al25 who reported a prevalence of 72.39% from Dire Dawa, Eastern Ethiopia, and by Abunna et al26 who recorded a prevalence of 66.12% in goats and 80.30% in sheep from Bedelle district. However, the present study revealed a prevalence of 69.09% in goats and 68.33% in sheep as compared to the earlier report described by Desalegn et al27 from Haramaya district, eastern Hararghe who reported a prevalence of 87.5% in goats and 89.9% in sheep, by Kifle et al28 who reported prevalence of 85.26% in goats from Benatsemay district, south omo zone, south-western Ethiopia, by Eyob and Matios29 who reported a prevalence of 97.58% in goats and 69.86% in sheep from Dhas district of Borana pastoral area, southern Ethiopia.
This research also found a greater prevalence of tick infestation than earlier studies done by Yacob et al6 from in and around Wolaita Sodo who recorded a prevalence of 18.6% in goats and 31.8% in sheep, by Sertse and Wossene30 from the Eastern part of Amhara, North East Ethiopia who recorded a prevalence of 3.4% in goats and 22.2% in sheep and by Abebe et al31 from selected districts of Tigray region who recorded a prevalence of 58.8% in goats and 40% in sheep. Geographical differences, seasons of investigation, and regular exposure to the same community grazing lands may have contributed to the variation in prevalence.
The age-wise tick prevalence of the current study showed that a higher (87.18%) tick burden in older animals followed by young (64.37%) and adults (64.26%) and showed a statistically significant association (p<0.05) with the occurrence of tick infestation. According to Ahmed et al25 in Dire Dawa, eastern Ethiopia, there was a significant difference in age groups, which is in line with the present study. Tick infestation in older animals was shown to be considerably greater than in other age groups. This might be due to young animals were kept in the house and grazed around the house until they grew stronger, reducing their chances of acquiring ticks compared to older animals kept in an outdoor system. This, in turn, contributes to a lower risk of tick exposure since the number of ticks in the household is lower, and is likely linked to older animals that have low immunity and resistance.
Tick infestation has a significant (p<0.005) effect on the animal body conditions of the small ruminants. Animals with poor body condition scores, medium and good revealed an infestation rate of 86.36%, 69.39%, and 63.56%, respectively. This was incomparable with the study conducted by Ahmed et al25 in Dire Dawa, eastern Ethiopia, and by Seid32 in Mizzen Teferi Bench Maji Zone, SNNPRS. This might be due to the fact that animals in poor physical condition have low tick resistance and lack the body ability to develop resistance, whereas animals in good physical condition are able to combat the infestation effectively.33 Furthermore, because ticks consume a huge quantity of blood and fluid, a major tick infestation may result in poor body health and condition.
The sex-wise comparison revealed that females had a higher rate of tick infestation (78.1%) than males (58.42%). Although the actual explanation of female animals having a greater frequency of tick infestation is unknown, females may feed on fields where they are in touch with other animals and they are also allowed to live a long time for birth-giving purposes that increase the probability of being infested with tick than males that are mainly kept at home for fattening (meat purpose) for a short period. Furthermore, production stress, such as pregnancy and lactation, may have made female animals more vulnerable to infestation. In the current study, the analysis of logistic regression reporting odds ratio revealed that the odds of older, male and small ruminants with poor body conditions were more infested by hard ticks than young, female, and medium body conditioned ones while adult and good body conditioned small ruminants were held constant. This could probably be due to those animals with old age and poor condition became immunocompromised, but its higher occurrence in a male could be related to male small ruminants that were mostly kept under extensive management while those female counterparts were kept indoors for the sake of gestation.
This study found no significant association (p>0.05) between tick prevalence and factors, such as origin and species. These include Amblyomma variegatum, Boophilus decoloratus, Rhipicephalus pulchellus, and Hyalomma truncatum as the most common tick species infesting small ruminants in the research region at the time of the study. The most common and numerous tick species was Amblyomma variegatum (44.97%), which was in accordance with earlier investigations in Ethiopia by Pegram et al34 and Kumsa et al.35 The observation of Rh. (Bo.) decoloratus (30.79%) as the second most abundant tick species in the present study agrees with the previous findings of Kassa and Yalew36; Wasihun and Doda37; Meaza et al38 and Bedaso et al39 who reported that Rh. (Bo.) decoloratus was the most abundant tick species in their study.
The findings of Rh. pulchellus (20.47%) as the third predominant tick species were in agreement with previous studies from Borena province by Regassa40 and from West Hararghe Zone, East Ethiopia, by Ababa et al.41 Conversely, this is in contrast to previous reports of the least abundance of this tick species from Western Amhara by Alemu et al42 and Haramaya, Eastern Ethiopia by Kassa and Yalew.36 In Ethiopia, this tick prefers semi-arid and lowland environments as described by Pegram et al.34 Contrary to the previous research by Ahmed et al,25 Hyalomma truncatum was the least common tick encountered in small ruminants of the study area. This could be due to the difference in the season as reported by De Castro43 and Tiki and Addis.44
The male-to-female ratio of ticks was 0.86:1.16 and this was comparable to previous reports of Ahmed et al25 who reported that in all but B. decoloratus, male ticks dominated females because males typically stay on the host longer than females. Female ticks that are fully engorged will drop to the ground to lay eggs, but male ticks will stay on the host for several months to continue eating and mating with other females before falling off.45 In this study, females of B. decoloratus outnumbered males, most likely due to the male’s small size, which may not be visible during collecting.46
The current study suggested that all Amblyomma tick species have a similar preference for head, tail, ear, and leg which is in line with the previous observation of Wasihun and Doda37 in southern Ethiopia. Members of the genus Hyalomma showed similar preferences for the ear, tail, and leg which is consistent with the earlier report by Ayalew et al47 in central Oromia, Ethiopia. Members of the Rhipicephalus species were encountered mainly in the head, ears, and under the tail, which has been reported earlier by Kassa and Yalew36. The Rh. (Bo.) decoloratus showed a preference for attachment sites head, ears, and tail which is again in agreement with the finding of Kariuki et al48 from Kenya.
Conclusion
The current investigation revealed a significant prevalence of tick infestation in small ruminants of Boloso sore district and this causes a major health constraint that results in huge economic losses through blood-sucking, damage to hide, and transmitting a variety of vector-borne diseases. This study showed that A. variegatum and B. decoloratus are the predominant and common tick species followed by Rh. pulchellus and Hyalomma truncatum in the study district. The prevalence of tick infestation was shown to be statistically significant (p<0.05) across the sexes, ages, and body conditions of sheep and goats. Due to the high prevalence of small ruminant ticks in the study area, districts require immediate attention at all levels to reduce the impact on the health and production of small ruminants and thereby enhance the living conditions of farmers in the study area. The strategic application of acaricides and minimizing frequent contact between different herds might minimize the infestation of ticks. Awareness creation to the livestock owners on the potential effect of ticks is also needed.
Abbreviations
CI, confidence interval; CSA, Central Statistical Agency; ESGPIP, Ethiopian Sheep and Goat Productivity Improvement Program; STATA, Software for Statistics and Data Science; WZLFR, Wolaita Zone Livestock and Fishery Resources office.
Data Sharing Statement
The corresponding author will provide the datasets that were used and analyzed during this work on reasonable request.
Ethics Approval and Consent to Participate
Ethical permission was granted for this study by the Wolaita Sodo University of Research Ethics Committee. Before gathering samples from small ruminant owners’ goats and sheep, a verbal agreement was obtained, and appropriate sanitary precautions were followed when taking tick samples from the body’s surface. The best veterinary practice guidelines were followed, and the study’s aim was explained to sheep and goat owners, and the Wolaita Sodo University of Research Ethics and Review Committee accepted the oral informed consent method described in the paper.
Acknowledgments
The authors would like to acknowledge animal owners and animal health professionals for their collaboration during tick collection in the field.
Author Contributions
All authors contributed significantly to the conception and design, data acquisition, and data analysis and interpretation; participated in the drafting of the article or critically revised it for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work.
Funding
This work was not supported by any funding source or institution.
Disclosure
There were no competing interests disclosed by any of the authors.
References
1. Central Statistical Agency. Federal Democratic Republic of Ethiopia Agricultural Sample Survey. Report on Livestock and Livestock Characteristics. Addis Ababa, Ethiopia: Central Statistical Agency; 2019.
2. Sheferaw D, Degefu H, Banteyirgu D. Epidemiological study of small ruminant mange mites in three agro-ecological zones of Wolaita, Southern Ethiopia. Ethiop Vet J. 2010;14:31–38.
3. Solomon A, Authority ELM. Livestock marketing in Ethiopia: a review of structure, performance, and development initiatives. 2003.
4. GebreMariam S, Amare S, Baker D, et al. Diagnostic study of live cattle and beef production and marketing: constraints and opportunities for enhancing the system. Gates Open Res. 2019;3:181.
5. Tefera S. Investigation on Ectoparasites of Small Ruminants in Selected Sites of Amhara Regional State and Their Impact on the Tanning Industry. Addis Ababa University, Faculty of Veterinary Medicine; 2004.
6. Yacob H, Yalew T, Dinka A. Part I: ectoparasite prevalences in sheep and in goats in and around Wolaita soddo, Southern Ethiopia. Revue de Médecine Vétérinaire. 2008;159:8–9.
7. Kassa B. Cockle, mange and pox: major threats to the leather industry in Ethiopia. Ethiopian leather industry: perseverance towards value addition.
8. Minjauw B, McLeod A. Tick-Borne Diseases and Poverty: The Impact of Ticks and Tick-Borne Diseases on the Livelihoods of Small-Scale and Marginal Livestock Owners in India and Eastern and Southern Africa. DFID Animal Health Programme, Centre for Tropical Veterinary Medicine; 2003.
9. Zeleke M, Bekele T. Species of ticks on camels and their seasonal population dynamics in Eastern Ethiopia. Trop Anim Health Prod. 2004;36:225–231. doi:10.1023/B:TROP.0000016830.30194.2a
10. Bekele T. Studies on seasonal dynamics of ticks of Ogaden cattle and individual variation in resistance to ticks in eastern Ethiopia. J Vet Med Series B. 2002;49:285–288. doi:10.1046/j.1439-0450.2002.00567.x
11. Latif A, Walker A. An introduction to the biology and control of ticks in Africa. ICTTD-2 project. 2004:1–29.
12. Wall R, Shearer D. Veterinary Ectoparasites: Biology, Pathology and Control. Oxford: Blackwells Science Ltd; 2001.
13. Wall RL, Shearer D. Veterinary Ectoparasites: Biology, Pathology and Control. John Wiley & Sons; 2008.
14. Ellse L, Wall R. The use of essential oils in veterinary ectoparasite control: a review. Med Vet Entomol. 2014;28:233–243. doi:10.1111/mve.12033
15. Abebaw G. ’Seasonal dynamics and host preference of Boophilus decoloratus on naturally infested cattle in Jimma zone, south western Ethiopia. Ethiop Vet J. 2004;18:19–28.
16. Nibret M, Basaznew B, Tewodros F. Hard ticks (Ixodidae): species composition, seasonal dynamics and body site distribution on cattle in Chilga District, Northwest Ethiopia. Asian J Agric Sci. 2012;4:341–345.
17. Ayana M, Gelaye A, Fesseha H, et al. Study on the distribution of ixodid ticks of cattle in pastoral areas of Yabello district, Borana zone, Oromia, Ethiopia. Parasite Epidemiol Control. 2021;12:e00200. doi:10.1016/j.parepi.2021.e00200
18. Misebo F, Gashaw T, Yilma M. Assessment on major reproductive health problems of dairy cattle in Boloso Sore, Southern Ethiopia. J Vet Med Anim Health. 2018;10:224–230. doi:10.5897/JVMAH2018.0673
19. Gatenby M. Sheep.
20. Molla B, Haile H, Alemu S. Prevalence and risk factors associated to skin diseases in small ruminants in Gamo Gofa zone, south-Western Ethiopia. J Vet Med Anim Health. 2017;9:228–234.
21. Thrusfield M. Veterinary Epidemiology. John Wiley & Sons; 2018.
22. Taylor M, Coop R, Wall R. Text Book of Veterinary Parasitology.
23. Walker AR. Ticks of Domestic Animals in Africa: A Guide to Identification of Species. Edinburgh: Bioscience Reports; 2003.
24. Lemma S, Redii A. Prevalence of tick and mange mites’infestation in Goats in Benatsemay district of South Omo Zone, Ethiopia. Int J Res. 2019;7:111–118.
25. Ahmed J, Wendemagegn D, Tsehay A, et al. Prevalence of tick infestation on small ruminants in and around Dire Dawa, Eastern Ethiopia. Int J Res. 2017;5:326–336. doi:10.29121/granthaalayah.v5.i5.2017.1864
26. Abunna F, Kasasa D, Shelima B, et al. Survey of tick infestation in small ruminants of Miesso district, West Harergie, Oromia Region, Ethiopia. Trop Anim Health Prod. 2009;41:969–972. doi:10.1007/s11250-008-9286-3
27. Desalegn T, Fikru A, Kasaye S. Survey of tick infestation in domestic ruminants of Haramaya District, Eastern Hararghe, Ethiopia. J Bacteriol Parasitol. 2015;6:1.
28. Kifle T, Mathewos M, Fesseha H, et al. Study on prevalence of ixodid ticks of goats and acaricide utilization practices of herd owners in Benatsemay District, South Omo Zone, South-Western Ethiopia. Vet Med Res Rep. 2021;12:225.
29. Eyob E, Matios L. Preliminary survey on the distribution of ixodid ticks in small ruminants of Dhas District of Borena pastoral area, Southern Rangelands of Ethiopia. Adv Biores. 2014;5:87–91.
30. Sertse T, Wossene A. A study on ectoparasites of sheep and goats in eastern part of Amhara region, northeast Ethiopia. Small Ruminant Res. 2007;69:62–67. doi:10.1016/j.smallrumres.2005.12.010
31. Abebe R, Tatek M, Megersa B, et al. Prevalence of small ruminant ectoparasites and associated risk factors in selected districts of Tigray Region, Ethiopia. Glob Vet. 2011;7:433–437.
32. Seid B. A Survey of Cattle Tick Species in and Around Mizan- Teferi, Bench Maji Zone and SNNPRs. Faculty of Veterinary Medicine. Deberezeit, Ethiopia: Addis Ababa University; 2004.
33. Manan A, Khan Z, Ahmad B. Prevalence and Identification of Ixodid. Ministry of Economic Development and Cooperation Survey of Livestock and Fisheries Development: Agricultural Development Department. Addis Ababa, Ethiopia: Livestock Team; 2007.
34. Pegram RG, Hoogstraal H, Wassef HY. Ticks (Acari: ixodoidea) of Ethiopia. I. Distribution, ecology and host relationships of species infesting livestock. Bull Entomol Res. 1981;71:339–359. doi:10.1017/S0007485300008373
35. Kumsa B, Socolovschi C, Parola P, et al. Molecular detection of Acinetobacter species in lice and keds of domestic animals in Oromia Regional State, Ethiopia. PLoS One. 2012;7:e52377. doi:10.1371/journal.pone.0052377
36. Kassa S, Yalew A. Identification of Ixodide ticks of cattle in and around Haramaya district, Eastern Ethiopia. Sci J Crop Sci. 2012;1:32–38.
37. Wasihun P, Doda D. Study on prevalence and identification of ticks in Humbo district, Southern Nations, Nationalities, and Peoples Region (SNNPR), Ethiopia. J Vet Med Anim Health. 2013;5:73–80.
38. Meaza G, Abdu M, Yisehak K. Determination of the prevalence of ixodid ticks of cattle breeds, their predilection sites of variation and tick burden between different risk factors in Bahir Dar, Ethiopia. Glob Vet. 2014;13:520–529.
39. Bedasso M, Abebe B, Degefu H. Species composition, prevalence and seasonal variations of ixodid cattle ticks in and around Haramaya town, Ethiopia. J Vet Med Anim Health. 2014;6:131–137. doi:10.5897/JVMAH2014.0275
40. Regassa A. Tick infestation of Borana cattle in the Borana Province of Ethiopia. 2001.
41. Ababa H, Negese T, Birru B, et al. Study on the status of Bovine Tick Infestation, in Guba-Koricha District in West Hararghe Zone, East-Ethiopia. Int J Res. 2017;5:202–213.
42. Alemu G, Chanie M, Mengesha D, et al. Prevalence of ixodid ticks on cattle in Northwest Ethiopia. Acta Parasitol Glob. 2014;5:139–145.
43. De Castro J. A Survey of the Tick Species in Western Ethiopia, Including Previous Findings and Recommendations for Further Tick Surveys in Ethiopia. FAO; 1994.
44. Tiki B, Addis M. Distribution of ixodid ticks on cattle in and around Holeta town, Ethiopia. Glob Vet. 2011;7:527–531.
45. Solomon G. Seasonal variation of ticks on calves at Sabetha, Western Shewa zone. 2001.
46. Tessema T, Gashaw A. Prevalence of ticks on local and crossbred cattle in and around Asella town, southeast Ethiopia. Ethiop Vet J. 2010;14:79–89.
47. Ayalew T, Hailu Y, Kumsa B. Ixodid ticks infesting cattle in three agroecological zones in central Oromia: species composition, seasonal variation, and control practices. Comp Clin Path. 2014;23:1103–1110. doi:10.1007/s00580-013-1748-y
48. Kariuki EK, Penzhorn BL, Horak IG. Ticks (Acari: Ixodidae) infesting cattle and African buffaloes in the Tsavo conservation area, Kenya: research communication. Onderstepoort J Vet Res. 2012;79:1–4. doi:10.4102/ojvr.v79i1.437
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.