Back to Journals » Journal of Inflammation Research » Volume 14
Sterculia tragacantha Lindl Leaf Extract Ameliorates STZ-Induced Diabetes, Oxidative Stress, Inflammation and Neuronal Impairment
Authors Onikanni AS , Lawal B , Olusola AO, Olugbodi JO, Sani S, Ajiboye BO, Ilesanmi OB , Alqarni M, Mostafa-Hedeab G, Obaidullah AJ, Batiha GES, Wu ATH
Received 15 October 2021
Accepted for publication 17 November 2021
Published 9 December 2021 Volume 2021:14 Pages 6749—6764
DOI https://doi.org/10.2147/JIR.S319673
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ning Quan
Amos Sunday Onikanni,1– 3,* Bashir Lawal,4,5,* Augustine O Olusola,1 Janet O Olugbodi,6 Saidu Sani,7 Basiru Olaitan Ajiboye,8 Omotayo B Ilesanmi,9 Mohammed Alqarni,10 Gomaa Mostafa-Hedeab,11,12 Ahmad J Obaidullah,13,14 Gaber El-Saber Batiha,15 Alexander TH Wu16– 21
1Toxicology and Environmental Laboratory, Department of Biochemistry, Faculty of Science, Adekunle Ajasin University, Akungba-Akoko, Ondo State, Nigeria; 2Biochemistry Unit, Department of Chemical Sciences, Afe Babalola University, Ado-Ekiti, Ekiti State, Nigeria; 3Graduate Institute of Biomedical Science, College of Medicine, China Medical University, Taipei, Taiwan; 4PhD Program for Cancer Molecular Biology and Drug Discovery, College of Medical Science and Technology, Taipei Medical University and Academia Sinica, Taipei, 11031, Taiwan; 5Graduate Institute of Cancer Biology & Drug Discovery, College of Medical Science and Technology, Taipei Medical University, Taipei, 11031, Taiwan; 6Department of Biochemistry, Bingham University, Karu, Nigeria; 7Department of Biochemistry, Faculty of Biological Science, Alex Ekwueme Federal University Ndufu Alike IkwoD, Abakaliki, Ebonyi State, Nigeria; 8Phytomedicine and Molecular Toxicology Research Laboratory, Department of Biochemistry, Faculty of Science, Federal University, Oye-Ekiti, Ekiti State, Nigeria; 9Department of Biochemistry, Faculty of Science, Federal University Otuoke, Ogbia, Bayelsa State, 23401, Nigeria; 10Department of Pharmaceutical Chemistry, College of Pharmacy, Taif University, Taif, 21944, Saudi Arabia; 11Pharmacology Department & Health Research Unit, Medical College, Jouf University, Sakakah, Saudi Arabia; 12Pharmacology Department, Faculty of Medicine, Beni-Suef University, Beni Suef, Egypt; 13Department of Pharmaceutical Chemistry, College of Pharmacy, King Saud University, Riyadh, 11451, Saudi Arabia; 14Drug Exploration and Development Chair (DEDC), Department of Pharmaceutical Chemistry, College of Pharmacy, King Saud University, Riyadh, 11451, Saudi Arabia; 15Department of Pharmacology and Therapeutics, Faculty of Veterinary Medicine, Damanhour University, Damanhour, 22511, AlBeheira, Egypt; 16The PhD Program of Translational Medicine, College of Medical Science and Technology, Taipei Medical University, Taipei, 11031, Taiwan; 17International Ph.D. Program for Translational Science, College of Medical Science and Technology, Taipei Medical University, Taipei, 11031, Taiwan; 18TMU Research Center of Cancer Translational Medicine, Taipei Medical University, Taipei, 11031, Taiwan; 19Clinical Research Center, Taipei Medical University Hospital, Taipei Medical University, Taipei, 11031, Taiwan; 20Taipei Heart Institute, Taipei Medical University, Taipei, 11031, Taiwan; 21Graduate Institute of Medical Sciences, National Defense Medical Center, Taipei, 11490, Taiwan
*These authors contributed equally to this work
Correspondence: Alexander TH Wu Tel +886 2 2697 2035
Email [email protected]
Background: Sterculia tragacantha is a medicinal plant commonly used in the western part of Nigeria, for managing diabetes mellitus. However, there is a dearth of scientific information on the antidiabetic and neuroprotective properties of the plant.
Methods: The in silico, in vitro and in vivo models were used to evaluate the antioxidants, antidiabetic, anti-inflammatory and neuroprotective potential of aqueous extract of Sterculia tragacantha leaf (AESTL) in streptozotocin (STZ)-induced diabetic rats. Thirty (30) male albino rats (155.34± 6.33 g) were intraperitoneal injected with 40 mg/kg of freshly prepared streptozotocin and were divided into 5 groups (A-E) of 6 animals each. Groups A–D were treated with 0, 150 and 300 mg/kg of AESTL, and 200 mg/kg body weight of metformin respectively, while group E serve as the normal control.
Results: The results of in vitro analysis revealed dose-dependent antioxidant activities; ABTS (IC50 = 63.03± 2.57 μg/mL), DPPH (117.49± 2.35 μg/mL), FRAP (15.19± 0.98 mmol/100g), TAC (43.38± 0.96 mg/100g), hypoglycaemic effect; α-amylase (IC50 = 77.21± 4.35 μg/mL) and α-glucosidase (IC50 = 443.25± 12.35), and anti-cholinesterase; AChE (IC50 = 113.07± 3.42 μg/mL) and BChE (IC50 = 87.50± 4.32 μg/mL) activities of AESTL. In vivo study revealed dose-dependent hypoglycemic effect and body weight improvement in rats treated with the AESTL. In addition, AESTL improved the antioxidant status and attenuated STZ-induced dysregulations of Na+-K+-ATPase, cholinesterases and neurotransmitters in the brain tissue of experimental rats. The results also demonstrated that AESTL could regulate anti-inflammatory response via inhibition of COX-2/NO signaling axis in the brain of diabetic rats. Molecular docking analysis revealed that epicatechin and procyanidin B2, the bioactive compounds from AESTL, docked well to the binding cavities of acetylcholinesterase, butyrylcholinesterase, α-amylase and α-glucosidase with binding affinities ranges between – 8.0 and – 11.4 kcal/mol, suggesting that these compounds are the bioactive component that could be responsible for the antidiabetic and neuroprotective activities of AESTL.
Conclusion: The results of the present study strongly suggested that the AESTL extract could be very useful for halting diabetes progression and its associated neuroinflammation complications.
Keywords: Sterculia tragacantha, cholinesterase, antidiabetic, neurotransmitters, oxidative stress markers
Introduction
Diabetes is a metabolic disorder characterized by inadequate insulin secretion or abnormality in the action of insulin or both.1 The most common types of diabetes mellitus (DM) included Type 2 diabetes mellitus (T2DM), type 1 diabetes mellitus (T1DM) and gestational diabetes mellitus (GDM). According to the current statistic, DM affects more than 463 million of the global population and is projected to be 700 million by 2045.2 An increase in its prevalence and enormous social and economic significance together with the severity make DM a global challenge.3
Free radical generation has been implicated in the pathogenesis of DM. High generations of free radicals coupled with depleted levels of endogenous antioxidants result in the impairment of cellular organelles, excessive lipid peroxidation, destruction of pancreatic β-cells, oxidative stress and development of diabetic condition.4 The progression of diabetes complications is characterized by the development of certain pathological conditions including nephropathy, retinopathy, and neuropathy.5 Early and adequate management of these diseases are critical to the prevention of diabetes-induced pathological impairments of nerve tissues and other vital organs.6
The pathogenesis of diabetes-induced brain injury is complex and includes combination of oxidative stress, neuroinflammation, mitochondrial dysfunction, reduction of neurotrophic factors, vascular disease, apoptosis, acetylcholinesterase (AChE) activation, neurotransmitters’ changes, and impairment of brain repair processes.7 Hyperglycemia generates excess NADH and overloads the electron transport chain, leading to the production of superoxide radicals and subsequent mitochondrial and cytosolic oxidative stress. Defects in metabolic and vascular pathways intersect with oxidative stress to induce inflammation, and onset and progression of neuronal injury.8 The association between DM and pathological changes in the central nervous system causes memory loss and affective retardation, which later increase the risk of vascular depletion of the brain.9 However, the currently available drugs for the treatment of these conditions merely alleviate the symptoms.10
Acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) are 2 major forms of cholinesterase implicated in the pathogenesis of neurological impairment, and inhibiting the activities of these enzymes has been accepted as an effective strategy for the treatment/management of neurodegenerative diseases.11 Recently, studies showed that the activity of BChE is significantly higher in degenerative disease than in the plaques of age-related non-demented brains.11 Most of the approved AChE inhibitors do not alter the activity of BChE, which is very critical when managing those degenerative diseases. Therefore, the use of antioxidants to counter the cellular oxidative stress within the nervous system has been suggested as a potential therapeutic strategy for neurological disorders.10 The α-glucosidase and α-amylase are important enzymes of the gastrointestinal tract and function to digest complex carbohydrates, thus contributing to postprandial hyperglycemia in T2DM.12 The inhibition of α-amylase and α-glucosidase activities have been proposed as therapeutic strategy for the control of hyperglycemia.6
In developing and some developed countries, medicinal plants are commonly used as source of therapeutic agent to meet the primary health care need, and have received significant scientific validation of their activities against several diseases.13–16 The use of herbal medicines is now common across the world due to their normoglycemic potentials with minimal side effects. These medicinal plants contain various phytochemicals with antioxidants and other biological activities for the treatment and management of many diseases.15 The WHO verdict also suggested the need to grow and access better pharmacological agents that will improve the antioxidant and normal glycemic status, ameliorate the destruction of beta cells, and restore the diabetes-associated complications.17 Hence, recent efforts have focused on plant phytochemicals as natural sources of effective AChE, BChE, α-amylase and α-glucosidase inhibitors with little or no side effects, which could serve as a dietary intervention in the management of diabetes-induced complications. One example of the curative plant used in the prevention and management of diabetes and its complications is Sterculia tragacanth.
S. tragacantha belongs to the family Sterculiaceae. It is commonly known as Uhobo and Abalo in the Eastern and Western regions of Nigeria, respectively, where it is widely used for the treatment of pain, diarrhoea, edema, gout, whitlow, diabetes, cold and infectious diseases.18 Pharmacologically, the plant has been reported for antioxidants, antinociceptive, anti-inflammatory,19–22 neuroprotective,23 mosquitocidal,24 antimicrobial25 and several other activities.26 However, there is a dearth of information on the hypoglycemic and neuroprotective properties of the plant. The present study demonstrated that aqueous extract of Sterculia tragacantha leaf (AESTL) exhibited in vitro antioxidant activities and inhibited the activities of AChE, BChE, α-amylase and α-glucosidase in a dose-dependent fashion. The extract exhibited hypoglycaemic effect in an animal model and attenuated diabetes-induced oxidative stress and neuronal impairment in streptozotocin-intoxicated rats.
Materials and Methods
Plant Collections and Extractions
S. tragacantha leaves were obtained from the Osogbo Central Market, Osun State, Nigeria. The leaves were authenticated at the Forestry Research Institute of Nigeria (FRIN), Ibadan, Oyo State, Nigeria, where the voucher number (FHI: 112856) was deposited. The leaves were air-dried at 25° C for two weeks and pulverized using a mechanical blender (Type VIII machine of Christy and Norris limited). Two hundred grams (200 g) of the powered sample was extracted in 2.5 L distilled water with constant shaking for 48 h and then filtered. Thereafter, the resulting filtrate was lyophilized using freeze dryer (Massion, UK) and the dried extract (AESTL) was stored in air-tight container.
Analysis of Phytochemical Compositions
The total flavonoid content of the extract was estimated using a spectrophotometer based on the formation of flavonoid–aluminium complex that absorb maximally at 415 nm.27 Total phenol was estimated using Folin–Ciocalteu reagent protocol,28 while the total terpenoid content was estimated as described by Ghorai et al.29
Analysis of in vitro Antioxidant Activities
DPPH Radical Scavenging Activity
The DPPH radical scavenging activity of AESTL was determined based on the ability to scavenge the stable DPPH free radical according to the procedure described by Tsado et al.30 Briefly, 0.5 mL of the DPPH (50 mg/mL) diluted in 4.5 mL of methanol) was mixed with 0.1 mL of AESTL (10–100mg/mL). The reaction mixture was incubated for 45 min in the dark. The decrease in absorbance was measured at 517 nm against a blank.
FRAP (Ferric Reducing-Antioxidant Power) Assay
The FRAP was assessed based on the reduction of a ferric tripyridyl triazine (TPTZ) complex to its ferrous, coloured form in the presence of antioxidant.31 Briefly, 3 mL of FRAP reagent was mixed with 100 μL of the extract and incubated for 30 min at 37°C; the reduction of Fe3+ complex to the ferrous form was monitored by measuring the change absorbance at 593 nm. The FRAP values were expressed as mmol of Fe2+ equivalents per kg of the extract.
Total Antioxidant Capacity (TAC)
The total antioxidant capacity of the extract was evaluated by the phosphomolybdenum method according to the procedure described by Kasangana et al.32 A 0.3 mL of extract (1 mg/mL extract in methanol) was incubated with 2.7 mL of phosphomolybdenum reagent (0.6 M sulfuric acid, 28 mM sodium phosphate and 4 mM ammonium molybdate) at 95°C for 90 min. The reaction was monitored using a spectrophotometer at 695 nm. The total antioxidant activity was expressed as the number of grams equivalent of ascorbic acid.
Analysis of in vitro Hypoglycemic Activities
α-Amylase Inhibition Assay
The α-amylase inhibitory activity of the extract was assayed following the protocol of Worthington.33 An aliquot of AESTL (20–100 μL) and 500 μL of 0.02 M sodium phosphate buffer (pH 6.9 with 0.006 M NaCl) containing α-amylase (0.5 mg/mL) were incubated at 25 °C for 10 min. One (1%) of starch solution (pH 6.9 with 0.006 M NaCl) was added and the reaction mixtures was incubated at 25 °C for 10 min. The reaction was stopped by the addition of dinitrosalicylic acid colour reagent followed by incubation in a boiling water bath for 5 min. The absorbance was recorded at 540 nm. The α-amylase inhibitory activity was expressed as percentage inhibition and the concentration of extract causing 50% inhibition of enzyme’s activity (IC50) was calculated.
α-Glucosidase Inhibition Assay
The α-Glucosidase inhibitory activities of the extract was assayed following the protocol of Apostolidis et al.34 Appropriate dilution of the extract and 100 μL of α-glucosidase solution (1.0 U/mL) in 0.1 M phosphate buffer (pH 6.9) were incubated for 10 min at 25 °C. A solution of 5 mM p-nitrophenyl-α-
In vivo Antidiabetic Study
Experimental Design
Male albino rats (155.34±6.33 g) were obtained from Suramos Farm Ltd, Ikere-Ekiti, Ekiti-State, Nigeria. The rats were fed with standard pelletized feeds (Ladokun Feeds, Nigeria Ltd) and provided with water ad libitum. All animal experiments were conducted in adherent to the regulations by the Ethics Committee on Animals Use of Afe Babalola University (Protocol approval number: AB235B). Diabetes was induced in the experimental animals after overnight fasting by a single intraperitoneal injection (i.p.) of 40 mg/kg of freshly prepared streptozotocin (STZ). Glucose (50%) was given to the animals to alleviate mortality rates. Fasting blood sugar (FBS) levels were measured using a glucometer (Finetest, Roche Diagnostics) after 48 h and rats having FBS levels more than 250 mg/dL were selected and used for the present study. Thirty (30) hyperglycemic (FBS >250 mg/dL) rats were divided into 5 groups (A–E) of 6 animals each. Groups A–D were treated with 0, 150 and 300 mg/kg body weight of AESTL, and 200 mg/kg body weight of metformin respectively, while group E serve as the normal control. The therapeutic doses used for this study was selected based on the pilot toxicity studies on S. tragacantha which indicated its safety profile at high doses of up to 3000 mg/kg bw in rats.20,35 The fasting blood sugar (FBS) and body weight were monitored throughout the study period. The percentage hypoglycaemic effect was calculated as follows: FBS ((Vehicle control - Treatment)/Vehicle control) × 100.
Sample Collection and Processing
On the 22nd day of the experiment, the animals were fasted overnight but had free access to water. Thereafter, the animals were anaesthetized using diethyl ether and were humanly sacrificed. The brain samples were harvested into plain bottles, and homogenized using 0.1 M phosphate buffer solution. The homogenates were further centrifuged for 10 minutes at 5000 rpm after which the resulting supernatants were processed immediately for biochemical analysis.
Analysis of Biochemical Parameters
Enzymatic and Non-Enzymatic Biomarkers of Oxidative Stress in the Brain Tissue
The activity of superoxide dismutase (SOD) in the brain homogenate was determined as described by Kakkar et al.36 The catalase activity (CAT) was determined using the hydrogen peroxide (H2O2) reaction protocol as described by Sinha.37 The activity of glutathione peroxidase (GPx) was determined by using the Ellman’s reagent (19.8 mg, DTNB in 100 mL 0.1% sodium nitrate) protocols as described by Rotruck et al,38 while the reduced glutathione (GSH) level was determined by a modified colorimetric method.39
Analysis of Lipid Peroxidation
The estimation of lipid peroxidation (LPO) in the brain homogenate was determined spectrophotometrically by measuring the thiobarbituric acid reactive substances (TBARS) as described by Shagirtha et al.40 Briefly, 0.5 mL of the brain homogenate sample was treated with 2 mL of TBA-trichloroacetic acid reagent (0.37% TBA, 0.25 N HCl, and TCA 15% in ratio 1:1:1). The reaction mixture was incubated in water bath for 15 min, then cooled and centrifuged for 10 min at room temperature. Change in absorbance against a reference blank was monitored at 535 nm.
Analysis of Inflammatory Biomarkers
The cyclooxygenase-2 activity (COX-2) in the brain tissue was estimated using the methods of Shimizu et al.41 Nitric oxide level was measured by calculating nitric oxide content in a reaction medium consisting of 0.4 mL of working reagent (weighed 2 g vanadium chloride in 400 mL 5% hydrochloric acid), 0.2 mL of 0.1% N-(l-naphthyl) ethylenediamine dihydrochloride then 0.2 mL of 2 g sulfanilamide in 5% of hydrochloric acid. The nitrite level, which has the same estimative of NO level, was measured using a spectrophotometer at 540 nm, after 60 minutes of incubation at 37 °C. The levels of nitrite and nitrate on the brain were computed in nanomole NO.42
Analysis of Cholinesterase (ChEs) and Neurotransmitters
Acetylcholinesterase (AChE) activity was assessed in the brain using acetylthiocholine iodide as a substrate as described by Shagirtha et al,40 while the activities of butyrylcholinesterase (BChE) in the brain were analyzed following a method described by Jońca et al.43 The levels of dopamine and serotonin were assayed based on the method of Pagel et al.,44 while the levels of the epinephrine and non-epinephrine were determined using the ELISA kits (Aviva Systems Biology, Corp., USA) according to the manufacturer protocols.
Analysis of Na+- K+-ATPase Activity
The activity of Na+-K+ATPase was estimated by a modified colorimetric method described previously.45 In brief, 0.2 mL of brain tissue homogenate was added to the mixture containing 1 mL 184 mM Tris–HCl buffer (pH 7.5), 0.2 mL 50 mM MgSO4, 0.2 mL 50 mM KCl, 0.2 mL 600 mM NaCl, 0.2 mL (1 mM) EDTA and 0.2 mL 10 mM ATP and incubated for 15 min at 37 °C. The reaction mixture was arrested by the addition of 1 mL ice-cold 10% TCA. The amount of Pi (inorganic phosphate) liberated was estimated in protein-free supernatant.
Molecular Docking Studies of Ligand Receptor Interaction
The bioactive compound was characterized, and two compounds (procyanidin B2 and epicatechin) were identified as the major bioactive components of the extract. The crystal structures of the receptors – AChE (PDB:1H23), BChE (PDB:5LKR), alpha-amylase, alpha-glucosidase (PDB:3WY4) – in PDB file format were obtained from the Protein Data Bank, while the three-dimensional (3D) structure of the ligands (procyanidin B2 and epicatechin) was obtained in mol2 format using the Avogadro molecular builder and visualization tool version 1.XX.46 The mol2 were converted to PDB files using the PyMOL Molecular Graphics System, version 1.2r3pre. All PDB files were subsequently converted to PDBQT files using AutoDock Vina (version 0.8, Scripps Research Institute, La Jolla, CA, USA).47 The receptors were prepared for docking by removing water molecules, adding polar hydrogen atoms and Kolman charges.48,49 Docking was conducted using AutoDock Vina as described in previous studies,48,50,51 and results were visualized using the PyMOL, Discovery studio visualizer version 19.1.0.18287 (BIOVIA, San Diego, CA, USA).52
Statistical Analysis
All analyses were conducted in triplicate and analyzed using a GraphPad prism version 8.0. One-way analysis of variance (ANOVA) was used to compare the significant differences between treatment groups. Results were considered significant at *p<0.05, **p<0.01 or ***p<0.001 and are presented mean ± standard error of the mean.
Results
In vitro Antioxidants and Anti-Hyperglycemic Effects of AESTL
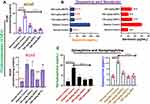
We used different in vitro approaches to evaluate the antioxidants’ effect of AESTL. The results revealed that AESTL contains total flavonoids, phenols and terpenes contents of 107.90±1.04, 12.12±0.03, and 31.29±0.94 mg/100g, respectively (Table 1). The result revealed that both the standard drugs and AESTL scavenged the free radicals in a concentration-dependent manner (Figure 1). The extract demonstrated dose-dependent ABTS and DPPH radicals scavenging effects with IC50 of 63.03±2.57 and 117.49±2.35 μg/mL, respectively. Furthermore, extract exhibited FRAP and TAC values of 15.19±0.98 mmol/100g and 43.38±0.96 mg/100g, respectively. However, as expected, the activities demonstrated by AESTL was lower than the standard drugs. We also evaluated the anti-hyperglycemic effects of the extract using the α- amylase and α- glucosidase assays, and found that AESTL exhibited dose-dependent inhibitions of α- amylase and α-glucosidase with IC50 values of 77.21±4.35 and 443.25±12.35, respectively. Altogether, the results of the study strongly suggested the antioxidants and hypoglycemic effects of the plant extract and hence form the basis for the in vivo evaluation of it efficacy.
 |
Table 1 Phytochemical Compositions and in vitro Antioxidants and Hypoglycemic Effects of Aqueous Extract of Sterculia tragacantha Leaf (AESTL) |
In vitro Cholinesterase Inhibitory Activities of AESTL
The AChE and BChE inhibitory potential of AESTL were estimated and the result revealed that AESTL significantly inhibited the activities of AChE (Figure 2A) and BChE (Figure 2B) in a dose-dependent manner, having IC50 values of 113.07±3.42 and 87.50±4.32 μg/mL for AChE and BChE, respectively. However, the standard drug (nicotinic acid) demonstrated higher activities and hence lower IC50 against the enzymes.
AESTL Demonstrated Hypoglycemic Effects in Streptozotocin-Induced Diabetic Rats
Injection of streptozotocin caused significant elevation of the fasting blood sugar (Table 2) and loss of body weight (Table 3) of the experimental rats. Interestingly, treatment with AESTL caused dose dependent and progressive decreases in the FBS (p<0.0001), having percentage hypoglycemic effects of 68.27±2.34 and 71.92±2.45 at 150 and 300 mg/kg BW, respectively. The in vivo hypoglycemic activities of the plant extract is comparable with the standard drug, metformin (72.59±3.45%) (Table 2). Furthermore, treatment with the plant extract caused significant improvement of the body weight (p<0.001) when compared with the vehicle control (Table 3).
 |
Table 2 Hypoglycaemic Effect of the Aqueous Extract of Sterculia tragacantha Leaf (AESTL) in Streptozotocin-Induced Diabetic Rats |
 |
Table 3 Effect of the Aqueous Extract of Sterculia tragacantha Leaf (AESTL) on Bodyweight Changes in Streptozotocin-Induced Diabetic Rats |
AESTL Attenuates the Dysregulation of Cholinesterase and Neurotransmitters in Brain Tissue of Streptozotocin-Induced Diabetic Rats
The effect of AESTL on the levels of cholinesterases (AChE and BChE) and neurotransmitters (dopamine, serotonine, epinephrine and norepinephrine) in the brain of STZ-induced diabetic rats is shown in Figure 3. Diabetic untreated rats demonstrated a significant increased levels of AChE and BChE (Figure 3A), dopamine and serotonin (Figure 3B), and epinephrine and norepinephrine (Figure 3C) when compared with the rats in the normal control group. However, treatment with AESTL significantly reversed the levels of AChE, BChE, dopamine, serotonins, epinephrine and norepinephrine (Figure 2B) towards normalization compared to untreated diabetic rats. Collectively, our results demonstrated that AESTL attenuated the dysregulation of cholinesterase and neurotransmitters in brain tissue of STZ-induced diabetic rats.
AESTL Regulates Na+-K+-ATPase Anti-Inflammatory Response via Inhibition of COX-2/NO Signaling Axis in Brain of Streptozotocin-Induced Diabetic Rats
The role of nitric oxide synthase and cyclooxygenase 2 in inflammation and pathology of neurodegenerative diseases has been well described. We evaluated the protective effect of AESTL on STZ-induced dysregulation of Na+-K+-ATPase and inflammation makers (Figure 4). Our results revealed that the activities of cyclooxygenase-2 (COX-2), (Figure 4A), Na+-K+-ATPase (Figure 4B) and the level of NO (Figure 4C) in the brain of STZ-induced diabetic untreated rats were significantly (p < 0.05) elevated when compared with the normal control. Treatment with AESTL significantly (p < 0.05) reduced the levels of these biomarkers when compared with the diabetic untreated rats.
AESTL Attenuates Oxidative Stress in Brain of Streptozotocin-Induced Diabetic Rats
Oxidative stress has been implicated in the pathogenesis of diabetes and neurodegenerative diseases. We evaluated the protective effect of AESTL in STZ-induced oxidative stress in brain tissue of diabetic rats. Our results revealed that AESTL demonstrated dose-dependent antioxidant activities by significantly (p<0.05) improving the activities of enzymatic antioxidants including the SOD (Figure 5A), GPx (Figure 5B) and CAT (Figure 5C), and level of non-enzymatic antioxidant (GSH) (Figure 5D), and lipid peroxidation (Figure 5E) in the brain of diabetic rats when compared with the diabetic untreated rats. Interestingly, the antioxidant activities demonstrated by AESTL at 300 mg/kg are significantly higher than the antioxidant activities demonstrated by the standard drug (metformin). Collectively, our results demonstrated that AESTL exhibited significant antioxidant activities in in vitro as well as in vivo in the brain of STZ-intoxicated rats.
Epicatechin and Procyanidin B2, the Bioactive Compounds From AESTL, Have the Molecular Properties to Interact Efficiently with Cholinesterase, α-Amylase and α-Glucosidase
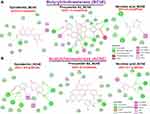
We evaluated the ligand–receptor interactions between two bioactive compounds from AESTL (epicatechin and procyanidin B2) and cholinesterases, α-amylase and α-glucosidase using the molecular docking studies. Interestingly, our results revealed that epicatechin and procyanidin B2 docked well to the binding cavities of acetylcholinesterase, butyrylcholinesterase, α-amylase and α-glucosidase with respective binding affinities ranges between –8.0 and –11.4 kcal/mol (Figures 6 and 7). Comparatively, procyanidin B2 demonstrated higher affinities for BChE, α-amylase and α-glucosidase than does the epicatechin. Both epicatechin and procyanidin B2 are bound to the binding pocket of the receptors by several conventional H-bonds, alkyl and multiple π-interactions. Furthermore, several van der Waals forces were found around the ligand backbones with the respective amino acid residues of receptors binding pockets. Compared to the epicatechin and procyanidin B2, the standard drugs acarbose and nicotinic acid demonstrated lower binding affinities to their respective receptors (Figures 6 and 7). Altogether, our molecular docking analysis revealed that both epicatechin and procyanidin B2 have molecular properties to interact efficiently with cholinesterase, α-amylase and α-glucosidase, suggesting that these compounds are the bioactive components that could be responsible for the biological activities of AESTL reported in this study.
Discussion
Medicinal plants are important therapeutic agents used in the management of several diseases including diabetes. Herein, Sterculia tragacantha, one of the commonly used herbal remedies in the management of diabetes, was investigated for antidiabetic, antioxidants, anti-inflammatory and neuroprotective potential in streptozotocin-induced diabetic rats. In addition, we demonstrated that two bioactive compounds from the plant extract, epicatechin and procyanidin B2, has molecular properties to interact with molecular targets, suggesting that these compounds are the bioactive component that could be responsible for the antidiabetic and neuroprotective activities of AESTL.
Free radicals play an important etiologic role in the pathogenesis of metabolic and neurodegenerative diseases.5 Therefore, antioxidants serve as an important agent for the prevention of free radical-induced oxidative stress and tissue damage.15 The reducing power due to hydrogen donating ability has been attributed to the biological activities of medicinal plants.5 Interestingly, the results of the present study revealed that AESTL induced the scavenging of free radicals and improved the antioxidants status of glycemic and neuronal impaired rats, thus serving as a potential therapeutic agent in the management of oxidative stress and associated disorders.
Impaired cholinesterase activities and neuronal dysfunction are diabetes-mediated complications.7 The increased inhibition of BChE and AChE activities following treatment with AESTL extract could be attributed to the significant amounts of its phytochemical composition, particularly the flavonoids and phenols (Table 1), which have been reported to exhibit cholinesterase inhibition activities.53 Lunke’s et al54 reported that serum cholinesterase activity is highly over expressed in diabetes and associated pathologies. Therefore, results of the present study strongly suggested that the AESTL extract could be very useful for halting diabetes progression and its associated neuronal complications. In line with the findings from the present study, previous studies had proved that pharmacological inhibition of acetylcholinesterase represents an important therapeutic strategy for the treatment of various metabolic and neurodegenerative diseases.11,55
α-Amylase and α-glucosidase are important enzymes of the gastrointestinal tract and function to digest complex carbohydrates, thus contributing to postprandial hyperglycemia in type 2 diabetes.12 The inhibition of α-amylase and α-glucosidase activities has been suggested as an effective strategy for the control of hyperglycemic conditions in type 2 diabetes mellitus6; hence, the search for pharmacologically active phytoconstituents that could inhibit the activities of these enzymes is of increasing scientific interest.56 Therefore, the inhibition of α-glucosidase and α-amylase by AESTL extract is an appraisable property. This would facilitate the retardation of elevation of blood sugar and suppress postprandial hyperglycemia by decreasing the rate of blood sugar absorption.56
The increased blood glucose levels in diabetic untreated rats could be attributed to the oxidative status induced by the STZ treatment, which is in line with the results observed in previous studies.57,58 Similarly, lowered level of the bodyweight observed in untreated diabetic rats corroborates with the finding of Ajiboye et al.23 This loss of bodyweight could be attributed to the increased catabolism of fats and proteins, leading to muscle reduction and weight loss. Importantly, our results revealed hypoglycaemia and improvement of body weight in AESTL-treated rats, suggesting a metabolic restoration and antidiabetic effect of the plant.
The in vivo inhibition of AChE and BChE in the brain tissue of rats treated with the AESTL corroborates with our in vitro findings and thus suggests that the plant has potential therapeutic importance for the management of neuronal impairment in diabetes mellitus. Norepinephrine (NE) and epinephrine (E) are very similar neurotransmitters and hormones associated with metabolic and neurodegenerative disorders.59 The high levels of NE and E in diabetic untreated rats could be attributed to their accumulation due to the inhibition of presynaptic release of NE and/or increased reuptake of released NE andE60 in the brain tissue of STZ-induced diabetic rats. The decreased levels of NE and E following treatment with AESTL could be due to either decreased synthesis or an increased rate of degradation.
Dopamine and serotonin (5-HT) are important neurotransmitters that are widely distributed in the central nervous system and have been explored as a biomarker in metabolic and neurological disorders.61 Results of the present study revealed significantly high levels of dopamine and serotonin (5-HT) in brain tissue of STZ-intoxicated rats, which is in agreement with the result of the previous study.23 The reversal effect of AESTL on the tissue levels of neurotransmitters suggests the preservation of neuronal integrity, a finding which is in line with previous pharmacological evaluations of some natural products.23,62
Hyperglycaemic-induced alteration of cyclooxygenase (COX) pathway activity with subsequent impaired production and function of prostaglandins (PGs) is one mechanism that is associated with the pathogenesis of diabetic neuropathy.63 COX-2, the inducible COX isoform, is upregulated in a variety of pathophysiological conditions including diabetes. In addition, the upregulation of COX-2 has tissue-specific consequences and is associated with the activation of downstream inflammatory reactions.63,64 Alterations in Na+-K+-ATPase activity also represent an important mechanism of diabetics-induced neurotoxicity.65 In the same vein, nitric oxide, as a neurotransmitter, is reported to mediate synaptic activity, neural plasticity, and memory function.66 Interestingly, we found that treatment with AESTL reverses the overexpression of Na+-K+-ATPase, COX-2 and NO to the baseline value, hence suggesting the anti-inflammatory and therapeutic role of AESTL in the maintenance of ion gradients across biological membranes and thus confer significant protection to the brain by stabilizing the functional integrity of the membrane.67
Antioxidant enzymes such as SOD, catalase, glutathione peroxidase as well as glutathione form the defense system, which usually protects the cell against oxidative damage. The depleted levels of these antioxidants in diabetic untreated rats could be attributed to the STZ-mediated generation of free radicals in the brain of diabetic rats, which results in oxidative damages to membrane lipid, protein and decreased levels of the endogenous antioxidants.68 It is noteworthy that treatment with AESTL significantly reversed the depleted levels of GSH, SOD, catalase and GPx which is supported by earlier findings where natural antioxidants are used as a remedy in oxidative stressed rats.69 Cytotoxic agents such as malondialdehyde become stable and easily diffused in secondary injuries caused by oxidative stress.70 The increased LPO in STZ-intoxicated rats corroborates the research carried out by Oyinloye et al,71 where an elevated generation of reactive free radicals when exposed to toxic compounds resulted in increased LPO that eventually compromised cellular integrity of the neurons. However, the inhibition of LPO by AESTL further strengthened its antioxidants’ capacity in STZ-intoxicated rats. Although the present study is limited by the lack of histopathological examination of brain tissue, the biochemical analysis of the tissue homogenate revealed the ability of AESTL to attenuate diabetic-mediated oxidative stress, inflammation and neuronal impairment in STZ-induced diabetic rats.
Molecular docking simulation of the interaction between a receptor and ligand has been widely used in elucidating the binding affinities between a target and a drug candidate and for unveiling the behavior of a drug molecule within the binding cavities of the targets.72–74 Consequently, we conducted a molecular docking study to elucidate the potential druggability of cholinesterase α-amylase and α-glucosidase by two bioactive compounds from AESTL (epicatechin and procyanidin B2). Interestingly, our results revealed that epicatechin and procyanidin B2 docked well to the binding cavities of acetylcholinesterase, butyrylcholinesterase, α-amylase and α-glucosidase with respective binding affinities ranges between –8.0 and –11.4 kcal/mol (Figures 6 and 7). Comparatively, procyanidin B2 demonstrated higher affinities for BChE, α-amylase and α-glucosidase than does the epicatechin. Both epicatechin and procyanidin B2 are bound to the binding pocket of the receptors by several conventional H-bonds, alkyl and multiple π-interactions. Furthermore, several van der Waals forces were found around the ligand backbones with the respective amino acid residues of receptors binding pockets would create strong cohesive environments, that stabilizes the receptor–ligand complexes.75–77 Compared to epicatechin and procyanidin B2, the standard drugs acarbose and nicotinic acid demonstrated lower binding affinities to their respective receptors (Figures 6 and 7). Altogether, our molecular docking analysis revealed that both epicatechin and procyanidin B2 has molecular properties to interact efficiently with cholinesterase, α-amylase and α-glucosidase, suggesting that these compounds are the bioactive components that could be responsible for the biological activities of AESTL reported in this study. Another limitation of the present study, therefore, lies in the individual isolation and biological evaluation of these compounds against STZ-induced oxidative and neuronal impairment. This is however under investigation in our laboratory.
Conclusion
Conclusively, the results of the present study revealed that Sterculia tragacantha Lindl (AESTL) exhibited therapeutic efficacy against STZ-mediated oxidative stress, inflammation and neuronal impairment thus represents a source of bioactive agents for the management of diabetes and associated complications. Interestingly, epicatechin and procyanidin B2, the bioactive compounds from AESTL, have the molecular properties to interact with high affinities to the binding cavity of acetylcholinesterase, butyrylcholinesterase, α-amylase and α-glucosidase, suggesting that these compounds are the bioactive components that could be responsible for the antidiabetic and neuroprotective activities of AESTL.
Institutional Review Board Statement
Institutional review board approval is not required for original research with animal studies.
Informed Consent Statement
This study did not require nor include human participants or tissues.
Acknowledgment
The authors would like to extend their sincere appreciation to Taif University Researchers Supporting Project number (TURSP-2020/309), Taif University, Taif, Saudi Arabia.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article was submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
Alexander TH Wu is funded by the Ministry of Education, Taipei Medical University (DP2-110-21121-03-C-09 and DP2-110-21121-01-H-03-03).
Disclosure
The authors declare no conflicts of interest for this work.
References
1. Bilous R, Donnelly R, Idris I. Handbook of Diabetes. John Wiley & Sons; 2021.
2. Saeedi P, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas. Diabetes Res Clin Pract. 2019;157:107843. doi:10.1016/j.diabres.2019.107843
3. Deng Y, Li N, Wu Y, et al. Global, regional, and national burden of diabetes-related chronic kidney disease from 1990 to 2019. Front Endocrinol (Lausanne). 2021;12:809. doi:10.3389/fendo.2021.672350
4. Darenskaya M, Kolesnikova L, Kolesnikov S. Oxidative stress: pathogenetic role in diabetes mellitus and its complications and therapeutic approaches to correction. Bull Exp Biol Med. 2021;171(2):179–189. doi:10.1007/s10517-021-05191-7
5. Park S, Park S-Y. Can antioxidants be effective therapeutics for type 2 diabetes? Yeungnam Univ J Med. 2021;38(2):83. doi:10.12701/yujm.2020.00563
6. Agada R, Usman WA, Shehu S, Thagariki D. In vitro and in vivo inhibitory effects of Carica papaya seed on α-amylase and α-glucosidase enzymes. Heliyon. 2020;6(3):e03618. doi:10.1016/j.heliyon.2020.e03618
7. Hamed SA. Brain injury with diabetes mellitus: evidence, mechanisms and treatment implications. Expert Rev Clin Pharmacol. 2017;10(4):409–428. doi:10.1080/17512433.2017.1293521
8. Jin HY, Moon -S-S, Calcutt NA. Lost in translation? Measuring diabetic neuropathy in humans and animals. Diabetes Metab J. 2021;45(1):27–42. doi:10.4093/dmj.2020.0216
9. Azmi S, Alam U, Burgess J, Malik RA. State-of-the-art pharmacotherapy for diabetic neuropathy. Expert Opin Pharmacother. 2021;22(1):55–68. doi:10.1080/14656566.2020.1812578
10. Pohl F, Kong Thoo Lin P. The potential use of plant natural products and plant extracts with antioxidant properties for the prevention/treatment of neurodegenerative diseases: in vitro, in vivo and clinical trials. Molecules. 2018;23(12):3283. doi:10.3390/molecules23123283
11. Orhan I, Aslan S, Kartal M, Şener B, Başer KHC. Inhibitory effect of Turkish Rosmarinus officinalis L. on acetylcholinesterase and butyrylcholinesterase enzymes. Food Chem. 2008;108(2):663–668. doi:10.1016/j.foodchem.2007.11.023
12. Shim Y-J, Doo H-K, Ahn S-Y, et al. Inhibitory effect of aqueous extract from the gall of Rhus chinensis on alpha-glucosidase activity and postprandial blood glucose. J Ethnopharmacol. 2003;85(2–3):283–287. doi:10.1016/S0378-8741(02)00370-7
13. Bashir L, Shittu O, Sani S, Busari M, Adeniyi K. African natural products with potential antitrypanosomal properties: a review. Int J Biochem Res Rev. 2015;7(2):45–79. doi:10.9734/IJBCRR/2015/16039
14. Lawal B, Shittu OK, Kabiru AY, et al. Potential antimalarials from African natural products: a review. J Intercult Ethnopharmacol. 2015;4(4):318. doi:10.5455/jice.20150928102856
15. Lawal B, Shittu OK, Oibiokpa FI, Berinyuy EB, Mohammed H. African natural products with potential antioxidants and hepatoprotectives properties: a review. Clin Phytosci. 2017;2(1):1–66.
16. Ndako M, Jigam AA, Kabiru AY, Umar SI, Lawal B. Polar extracts from Gymnosporia senegalensis (syn. Maytenus senegalensis) root bark, its effects on nociception, edema, and malarial infection. Phytomed Plus. 2021;1(4):100113. doi:10.1016/j.phyplu.2021.100113
17. Zdrowia ŚO; Organization WH. World Report on Knowledge for Better Health: Strengthening Health Systems. World Health Organization; 2004.
18. Sadeer NB, Llorent-Martínez EJ, Bene K, et al. Chemical profiling, antioxidant, enzyme inhibitory and molecular modelling studies on the leaves and stem bark extracts of three African medicinal plants. J Pharm Biomed Anal. 2019;174:19–33. doi:10.1016/j.jpba.2019.05.041
19. Udegbunam R, Asuzu U, Kene R, Udegbunam S, Nwaehujor C. Anti-nociceptive, anti-inflammatory and anti-oxidant effects of the methanol leaf extract of Sterculia tragacantha Lindl. J Pharmacol Toxicol. 2011;6(5):516–524. doi:10.3923/jpt.2011.516.524
20. Mogbojuri OM, Adedapo AA, Abatan MO. Phytochemical screening, safety evaluation, anti-inflammatory and analgesic studies of the leaf extracts of Sterculia tragacantha. J Complement Integr Med. 2016;13(3):221–228. doi:10.1515/jcim-2015-0114
21. Ajiboye BO, Oyinloye BE, Awurum JC, Onikanni SA, Adefolalu A, Oluba OM. Protective role of Sterculia tragacantha aqueous extract on pancreatic gene expression and oxidative stress parameters in streptozotocin-induced diabetic rats. J Complement Integr Med. 2021. doi:10.1515/jcim-2021-0020
22. Udegbunam RI, Asuzu UI, Kene RO, Oyiga CT, Udegbunam SO, Nwaehujor CC. Anti-inflammatory and anti-oxidant effects of Sterculia tragacantha fractions in mice. Afr J Biotechnol. 2013;12:6.
23. Ajiboye BO, Oyinloye BE, Essien PE, Onikanni SA, Ojo OA, Kappo AP. Ameliorative potential of Sterculia tragacantha aqueous extract on renal gene expression and biochemical parameters in streptozotocin-induced diabetic rats. J Pharm Invest. 2021;51(1):103–113. doi:10.1007/s40005-020-00506-8
24. Jerome SZ, Benson BB, Foungoye OA, M-bj A, Yves-Alain B. Analysis by GC (Ir), GC/MS and mosquito repellent effect of essential oils against Anopheles gambiae: case of stem bark of Sterculia tragacantha Lindl (Sterculiaceae) from Côte d'Ivoire. J Med Plants Res. 2021;15(7):309–320. doi:10.5897/JMPR2021.7132
25. Palve A, Shetty P, Pimpliskar M, Jadhav R. Study on antibacterial and antifungal activities of Sterculia lychnophora extracts [J]. Int J Curr Microbiol Appl Sci. 2015;4(11):336–341.
26. Prista LN, Alves AC, Da Costa M. A pharmacological study of Sterculia tragacantha. Garcia de Orta. 1960;8:67–80.
27. Sofowora A. Research on medicinal plants and traditional medicine in Africa. J Altern Complement Med. 1996;2(3):365–372. doi:10.1089/acm.1996.2.365
28. Hagerman AE, Riedl KM, Jones Ga, et al. High molecular weight plant polyphenolics (Tannins) as biological antioxidants. J Agric Food Chem. 1998;46(5):1887–1892. doi:10.1021/jf970975b
29. Ghorai N, Chakraborty S, Gucchait S, Saha SK, Biswas S. Estimation of Total Terpenoids Concentration in Plant Tissues Using a Monoterpene. Linalool as standard reagent; 2012.
30. Tsado N, Lawal B, Ossa P, et al. Antioxidants and antimicrobial activities of methanol extract of Newbouldia laevis and Crateva adansonii. J Pharm Allied Health Sci. 2016;6:14–19. doi:10.3923/jpahs.2016.14.19
31. Hajimahmoodi M. Antioxidant activity, reducing power and total phenolic content of Iranian olive cultivar. 第十四届世界食品科技大会. 2008;8:676.
32. Kasangana PB, Haddad PS, Stevanovic T. Study of polyphenol content and antioxidant capacity of Myrianthus arboreus (Cecropiaceae) root bark extracts. Antioxidants. 2015;4(2):410–426. doi:10.3390/antiox4020410
33. Worthington K. Alpha Amylase Worthington Enzyme Manual. Lakewood, NJ: Worthington Biochemical Corporation[Google Scholar]; 1993:36–41.
34. Apostolidis E, Y-i K, Shetty K. Inhibitory potential of herb, fruit, and fungal-enriched cheese against key enzymes linked to type 2 diabetes and hypertension. Innovat Food Sci Emerg Technol. 2007;8(1):46–54. doi:10.1016/j.ifset.2006.06.001
35. Abatan M, Mogbojuri O. Evaluation of the anti-inflammatory and analgesic activities of leaf-extracts of Sterculia tragacantha in rats. FASEB J. 2015;29(S1):
36. Kakkar P, Das B, Viswanathan P. A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys. 1984;21(2):130–132.
37. Sinha AK. Colorimetric assay of catalase. Anal Biochem. 1972;47(2):389–394. doi:10.1016/0003-2697(72)90132-7
38. Rotruck JT, Pope AL, Ganther HE, Swanson A, Hafeman DG, Hoekstra W. Selenium: biochemical role as a component of glutathione peroxidase. Science. 1973;179(4073):588–590. doi:10.1126/science.179.4073.588
39. Kum-Tatt L, Tan I-K. A new colorimetric method for the determination of glutathione in erythrocytes. Clinica Chimica Acta. 1974;53(2):153–161. doi:10.1016/0009-8981(74)90093-X
40. Shagirtha K, Bashir N, MiltonPrabu S. Neuroprotective efficacy of hesperetin against cadmium induced oxidative stress in the brain of rats. Toxicol Ind Health. 2017;33(5):454–468. doi:10.1177/0748233716665301
41. Shimizu T, Kondo K, Hayaishi O. Role of prostaglandin endoperoxides in the serum thiobarbituric acid reaction. Arch Biochem Biophys. 1981;206(2):271–276. doi:10.1016/0003-9861(81)90091-6
42. Miranda HF, Puig MM, Prieto JC, Pinardi G. Synergism between paracetamol and nonsteroidal anti-inflammatory drugs in experimental acute pain. Pain. 2006;121(1–2):22–28. doi:10.1016/j.pain.2005.11.012
43. Jońca J, Żuk M, Wasąg B, et al. New insights into butyrylcholinesterase activity assay: serum dilution factor as a crucial parameter. PLoS One. 2015;10(10):e0139480. doi:10.1371/journal.pone.0139480
44. Pagel P, Blome J, Wolf HU. High-performance liquid chromatographic separation and measurement of various biogenic compounds possibly involved in the pathomechanism of Parkinson’s disease. J Chromatogr B Biomed Sci Appl. 2000;746(2):297–304. doi:10.1016/S0378-4347(00)00348-0
45. Shyjan AW, Cena V, Klein DC, Levenson R. Differential expression and enzymatic properties of the Na+, K (+)-ATPase alpha 3 isoenzyme in rat pineal glands. Proc Natl Acad Sci. 1990;87(3):1178–1182. doi:10.1073/pnas.87.3.1178
46. Marcus D, Hanwell DEC, Lonie DC, et al. Avogadro: an advanced semantic chemical editor, visualization, and analysis platform. J Cheminform. 2012;4:17.
47. Trott O, Olson AJ. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem. 2010;31(2):455–461. doi:10.1002/jcc.21334
48. Lawal B, Liu Y-L, Mokgautsi N, et al. Pharmacoinformatics and preclinical studies of NSC765690 and NSC765599, potential STAT3/CDK2/4/6 inhibitors with antitumor activities against NCI60 human tumor cell lines. Biomedicines. 2021;9(1):92. doi:10.3390/biomedicines9010092
49. Wu S-Y, Lin K-C, Lawal B, Wu ATH, Wu C-Z. MXD3 as an onco-immunological biomarker encompassing the tumor microenvironment, disease staging, prognoses, and therapeutic responses in multiple cancer types. Comput Struct Biotechnol J. 2021;19:4970–4983. doi:10.1016/j.csbj.2021.08.047
50. Lawal B, Lee C-Y, Mokgautsi N, et al. mTOR/EGFR/iNOS/MAP2K1/FGFR/TGFB1 are druggable candidates for N-(2,4-difluorophenyl)-2′,4′-difluoro-4-hydroxybiphenyl-3-carboxamide (NSC765598), with consequent anticancer implications. Front Oncol. 2021;11:932. doi:10.3389/fonc.2021.656738
51. Olugbodi JO, Samaila K, Lawal B, et al. Computational and preclinical evidence of anti-ischemic properties of l-carnitine-rich supplement via stimulation of anti-inflammatory and antioxidant events in testicular torsed rats. Oxid Med Cell Longev. 2021;2021:5543340. doi:10.1155/2021/5543340
52. Visualizer DS. BIOVIA, Dassault Systèmes, BIOVIA Workbook, Release 2020; BIOVIA Pipeline Pilot, Release 2020. San Diego: Dassault Systèmes; 2020.
53. Orhan I, Kartal M, Tosun F, Şener B. Screening of various phenolic acids and flavonoid derivatives for their anticholinesterase potential. Zeitschrift für Naturforschung C. 2007;62(11–12):829–832. doi:10.1515/znc-2007-11-1210
54. Inácio Lunkes G, Stefanello F, Sausen Lunkes D, Maria Morsch V, Schetinger MRC, Gonçalves JF. Serum cholinesterase activity in diabetes and associated pathologies. Diabetes Res Clin Pract. 2006;72(1):28–32. doi:10.1016/j.diabres.2005.08.009
55. Pari L, Murugavel P. Diallyl tetrasulfide improves cadmium induced alterations of acetylcholinesterase, ATPases and oxidative stress in brain of rats. Toxicology. 2007;234(1–2):44–50. doi:10.1016/j.tox.2007.01.021
56. Papoutsis K, Zhang J, Bowyer MC, Brunton N, Gibney ER, Lyng J. Fruit, vegetables, and mushrooms for the preparation of extracts with α-amylase and α-glucosidase inhibition properties: a review. Food Chem. 2021;338:128119. doi:10.1016/j.foodchem.2020.128119
57. Asayama K, Nakane T, Uchida N, Hayashibe H, Dobashi K, Nakazawa S. Serum antioxidant status in streptozotocin-induced diabetic rat. Hormone Metabol Res. 1994;26(07):313–315. doi:10.1055/s-2007-1001693
58. Garg MC, Ojha S, Bansal D. Antioxidant status of streptozotocin diabetic rats. Indian J Exp Biol. 1996;34(3):264–266.
59. Paul A, Anandabaskar N, Mathaiyan J, Raj GM, editors. Introduction to Basics of Pharmacology and Toxicology. Volume 2 : Essentials of Systemic Pharmacology: From Principles to Practice. Singapore: Springer Nature Singapore Pte Ltd; 2021. doi:10.1007/978-981-33-6009-9S
60. Ramakrishnan R, Namasivayam A. Norepinephrine and epinephrine levels in the brain of alloxan diabetic rats. Neurosci Lett. 1995;186(2–3):200–202. doi:10.1016/0304-3940(95)11315-N
61. Chen Y, Xu J, Chen Y. Regulation of neurotransmitters by the gut microbiota and effects on cognition in neurological disorders. Nutrients. 2021;13(6):2099. doi:10.3390/nu13062099
62. Okesola MA, Ajiboye BO, Oyinloye BE, et al. Effect of Solanum macrocarpon Linn leaf aqueous extract on the brain of an alloxan-induced rat model of diabetes. J Int Med Res. 2020;48(6):300060520922649. doi:10.1177/0300060520922649
63. Bagi Z, Erdei N, Papp Z, Édes I, Koller A. Up-regulation of vascular cyclooxygenase-2 in diabetes mellitus. Pharmacol Rep. 2006;58:52.
64. Pop-Busui R, Kellogg AP, Cheng HT. Cyclooxygenase-2 pathway as a potential therapeutic target in diabetic peripheral neuropathy. Curr Drug Targets. 2008;9(1):68–76. doi:10.2174/138945008783431691
65. Lees JA, Saito M, Vidal M, et al. The retinoblastoma protein binds to a family of E2F transcription factors. Mol Cell Biol. 1993;13(12):7813–7825. doi:10.1128/mcb.13.12.7813-7825.1993
66. Wrzos HF, Cruz A, Polavarapu R, Shearer D, Ouyang A. Nitric oxide synthase (NOS) expression in the myenteric plexus of streptozotocin-diabetic rats. Dig Dis Sci. 1997;42(10):2106–2110. doi:10.1023/A:1018830820537
67. Babu PVA, Sabitha KE, Shyamaladevi CS. Green tea impedes dyslipidemia, lipid peroxidation, protein glycation and ameliorates Ca2+-ATPase and Na+/K+-ATPase activity in the heart of streptozotocin-diabetic rats. Chem Biol Interact. 2006;162(2):157–164. doi:10.1016/j.cbi.2006.05.020
68. Ajayi AM, Adedapo AD, Badaki VB, Oyagbemi AA, Adedapo AA. Chrysophyllum albidum fruit ethanol extract ameliorates hyperglycaemia and elevated blood pressure in streptozotocin-induced diabetic rats through modulation of oxidative stress, NF-κB and PPAR-γ. Biomed Pharmacother. 2021;141:111879. doi:10.1016/j.biopha.2021.111879
69. Kunte KB, Kuna Y. Neuroprotective effect of Bacopa monniera on memory deficits and ATPase system in Alzheimer’s disease (AD) induced mice. J Sci Innov Res. 2013;2(4):719–735.
70. Slatter D, Bolton C, Bailey A. The importance of lipid-derived malondialdehyde in diabetes mellitus. Diabetologia. 2000;43(5):550–557. doi:10.1007/s001250051342
71. Oyinloye BE, Adenowo AF, Osunsanmi FO, Ogunyinka BI, Nwozo SO, Kappo AP. Aqueous extract of Monodora myristica ameliorates cadmium-induced hepatotoxicity in male rats. SpringerPlus. 2016;5(1):1–7. doi:10.1186/s40064-016-2228-z
72. Kitchen DB, Decornez H, Furr JR, Bajorath J. Docking and scoring in virtual screening for drug discovery: methods and applications. Nat Rev Drug Discov. 2004;3(11):935–949. doi:10.1038/nrd1549
73. Chen J-H, Wu ATH, Lawal B, et al. Identification of cancer hub gene signatures associated with immune-suppressive tumor microenvironment and ovatodiolide as a potential cancer immunotherapeutic agent. Cancers. 2021;13(15):3847. doi:10.3390/cancers13153847
74. Yeh Y-C, Lawal B, Hsiao M, Huang T-H, Huang C-YF. Identification of NSP3 (SH2D3C) as a prognostic biomarker of tumor progression and immune evasion for lung cancer and evaluation of organosulfur compounds from Allium sativum L. as therapeutic candidates. Biomedicines. 2021;9(11):1582. doi:10.3390/biomedicines9111582
75. Wu ATH, Lawal B, Wei L, Wen Y-T, Tzeng DTW, Lo W-C. Multiomics identification of potential targets for Alzheimer disease and antrocin as a therapeutic candidate. Pharmaceutics. 2021;13(10):1555. doi:10.3390/pharmaceutics13101555
76. Lawal B, Kuo Y-C, Tang S-L, et al. Transcriptomic-based identification of the immuno-oncogenic signature of cholangiocarcinoma for HLC-018 multi-target therapy exploration. Cells. 2021;10(11):2873. doi:10.3390/cells10112873
77. Lawal B, Kuo YC, Sumitra MR, Wu ATH, Huang HS. In vivo pharmacokinetic and anticancer studies of HH-N25, a selective inhibitor of Topoisomerase I, and hormonal signaling for treating breast cancer. J Inflamm Res. 2021;14:4901–4913. doi:10.2147/JIR.S329401
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.