Back to Journals » Drug Design, Development and Therapy » Volume 17
Statistical Analysis in Clinical and Experimental Medical Research: Simplified Guidance for Authors and Reviewers
Authors Panos GD , Boeckler FM
Received 24 June 2023
Accepted for publication 30 June 2023
Published 3 July 2023 Volume 2023:17 Pages 1959—1961
DOI https://doi.org/10.2147/DDDT.S427470
Checked for plagiarism Yes
Georgios D Panos,1,2 Frank M Boeckler3,4
1Department of Ophthalmology, Queen’s Medical Centre, Nottingham University Hospitals NHS Trust, Nottingham, UK; 2Division of Ophthalmology and Visual Sciences, School of Medicine, Faculty of Medicine and Health Sciences, University of Nottingham, Nottingham, UK; 3Department of Pharmacy and Biochemistry, Eberhard Karls Universität Tübingen, Laboratory for Molecular Design and Pharmaceutical Biophysics, Institute of Pharmaceutical Sciences, Tübingen, 72076, Germany; 4Institute for Bioinformatics and Medical Informatics (IBMI), Eberhard Karls Universität Tübingen, Tübingen, 72076, Germany
Correspondence: Georgios D Panos, Department of Ophthalmology, Eye & ENT Centre, Queen’s Medical Centre, Derby Road, Lenton, Nottingham, NG7 2UH, UK, Tel +44 115 924 9924, Email [email protected]
There has been a growing emphasis in recent years on the importance of statistical analysis in medical research. Accurate statistical analysis is essential for ensuring the validity and reliability of research findings, as well as experiment reproducibility. However, many researchers may not have a strong background in statistics, which can lead to problems with study design, data analysis, and result interpretation.
The main takeaway from our extensive editorial experience is that basic statistical rules are not routinely followed by the authors, including extremely small group size, data distribution assessment, inappropriate use of parametric tests and pseudoreplication, and this is missed during the peer review process.
To address this issue we are, hereby, providing guidance for authors and peer reviewers on medical statistics. These guidelines provide a helpful framework for the researchers to follow when designing studies and analysing data, and for the reviewers to use when assessing the quality and accuracy of the manuscripts. Our authors are kindly requested to provide a detailed data and statistical analysis paragraph within the Methods section to facilitate the implementation of these guidelines.
The case can be simplified to the following equation: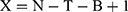
N = total number of observations,
T = number of treatments,
B = number of blocks
X = should be between 10 and 20.
Statistical analysis is the foundation of both clinical and experimental medical research. To ensure the credibility and replicability of their findings, authors must take a rigorous and transparent approach to data analysis and presentation. Reviewers must also understand statistical principles in order to evaluate the validity of submitted manuscripts. While the process may appear daunting, we hope that this information has helped to simplify some important aspects of statistical analysis. Remember that statistics do not constitute a necessary evil, but rather a necessary tool in our scientific endeavour. Collaboration between authors and statisticians is essential for the advancement of medical research and we strongly recommend that authors seek professional statistical advice when this is necessary.
Disclosure
The authors have no conflicts of interest in this work.
References
1. Fieberg JR, Vitense K, Johnson DH. Resampling-based methods for biologists. PeerJ. 2020;8:e9089. doi:10.7717/peerj.9089
2. Dwivedi AK, Mallawaarachchi I, Alvarado LA. Analysis of small sample size studies using nonparametric bootstrap test with pooled resampling method. Stat Med. 2017;36(14):2187–2205. doi:10.1002/sim.7263
3. Neuhauser M, Jockel KH. A bootstrap test for the analysis of microarray experiments with a very small number of replications. Appl Bioinformatics. 2006;5(3):173–179. doi:10.2165/00822942-200605030-00005
4. Gupta KK, Attri JP, Singh A, Kaur H, Kaur G. Basic concepts for sample size calculation: critical step for any clinical trials! Saudi J Anaesth. 2016;10(3):328–331. doi:10.4103/1658-354X.174918
5. Schulz KF, Grimes DA. Sample size calculations in randomised trials: mandatory and mystical. Lancet. 2005;365(9467):1348–1353. doi:10.1016/S0140-6736(05)61034-3
6. Festing MF. Design and statistical methods in studies using animal models of development. ILAR J. 2006;47(1):5–14. doi:10.1093/ilar.47.1.5
7. Arifin WN, Zahiruddin WM. Sample size calculation in animal studies using resource equation approach. Malays J Med Sci. 2017;24(5):101–105. doi:10.21315/mjms2017.24.5.11
8. Mancl LA, Leroux BG, DeRouen TA. Between-subject and within-subject statistical information in dental research. J Dent Res. 2000;79(10):1778–1781. doi:10.1177/00220345000790100801
9. Murdoch I. People and eyes: statistics in ophthalmology. Community Eye Health. 1998;11(27):43.
10. Cheng CY, Liu JH, Chiang SC, Chen SJ, Hsu WM. Statistics in ophthalmic research: two eyes, one eye or the mean? Zhonghua Yi Xue Za Zhi. 2000;63(12):885–892.
11. Nikinmaa M, Celander M, Tjeerdema R. Replication in aquatic biology: the result is often pseudoreplication. Aquat Toxicol. 2012;116–117:iii–iv.
12. Emmerich C. Accurate design of in vitro experiments – why does it matter? 2019. Available from: https://paasp.net/accurate-design-of-in-vitro-experiments-why-does-it-matter/.
13. Ghasemi A, Zahediasl S. Normality tests for statistical analysis: a guide for non-statisticians. Int J Endocrinol Metab. 2012;10(2):486–489. doi:10.5812/ijem.3505
14. Lee DK. Data transformation: a focus on the interpretation. Korean J Anesthesiol. 2020;73(6):503–508. doi:10.4097/kja.20137
15. Jan SL, Shieh G. Sample size determinations for Welch’s test in one-way heteroscedastic ANOVA. Br J Math Stat Psychol. 2014;67(1):72–93. doi:10.1111/bmsp.12006
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
