Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 17
Sodium–Glucose Cotransporter Protein 2 Inhibitors: Novel Application for the Treatment of Obesity-Associated Hypertension
Authors Hu Y , Bao J, Gao Z, Ye L, Wang L
Received 10 November 2023
Accepted for publication 13 January 2024
Published 26 January 2024 Volume 2024:17 Pages 407—415
DOI https://doi.org/10.2147/DMSO.S446904
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Konstantinos Tziomalos
Yilan Hu,1,2 Jiaqi Bao,1,2 Zhicheng Gao,1,2 Lifang Ye,2 Lihong Wang1,2
1The Second Clinical Medical College, Zhejiang Chinese Medical University, Hangzhou, People’s Republic of China; 2Heart Center, Department of Cardiovascular Medicine, Zhejiang Provincial People’s Hospital, Affiliated People’s Hospital, Hangzhou Medical College, Hangzhou, People’s Republic of China
Correspondence: Lihong Wang, Heart Center, Department of Cardiovascular Medicine, Zhejiang, Provincial People’s Hospital, Affiliated People’s Hospital, Hangzhou Medical College, Hangzhou, People’s Republic of China, Email [email protected]
Abstract: Obesity is becoming increasingly prevalent in China and worldwide and is closely related to the development of hypertension. The pathophysiology of obesity-associated hypertension is complex, including an overactive sympathetic nervous system (SNS), activation of the renin–angiotensin–aldosterone system (RAAS), insulin resistance, hyperleptinemia, renal dysfunction, inflammatory responses, and endothelial function, which complicates treatment. Sodium–glucose cotransporter protein 2 (SGLT-2) inhibitors, novel hypoglycemic agents, have been shown to reduce body weight and blood pressure and may serve as potential novel agents for the treatment of obesity-associated hypertension. This review discusses the beneficial mechanisms of SGLT-2 inhibitors for the treatment of obesity-associated hypertension. SGLT-2 inhibitors can inhibit SNS activity, reduce RAAS activation, ameliorate insulin resistance, reduce leptin secretion, improve renal function, and inhibit inflammatory responses. SGLT-2 inhibitors can, therefore, simultaneously target multiple mechanisms of obesity-associated hypertension and may serve as an effective treatment for obesity-associated hypertension.
Keywords: sodium-glucose cotransporter protein 2 inhibitors, obesity-associated hypertension, metabolism, neuro-humoral regulation, inflammation
Introduction
Since the 1990s, China’s economy has grown rapidly and people’s lifestyles have changed considerably,1 with a concurrent rapid increase in the prevalence of obesity. China now has the largest number of people with obesity worldwide.2,3 Obesity is a major risk factor for non-communicable diseases, presenting a great public health and economic burden.3,4
Hypertension is one of the most common complications of obesity, as well as a major risk factor for stroke, myocardial infarction, heart failure, and chronic kidney disease.5 It is estimated that by 2025, approximately 1.5 billion people worldwide will be affected by hypertension.6 A study including one million people in China from 2014 to 2017 showed that nearly half of adults aged 35–75 years suffer from hypertension, with approximately 22.5% of the people with hypertension being obese.7
Obesity-induced hypertension has diverse, interrelated pathogenic mechanisms, which complicates treatment. Many patients with obesity struggle to control their weight and blood pressure through diet and lifestyle changes and require treatment with drugs, such as orlistat, liraglutide, or bariatric surgery.8 First-line treatments for obesity-related hypertension currently include angiotensin-converting enzyme (ACE) inhibitors, angiotensin II (ANG II) receptor blockers, and calcium channel blockers.9
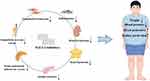
Sodium-glucose cotransporter 2 is mainly expressed in the proximal convoluted tubules of the kidney and is involved in glucose reabsorption. SGLT-2 inhibitors, a new class of drugs currently used to treat type 2 diabetes mellitus (T2DM), reduce blood glucose levels by increasing glucose excretion in the urine. This mechanism is insulin-independent, thus reducing the risk of hypoglycemia. More and more evidence suggests that SGLT-2 inhibitors have renal and cardiovascular protective effects in both diabetic and non-diabetic patients; thus, they are used in the treatment of heart failure and chronic kidney diseases.10–13 In addition, SGLT-2 inhibitors have been found to reduce body weight and blood pressure. Weight loss is a critical component in the treatment of patients with obesity-related hypertension. Therefore, SGLT-2 inhibitors may be a new option over the current first-line drug treatment of obesity-related hypertension. This review discusses the beneficial effects of SGLT-2 inhibitors targeting the pathogenic mechanisms of obesity-associated hypertension (Figure 1).
Obesity-Associated Hypertension, SGLT-2 Inhibitors, and the Sympathetic Nervous System
Over-activation of the sympathetic nervous system (SNS) in patients with obesity is associated with insulin resistance, hyperinsulinemia, obstructive sleep apnea, and renin–angiotensin–aldosterone system (RAAS) activation.14 Increased sympathetic nerve activity (SNA) is an important mechanism for the development of hypertension.15,16 However, SNS activation in patients with obesity varies between tissues. Animal and human studies using norepinephrine-spillover assessment and microneurographic measurements found increased SNA in the kidney and skeletal muscle, whereas cardiac SNA tends to be normal or may even be reduced.17–20 In a canine renal denervation model fed a high-fat diet, sodium retention and elevated blood pressure were greatly attenuated compared with controls,21 and in hypertensive patients with obesity, catheter-based renal denervation significantly reduced blood pressure for at least 3 years,22 which suggests that renal nerves play a key role in SNS activation and therefore in blood pressure in patients with obesity. Correlative studies in obese mice have shown that dapagliflozin decreases intrarenal tyrosine hydroxylase and norepinephrine.23 Dapagliflozin also reduced renal SNA, thereby reducing blood pressure and inhibiting weight gain in a mouse model of neurogenic hypertension.24 In a diabetic rabbit model, empagliflozin reduced SNS activity to similar levels as those of non-diabetic rabbits.25 Furthermore, ApoE-deficient mice treated with empagliflozin had significantly lower norepinephrine levels and suppressed SNA compared with controls.26 SGLT-2 inhibitors also improve the circadian rhythm of SNA,27,28 which may improve blood pressure. Although several studies have demonstrated that SGLT-2 inhibitors inhibit SNS activation, the exact mechanism remains unclear and requires experimental studies in humans.
Obesity-Associated Hypertension, SGLT-2 Inhibitors, and the Renin–Angiotensin–Aldosterone System
The renin–angiotensin–aldosterone system (RAAS) is a hormonal cascade that plays an important role in blood pressure regulation. Several important components of the RAAS, including plasma renin activity, angiotensinogen (AGT), ACE activity, ANG II, and aldosterone, are moderately elevated in patients with obesity, especially visceral obesity.29 RAAS activation occurs in patients with obesity even in the presence of obesity-related volume expansion and sodium retention, and may be associated with SNS hyperactivation, renal compression, and adipose tissue dysfunction.30 Adipose tissue can produce and secrete ANG II.31 In addition, AGT is closely related to ANG II production, and white adipose tissue is second only to the liver as a source of AGT.32 Moreover, adipocyte AGT-deficient mice fed a high-fat obesogenic diet do not develop elevated blood pressure, whereas control mice fed the same diet exhibit elevated plasma ANG II concentrations and blood pressure.33 In addition to ANG II stimulating adrenal aldosterone secretion in patients with obesity, adipocytes have been shown to produce aldosterone34 that induces sodium retention, which in turn leads to increased blood pressure. ACE inhibitors, ANG II receptor blockers, and saline cortical receptor antagonists have all been shown to reduce blood pressure in patients with obesity,35–37 highlighting the important role of the RAAS in obesity-associated hypertension. In high-fat diet-induced diabetic mice, canagliflozin inhibits AGT and attenuates hypertension.38 In obese rats with T2DM, 12 weeks of dapagliflozin treatment not only lowered glucose levels but also reduced RAAS activation.39 The macula densa, a chemoreceptor located in the glomerulus, regulates renin secretion in a sodium-dependent manner, thereby affecting RAAS activity. As SGLT-2 is a sodium-glucose cotransporter, SGLT-2 inhibitors reduce sodium reabsorption in the proximal tubule,40 which leads to increased sodium delivered to the macula densa, reducing renin release and activating the RAAS.41 In contrast, some studies have shown that sodium reabsorption is reduced in the proximal tubule and that polyureic natriuretic stimulation induced by SGLT-2 inhibitors activates systemic RAAS in patients with diabetes but does not affect intrarenal RAAS.42–44 Furthermore, no significant changes in plasma aldosterone levels are seen in patients treated with SGLT-2 inhibitors, which may be related to the aldosterone circadian rhythm.42,45 Overall, the effects of SGLT-2 inhibitors on the RAAS are complex and controversial, and although several studies have shown that SGLT-2 inhibitors attenuate RAAS activation, further research is required.
Obesity-Associated Hypertension, SGLT-2 Inhibitors, and Insulin Resistance
Obesity is often accompanied by hyperinsulinemia and insulin resistance, which play an important role in the development of hypertension.46 Animal experiments have shown that insulin promotes renal sodium reabsorption through activation of sodium/proton exchange protein 3 (NHE3) and epithelial sodium channels, thereby leading to sodium retention.47–49 In addition, under normal physiological conditions, insulin stimulates endothelial nitric oxide (NO) production, exerting anti-inflammatory and vasodilatory effects. Insulin-resistant states impair the selectivity of the insulin-stimulated O pathway, and compensatory hyperinsulinemia may activate mitogen-activated protein kinase, leading to vasoconstriction, water and sodium retention, and increased inflammatory responses.50 Furthermore, hyperinsulinemia may increase SNS activity and activate the RAAS, thereby elevating blood pressure,51–53 but this theory is currently controversial and requires further investigation. SGLT-2 inhibitors increase glucose excretion in an insulin-independent manner, lowering glucose levels, reducing glucose toxicity, and improving insulin sensitivity.54 In obese mice, dapagliflozin effectively reduces plasma insulin levels and improves pancreatic β-cell function after 10 weeks of treatment.55 Dapagliflozin also improves pancreatic injury, attenuates hyperinsulinemia, and reduces body weight in rats fed a high-fat diet by modulating the AMPK/mTOR signaling pathway, a major regulatory pathway for cell growth.56 Moreover, in a clinical study of pre-diabetic patients with insulin resistance, those treated with oral empagliflozin were found to have a more significant reversal of insulin resistance and lower blood pressure than the control group.57 Furthermore, several studies have reported that SGLT-2 inhibitors reduce insulin resistance in patients with diabetes58,59 with greater efficacy than metformin.60,61 Therefore, as insulin resistance is associated with metabolic activity, SGLT-2 inhibitors may be beneficial for the treatment of obesity and related metabolic diseases.
Obesity-Related Hypertension, SGLT-2 Inhibitors, and Hyperleptinemia
Leptin is an adipose-derived cytokine that suppresses appetite and increases energy expenditure.62 Leptin also stimulates the SNS, affecting cardiac and renal function.63 However, in people with obesity, high levels of leptin in circulating plasma induce a leptin-resistant state, which affects the appetite-suppressing effects of leptin but does not attenuate SNS activation.64 This selective leptin resistance suggests that hyperleptinemia may elevate blood pressure primarily through modulation of SNS activity, as demonstrated in experimental animal models.65,66 Furthermore, studies have shown that in the obese state, hyperleptinemia decreased NO availability and attenuated the vasodilatory effects of NO.67 Leptin is one of the most studied adipokines, and SGLT-2 inhibitors have been shown to improve metabolism by modulating adipokine levels.68 In obese rats, leptin levels and body mass index were significantly reduced by empagliflozin treatment.69 After 16 weeks of dapagliflozin treatment, serum leptin levels significantly decreased, and blood glucose and blood pressure significantly improved in patients with poor glycemic control.70 Furthermore, in patients with T2DM, dapagliflozin reduced circulating leptin levels, improved metabolism, and reduced cardiovascular risk.68,71,72 A recent clinical study showed that empagliflozin significantly reduced leptin levels compared with other glucose-lowering drugs used to treat patients with obesity and T2DM, such as biguanides and sulfonylureas.73 In addition, leptin levels are proportional to adiposity, and SGLT-2 may reduce leptin secretion via its weight-loss effect.74 As leptin is a major contributor to obesity and elevated blood pressure, leptin may be a potential target for SGLT-2 inhibitors in the treatment of obesity-associated hypertension.
Obesity-Associated Hypertension, SGLT-2 Inhibitors, and Renal Dysfunction
Obesity increases sodium reabsorption by the kidneys through physical compression of the kidneys, RAAS activation, and increased SNS activity,5 thereby leading to sodium retention, increased extracellular fluid, and increased blood pressure. During the early stages of weight gain, there is an increase in renal blood flow and glomerular hyperfiltration, which leads to increased sodium reabsorption. Following excessive weight gain, the kidneys are compressed by surrounding adipose tissue, which causes increased sodium reabsorption in the loop of Henle, thus indirectly increasing renin secretion and RAAS activation.75 RAAS and SNS activation increases sodium reabsorption in the renal tubules, causing sodium retention. Moreover, patients with obesity often exhibit hyperinsulinemia, dyslipidemia, and inflammation, the combination of which leads to renal insufficiency.76 Renal insufficiency has been demonstrated to cause hypertension in both animal experimental models and humans.77 In a double-blind, randomised trial involving patients with T2DM and kidney disease, long-term canagliflozin treatment reduced the risk of kidney failure, doubling of the creatinine level, or death from renal causes compared with placebo, with lower blood pressure and body weight.78 A further clinical study indicated that empagliflozin reduces the risk of kidney disease progression and hospitalisation of patients with chronic kidney disease, with or without T2DM.79 SGLT-2 inhibitors improve renal tissue viability by ameliorating oxidative damage through reduced glucose reabsorption.80 In addition, SGLT-2 inhibitors reduce uric acid concentration,81 thereby reducing renal injury.82 SGLT-2 inhibitors also inhibit NHE3 activity, which can cause sodium retention.83,84 Although the potent renoprotective activity of SGLT-2 inhibitors has been demonstrated, specific studies in patients with obesity-associated hypertension are still required.
Obesity-Associated Hypertension, SGLT-2 Inhibitors, and Inflammation
Obesity is recognised as a chronic low-grade inflammatory disease, and chronic inflammation plays an important role in the development and progression of hypertension.85 Obesity is characterised by excess adipose tissue, which secretes various adipokines that are involved in metabolic and inflammatory responses.86 Fat accumulation leads to adipocyte dysfunction and increased secretion of pro-inflammatory factors, such as tumor necrosis factor α, interleukin-6, interleukin-1 β, and resistin.87 In addition, several animal and human studies have shown that obesity increases the number of macrophages.88,89 Macrophages can alternate between the classically activated M1 phenotype, which is associated with increased production of pro-inflammatory factors, and the alternatively activated M2 phenotype, which repairs damaged tissue and prevents inflammation.90,91 In patients with obesity, macrophage polarisation is shifted from M2 to M1, resulting in increased production of pro-inflammatory factors,92 which induce inflammation, insulin resistance, oxidative stress, and endothelial dysfunction, thereby leading to vascular sclerosis and increased peripheral vascular resistance,93,94 and ultimately hypertension. Numerous studies have demonstrated the anti-inflammatory effects of SGLT-2 inhibitors.95–98 For example, empagliflozin reduces the amount of adipose tissue and the size of adipocytes and attenuates inflammation in ApoE-deficient mice fed a Western diet.99 Moreover, in spontaneously hypertensive rats, continuous feeding of empagliflozin for 30 days improved inflammatory response and blood pressure.100 Dapagliflozin pretreatment of macrophages obtained from healthy humans reduced M1 polarisation by bacterial lipopolysaccharide in vitro, thus reducing the M1/M2 ratio and blocking pro-inflammatory factor secretion.101 Additionally, in C57BL/6 mice with lipopolysaccharide-induced lung injury, canagliflozin reduced the M1/M2 macrophage ratio in vivo and contributed to attenuating the inflammatory response.102 In addition, empagliflozin attenuated inflammation, ameliorated endothelial dysfunction, and counteracted atherosclerosis in ApoE-deficient mice.103 Overall, SGLT-2 inhibitors reduce the amount of adipose tissue and the size of adipocytes, and modulate macrophage phenotypic transformation, thereby reducing inflammation.104 In recent years, numerous studies have demonstrated the anti-inflammatory effects of SGLT-2 inhibitors, suggesting additional indications for these drugs.
Obesity-Associated Hypertension, SGLT-2 Inhibitors, and Endothelial Function
Endothelial cells are involved in the regulation of vascular function through mediators such as NO.105 Our blood vessels are surrounded by adipose tissue known as perivascular adipose tissue (PVAT), which is able to regulate endothelial and vascular smooth muscle function through endocrine and paracrine effects and secretion of biologic factors (eg, contractile and relaxing factors).106 In obese patients, with the increased presence of PVAT, leading to dysregulation of its secretory function, it affects the homeostasis of the vascular system, leading to endothelial dysfunction.107 Other unfavourable factors of endothelial function, such as insulin resistance and inflammatory response, are also present in obese patients, as mentioned previously. When endothelial dysfunction occurs, endothelial nitric oxide synthase (eNOS) function and activity are altered, more reactive oxygen species (ROS) are produced, and enzyme “uncoupling” occurs, leading to an increased likelihood of cardiovascular disease, such as hypertension and atherosclerosis.108,109
Previous animal and clinical studies have demonstrated the effects of SGLT-2 inhibitors on vascular endothelial cells and vasoprotective effects. A prospective study in patients with T2DM revealed a decrease in blood pressure and a certain improvement in vascular function after 6 weeks of treatment with empagliflozin compared with a placebo control group.110 Furthermore, it was shown that patients with T2DM who had good glycaemic control after 2 days of acute treatment with dapagliflozin had a significant improvement in whole-body endothelial function, which may demonstrate that the effects of SGLT-2 inhibitors on the vascular system are rapid and direct.111 Although many studies have demonstrated the vasoprotective effect of SGLT-2 inhibitors, the mechanism has not been fully elucidated and may be related to their ability to reduce ROS production, increase NO utilisation, and reduce oxidative stress.112–115 Therefore, in patients with obese hypertension, early administration of SGLT-2 inhibitors may be able to improve their vascular function, benefiting the cardiovascular system and reducing the incidence of cardiovascular emergencies.
Conclusions
The increasing prevalence of obesity places a significant burden on human health and the healthcare system. The development of hypertension is closely related to obesity. Factors influencing obesity-associated hypertension are diverse and include, but are not limited to, SNS overactivity, RAAS activation, insulin resistance, hyperleptinemia, renal dysfunction, inflammatory responses, and endothelial function. Among them, SNS, RAAS, and renal function interact with each other. Insulin resistance and hyperleptinemia often exist at the same time, resulting in increased SNS activity and changes in vascular endothelial function. Inflammation is related to insulin resistance and dysfunction. These complex pathological mechanisms complicate the treatment of obesity-associated hypertension. SGLT-2 inhibitors have received significant research interest in recent years, and their clinical indications are constantly expanding. SGLT-2 inhibitors can simultaneously target multiple mechanisms of obesity-associated hypertension and may therefore be effective for the treatment of obesity-associated hypertension. However, few clinical studies have investigated SGLT-2 inhibitors for obesity-associated hypertension, and large-scale clinical trials are required to investigate the efficacy of SGLT-2 inhibitors for obesity-associated hypertension.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Wu Y, Xue H, Wang H, Su C, Du S, Wang Y. The impact of urbanization on the community food environment in China. Asia Pac J Clin Nutr. 2017;26(3):504–513. doi:10.6133/apjcn.032016.09
2. Wang Y, Wang L, Qu W. New national data show alarming increase in obesity and noncommunicable chronic diseases in China. Eur J Clin Nutr. 2017;71(1):149–150. doi:10.1038/ejcn.2016.171
3. Wang Y, Zhao L, Gao L, Pan A, Xue H. Health policy and public health implications of obesity in China. Lancet Diabetes Endocrinol. 2021;9(7):446–461.
4. Blüher M. Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol. 2019;15(5):288–298. doi:10.1038/s41574-019-0176-8
5. Hall JE, Do Carmo JM, da Silva AA, Wang Z, Hall ME. Obesity, kidney dysfunction and hypertension: mechanistic links. Nat Rev Nephrol. 2019;15(6):367–385. doi:10.1038/s41581-019-0145-4
6. Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365(9455):217–223. doi:10.1016/S0140-6736(05)17741-1
7. Lu J, Lu Y, Wang X, et al. Prevalence, awareness, treatment, and control of hypertension in China: data from 1·7 million adults in a population-based screening study (China PEACE Million Persons Project). Lancet. 2017;390(10112):2549–2558. doi:10.1016/S0140-6736(17)32478-9
8. Chang SH, Stoll CR, Song J, Varela JE, Eagon CJ, Colditz GA. The effectiveness and risks of bariatric surgery: an updated systematic review and meta-analysis, 2003–2012. JAMA Surgery. 2014;149(3):275–287. doi:10.1001/jamasurg.2013.3654
9. Shams E, Kamalumpundi V, Peterson J, Gismondi RA, Oigman W, de Gusmão Correia ML. Highlights of mechanisms and treatment of obesity-related hypertension. J Human Hypertens. 2022;36(9):785–793. doi:10.1038/s41371-021-00644-y
10. Kang A, Jardine MJ. SGLT2 inhibitors may offer benefit beyond diabetes. Nat Rev Nephrol. 2021;17(2):83–84. doi:10.1038/s41581-020-00391-2
11. Usman MS, Siddiqi TJ, Anker SD, et al. Effect of SGLT2 inhibitors on cardiovascular outcomes across various patient populations. J Am Coll Cardiol. 2023;81(25):2377–2387. doi:10.1016/j.jacc.2023.04.034
12. Docherty KF, Jhund PS. A welcome ‘failure’ of gliflozins: blood pressure reduction in heart failure. Eur Heart J. 2023;44(5):408–410. doi:10.1093/eurheartj/ehac712
13. Solomon J, Festa MC, Chatzizisis YS, Samanta R, Suri RS, Mavrakanas TA. Sodium-glucose co-transporter 2 inhibitors in patients with chronic kidney disease. Pharmacol Ther. 2023;242:108330.
14. Seravalle G, Grassi G. Sympathetic nervous system, hypertension, obesity and metabolic syndrome. High Blood Pressure Cardiovasc Prev. 2016;23(3):175–179. doi:10.1007/s40292-016-0137-4
15. Hall JE, da Silva AA, Do Carmo JM, et al. Obesity-induced hypertension: role of sympathetic nervous system, leptin, and melanocortins. J Biol Chem. 2010;285(23):17271–17276. doi:10.1074/jbc.R110.113175
16. Grassi G, Biffi A, Seravalle G, et al. Sympathetic neural overdrive in the obese and overweight state. Hypertension. 2019;74(2):349–358. doi:10.1161/HYPERTENSIONAHA.119.12885
17. Hall JE, Mouton AJ, da Silva AA, et al. Obesity, kidney dysfunction, and inflammation: interactions in hypertension. Cardiovasc Res. 2021;117(8):1859–1876. doi:10.1093/cvr/cvaa336
18. Gentile CL, Orr JS, Davy BM, Davy KP. Modest weight gain is associated with sympathetic neural activation in nonobese humans. Am J Physiol Regulatory Integr Comp Physiol. 2007;292(5):R1834–R1838. doi:10.1152/ajpregu.00876.2006
19. Armitage JA, Burke SL, Prior LJ, et al. Rapid onset of renal sympathetic nerve activation in rabbits fed a high-fat diet. Hypertension. 2012;60(1):163–171. doi:10.1161/HYPERTENSIONAHA.111.190413
20. Van Vliet BN, Hall JE, Mizelle HL, Montani JP, Smith MJ Jr. Reduced parasympathetic control of heart rate in obese dogs. A J Physiol. 1995;269(2 Pt 2):H629–37. doi:10.1152/ajpheart.1995.269.2.H629
21. Kassab S, Kato T, Wilkins FC, Chen R, Hall JE, Granger JP. Renal denervation attenuates the sodium retention and hypertension associated with obesity. Hypertension. 1995;25(4 Pt 2):893–897. doi:10.1161/01.hyp.25.4.893
22. Mahfoud F, Böhm M, Schmieder R, et al. Effects of renal denervation on kidney function and long-term outcomes: 3-year follow-up from the global SYMPLICITY registry. Eur Heart J. 2019;40(42):3474–3482. doi:10.1093/eurheartj/ehz118
23. Matthews VB, Elliot RH, Rudnicka C, Hricova J, Herat L, Schlaich MP. Role of the sympathetic nervous system in regulation of the sodium glucose cotransporter 2. J Hypertens. 2017;35(10):2059–2068. doi:10.1097/HJH.0000000000001434
24. Herat LY, Magno AL, Rudnicka C, et al. SGLT2 inhibitor-induced sympathoinhibition: a novel mechanism for cardiorenal protection. JACC. 2020;5(2):169–179. doi:10.1016/j.jacbts.2019.11.007
25. Gueguen C, Burke SL, Barzel B, et al. Empagliflozin modulates renal sympathetic and heart rate baroreflexes in a rabbit model of diabetes. Diabetologia. 2020;63(7):1424–1434. doi:10.1007/s00125-020-05145-0
26. Liu Y, Wu M, Xu B, Kang L. Empagliflozin alleviates atherosclerosis progression by inhibiting inflammation and sympathetic activity in a normoglycemic mouse model. J Inflamm Res. 2021;14:2277–2287. doi:10.2147/JIR.S309427
27. Rahman A, Fujisawa Y, Nakano D, Hitomi H, Nishiyama A. Effect of a selective SGLT2 inhibitor, luseogliflozin, on circadian rhythm of sympathetic nervous function and locomotor activities in metabolic syndrome rats. Clin Exp Pharmacol Physiol. 2017;44(4):522–525. doi:10.1111/1440-1681.12725
28. Wan N, Rahman A, Hitomi H, Nishiyama A. The effects of sodium-glucose cotransporter 2 inhibitors on sympathetic nervous activity. Front Endocrinol. 2018;9:421. doi:10.3389/fendo.2018.00421
29. Engeli S, Sharma AM. The renin-angiotensin system and natriuretic peptides in obesity-associated hypertension. J Mol Med. 2001;79(1):21–29. doi:10.1007/s001090000144
30. Schütten MT, Houben AJ, de Leeuw PW, Stehouwer CD. The link between adipose tissue renin-angiotensin-aldosterone system signaling and obesity-associated hypertension. Physiology. 2017;32(3):197–209. doi:10.1152/physiol.00037.2016
31. Cassis LA, Police SB, Yiannikouris F, Thatcher SE. Local adipose tissue renin-angiotensin system. Curr Hypertens Rep. 2008;10(2):93–98. doi:10.1007/s11906-008-0019-9
32. Phillips MI, Speakman EA, Kimura B. Levels of angiotensin and molecular biology of the tissue renin angiotensin systems. Regul Pept. 1993;43(1–2):1–20. doi:10.1016/0167-0115(93)90403-U
33. Yiannikouris F, Gupte M, Putnam K, et al. Adipocyte deficiency of angiotensinogen prevents obesity-induced hypertension in male mice. Hypertension. 2012;60(6):1524–1530. doi:10.1161/HYPERTENSIONAHA.112.192690
34. Briones AM, Nguyen Dinh Cat A, Callera GE, et al. Adipocytes produce aldosterone through calcineurin-dependent signaling pathways: implications in diabetes mellitus-associated obesity and vascular dysfunction. Hypertension. 2012;59(5):1069–1078. doi:10.1161/HYPERTENSIONAHA.111.190223
35. Grassi G, Seravalle G, Dell’Oro R, et al. Comparative effects of candesartan and hydrochlorothiazide on blood pressure, insulin sensitivity, and sympathetic drive in obese hypertensive individuals: results of the CROSS study. J Hypertens. 2003;21(9):1761–1769. doi:10.1097/00004872-200309000-00027
36. Dorresteijn JA, Schrover IM, Visseren FL, et al. Differential effects of renin-angiotensin-aldosterone system inhibition, sympathoinhibition and diuretic therapy on endothelial function and blood pressure in obesity-related hypertension: a double-blind, placebo-controlled cross-over trial. J Hypertens. 2013;31(2):393–403. doi:10.1097/HJH.0b013e32835b6c02
37. Williams B, MacDonald TM, Morant S, et al. Spironolactone versus placebo, bisoprolol, and doxazosin to determine the optimal treatment for drug-resistant hypertension (PATHWAY-2): a randomised, double-blind, crossover trial. Lancet. 2015;386(10008):2059–2068. doi:10.1016/S0140-6736(15)00257-3
38. Woods TC, Satou R, Miyata K, et al. Canagliflozin prevents intrarenal angiotensinogen augmentation and mitigates kidney injury and hypertension in mouse model of type 2 diabetes mellitus. Am J Nephrol. 2019;49(4):331–342. doi:10.1159/000499597
39. Shin SJ, Chung S, Kim SJ, et al. Effect of sodium-glucose co-transporter 2 inhibitor, dapagliflozin, on renal renin-angiotensin system in an animal model of type 2 diabetes. PLoS One. 2016;11(11):e0165703. doi:10.1371/journal.pone.0165703
40. Brady JA, Hallow KM. Model-based evaluation of proximal sodium reabsorption through SGLT2 in health and diabetes and the effect of inhibition with canagliflozin. J Clin Pharmacol. 2018;58(3):377–385. doi:10.1002/jcph.1030
41. Pruett JE, Romero DG, Yanes Cardozo LL. Obesity-associated cardiometabolic complications in polycystic ovary syndrome: the potential role of sodium-glucose cotransporter-2 inhibitors. Front Endocrinol. 2023;14:951099. doi:10.3389/fendo.2023.951099
42. Ansary TM, Nakano D, Nishiyama A. Diuretic effects of sodium glucose cotransporter 2 inhibitors and their influence on the renin-angiotensin system. Int J Mol Sci. 2019;20(3):629. doi:10.3390/ijms20030629
43. Gallo LA, Ward MS, Fotheringham AK, et al. Once daily administration of the SGLT2 inhibitor, empagliflozin, attenuates markers of renal fibrosis without improving albuminuria in diabetic db/db mice. Sci Rep. 2016;6:26428. doi:10.1038/srep26428
44. Cherney DZ, Perkins BA, Soleymanlou N, et al. Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation. 2014;129(5):587–597. doi:10.1161/CIRCULATIONAHA.113.005081
45. Hollenberg NK, Stevanovic R, Agarwal A, et al. Plasma aldosterone concentration in the patient with diabetes mellitus. Kidney Int. 2004;65(4):1435–1439. doi:10.1111/j.1523-1755.2004.00524.x
46. Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest. 2000;106(4):473–481. doi:10.1172/JCI10842
47. Horita S, Seki G, Yamada H, Suzuki M, Koike K, Fujita T. Insulin resistance, obesity, hypertension, and renal sodium transport. Int J Hypertens. 2011;2011:391762. doi:10.4061/2011/391762
48. Loffing J, Korbmacher C. Regulated sodium transport in the renal connecting tubule (CNT) via the epithelial sodium channel (ENaC). Pflug Arch. 2009;458(1):111–135. doi:10.1007/s00424-009-0656-0
49. Gesek FA, Schoolwerth AC. Insulin increases Na(+)-H+ exchange activity in proximal tubules from normotensive and hypertensive rats. A J Physiol. 1991;260(5 Pt 2):F695–703. doi:10.1152/ajprenal.1991.260.5.F695
50. Zhou MS, Wang A, Yu H. Link between insulin resistance and hypertension: what is the evidence from evolutionary biology? Diabetol Metab Syndr. 2014;6(1):12. doi:10.1186/1758-5996-6-12
51. da Silva AA, Do Carmo JM, Li X, Wang Z, Mouton AJ, Hall JE. Role of hyperinsulinemia and insulin resistance in hypertension: metabolic syndrome revisited. Can J Cardiol. 2020;36(5):671–682. doi:10.1016/j.cjca.2020.02.066
52. Anderson EA, Hoffman RP, Balon TW, Sinkey CA, Mark AL. Hyperinsulinemia produces both sympathetic neural activation and vasodilation in normal humans. J Clin Invest. 1991;87(6):2246–2252. doi:10.1172/JCI115260
53. Velloso LA, Folli F, Perego L, Saad MJ. The multi-faceted cross-talk between the insulin and angiotensin II signaling systems. Diabetes/Metab Res Rev. 2006;22(2):98–107. doi:10.1002/dmrr.611
54. Tanday N, Irwin N, Flatt PR, Moffett RC. Dapagliflozin exerts positive effects on beta cells, decreases glucagon and does not alter beta- to alpha-cell transdifferentiation in mouse models of diabetes and insulin resistance. Biochem Pharmacol. 2020;177:114009. doi:10.1016/j.bcp.2020.114009
55. Huang Z, Huang L, Wang C, et al. Dapagliflozin restores insulin and growth hormone secretion in obese mice. J Endocrinol. 2020;245(1):1–12.
56. Jaikumkao K, Promsan S, Thongnak L, et al. Dapagliflozin ameliorates pancreatic injury and activates kidney autophagy by modulating the AMPK/mTOR signaling pathway in obese rats. J Cell Physiol. 2021;236(9):6424–6440. doi:10.1002/jcp.30316
57. Kullmann S, Hummel J, Wagner R, et al. Empagliflozin improves insulin sensitivity of the hypothalamus in humans with prediabetes: a randomized, double-blind, placebo-controlled, phase 2 trial. Diabetes Care. 2022;45(2):398–406. doi:10.2337/dc21-1136
58. Min SH, Oh TJ, Baek SI, et al. Degree of ketonaemia and its association with insulin resistance after dapagliflozin treatment in type 2 diabetes. Diabetes Metabolism. 2018;44(1):73–76. doi:10.1016/j.diabet.2017.09.006
59. Mudaliar S, Henry RR, Boden G, et al. Changes in insulin sensitivity and insulin secretion with the sodium glucose cotransporter 2 inhibitor dapagliflozin. Diabetes Technol Ther. 2014;16(3):137–144. doi:10.1089/dia.2013.0167
60. Hao Z, Sun Y, Li G, Shen Y, Wen Y, Liu Y. Effects of canagliflozin and metformin on insulin resistance and visceral adipose tissue in people with newly-diagnosed type 2 diabetes. BMC Endocr Disord. 2022;22(1):37. doi:10.1186/s12902-022-00949-0
61. Koshizaka M, Ishikawa K, Ishibashi R, et al. Comparing the effects of ipragliflozin versus metformin on visceral fat reduction and metabolic dysfunction in Japanese patients with type 2 diabetes treated with sitagliptin: a prospective, multicentre, open-label, blinded-endpoint, randomized controlled study (PRIME-V study). Diabetes Obesity Metab. 2019;21(8):1990–1995.
62. Schwartz MW, Woods SC, Porte D Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404(6778):661–671.
63. Shek EW, Brands MW, Hall JE. Chronic leptin infusion increases arterial pressure. Hypertension. 1998;31(1 Pt 2):409–414. doi:10.1161/01.hyp.31.1.409
64. Poetsch MS, Strano A, Guan K. Role of leptin in cardiovascular diseases. Front Endocrinol. 2020;11:354.
65. Haynes WG, Morgan DA, Walsh SA, Mark AL, Sivitz WI. Receptor-mediated regional sympathetic nerve activation by leptin. J Clin Invest. 1997;100(2):270–278. doi:10.1172/JCI119532
66. Dunbar JC, Hu Y, Lu H. Intracerebroventricular leptin increases lumbar and renal sympathetic nerve activity and blood pressure in normal rats. Diabetes. 1997;46(12):2040–2043. doi:10.2337/diab.46.12.2040
67. Sweeney G. Cardiovascular effects of leptin. Nat Rev Cardiol. 2010;7(1):22–29. doi:10.1038/nrcardio.2009.224
68. Wu P, Wen W, Li J, et al. Systematic review and meta-analysis of randomized controlled trials on the effect of SGLT2 inhibitor on blood leptin and adiponectin level in patients with type 2 diabetes. Hormone Metab Res. 2019;51(8):487–494. doi:10.1055/a-0958-2441
69. Packer M, Zannad F, Butler J, et al. Influence of endpoint definitions on the effect of empagliflozin on major renal outcomes in the EMPEROR-preserved trial. Eur J Heart Fail. 2021;23(10):1798–1799.
70. Miura H, Sakaguchi K, Okada Y, et al. Effects of ipragliflozin on glycemic control, appetite and its related hormones: a prospective, multicenter, open-label study (SOAR-KOBE Study). J Diabetes Invest. 2019;10(5):1254–1261. doi:10.1111/jdi.13015
71. Vickers SP, Cheetham SC, Headland KR, et al. Combination of the sodium-glucose cotransporter-2 inhibitor empagliflozin with orlistat or sibutramine further improves the body-weight reduction and glucose homeostasis of obese rats fed a cafeteria diet. Diabetes Metab Syndr Obes. 2014;7:1254–1261. doi:10.2147/DMSO.S58786
72. Bailey CJ, Iqbal N, T’Joen C, List JF. Dapagliflozin monotherapy in drug-naïve patients with diabetes: a randomized-controlled trial of low-dose range. Diabetes Obesity Metab. 2012;14(10):951–959.
73. Szekeres Z, Sandor B, Bognar Z, et al. Clinical study of metabolic parameters, leptin and the SGLT2 inhibitor empagliflozin among patients with obesity and type 2 diabetes. Int J Mol Sci. 2023;24(5):4405. doi:10.3390/ijms24054405
74. McMillin SM, Pham ML, Sherrill CH. Effects of sodium-glucose cotransporter-2 inhibitors on appetite markers in patients with type 2 diabetes mellitus. Nutr Metab Cardiovasc Dis. 2021;31(8):2507–2511. doi:10.1016/j.numecd.2021.05.005
75. Grigoraș A, Balan RA, Căruntu ID, et al. Perirenal adipose tissue-current knowledge and future opportunities. J Clin Med. 2021;10(6):1291. doi:10.3390/jcm10061291
76. Hirohama D, Fujita T. Evaluation of the pathophysiological mechanisms of salt-sensitive hypertension. Hypertens Res. 2019;42(12):1848–1857.
77. DeMarco VG, Aroor AR, Sowers JR. The pathophysiology of hypertension in patients with obesity. Nat Rev Endocrinol. 2014;10(6):364–376. doi:10.1038/nrendo.2014.44
78. Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295–2306. doi:10.1056/NEJMoa1811744
79. Herrington WG, Staplin N, Wanner C, et al. Empagliflozin in patients with chronic kidney disease. N Engl J Med. 2023;388(2):117–127. doi:10.1056/NEJMoa2204233
80. Di Costanzo A, Esposito G, Indolfi C, Spaccarotella CAM. SGLT2 inhibitors: a new therapeutical strategy to improve clinical outcomes in patients with chronic kidney diseases. Int J Mol Sci. 2023;24(10):1.
81. Katsiki N, Rizzo M, Mikhailidis DP. Sodium-glucose co-transporter-2 (SGLT-2) inhibitors and uric acid: more good news! J Diabet Complicat. 2023;37(7):108510.
82. Bailey CJ. Uric acid and the cardio-renal effects of SGLT2 inhibitors. Diabetes Obesity Metab. 2019;21(6):1291–1298.
83. Trum M, Riechel J, Lebek S, et al. Empagliflozin inhibits Na(+) /H(+) exchanger activity in human atrial cardiomyocytes. ESC Heart Fail. 2020;7(6):4429–4437. doi:10.1002/ehf2.13024
84. Uthman L, Baartscheer A, Bleijlevens B, et al. Class effects of SGLT2 inhibitors in mouse cardiomyocytes and hearts: inhibition of Na(+)/H(+) exchanger, lowering of cytosolic Na(+) and vasodilation. Diabetologia. 2018;61(3):722–726. doi:10.1007/s00125-017-4509-7
85. Grundy SM. Inflammation, hypertension, and the metabolic syndrome. JAMA. 2003;290(22):3000–3002. doi:10.1001/jama.290.22.3000
86. Chang L, Garcia-Barrio MT, Chen YE. Perivascular adipose tissue regulates vascular function by targeting vascular smooth muscle cells. Arteriosclerosis Thrombosis Vasc Biol. 2020;40(5):1094–1109.
87. Lau WB, Ohashi K, Wang Y, et al. Role of adipokines in cardiovascular disease. Circ J. 2017;81(7):920–928. doi:10.1253/circj.CJ-17-0458
88. Harman-Boehm I, Blüher M, Redel H, et al. Macrophage infiltration into omental versus subcutaneous fat across different populations: effect of regional adiposity and the comorbidities of obesity. J Clin Endocrinol Metab. 2007;92(6):2240–2247. doi:10.1210/jc.2006-1811
89. Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112(12):1796–1808. doi:10.1172/JCI200319246
90. Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32(5):593–604. doi:10.1016/j.immuni.2010.05.007
91. Sun S, Ji Y, Kersten S, Qi L. Mechanisms of inflammatory responses in obese adipose tissue. Annu Rev Nutr. 2012;32:261–286. doi:10.1146/annurev-nutr-071811-150623
92. Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117(1):175–184. doi:10.1172/JCI29881
93. Britton KA, Fox CS. Ectopic fat depots and cardiovascular disease. Circulation. 2011;124(24):e837–41. doi:10.1161/CIRCULATIONAHA.111.077602
94. Su X, Peng D. Emerging functions of adipokines in linking the development of obesity and cardiovascular diseases. Mol Biol Rep. 2020;47(10):7991–8006. doi:10.1007/s11033-020-05732-9
95. Feijóo-Bandín S, Aragón-Herrera A, Otero-Santiago M, et al. Role of sodium-glucose co-transporter 2 inhibitors in the regulation of inflammatory processes in animal models. Int J Mol Sci. 2022;23(10). doi:10.3390/ijms23105634
96. Al Mamun A, Akter A, Hossain S, et al. Role of NLRP3 inflammasome in liver disease. J Dig Dis. 2020;21(8):430–436. doi:10.1111/1751-2980.12918
97. Kim SR, Lee SG, Kim SH, et al. SGLT2 inhibition modulates NLRP3 inflammasome activity via ketones and insulin in diabetes with cardiovascular disease. Nat Commun. 2020;11(1):2127. doi:10.1038/s41467-020-15983-6
98. Elrakaybi A, Laubner K, Zhou Q, Hug MJ, Seufert J. Cardiovascular protection by SGLT2 inhibitors - do anti-inflammatory mechanisms play a role? Mol Metab. 2022;64:101549. doi:10.1016/j.molmet.2022.101549
99. Han JH, Oh TJ, Lee G, et al. The beneficial effects of empagliflozin, an SGLT2 inhibitor, on atherosclerosis in ApoE (-/-) mice fed a western diet. Diabetologia. 2017;60(2):364–376. doi:10.1007/s00125-016-4158-2
100. Tan F, Long X, Du J, Yuan X. RNA-Seq transcriptomic landscape profiling of spontaneously hypertensive rats treated with a sodium-glucose cotransporter 2 (SGLT2) inhibitor. Biomed Pharmacothe. 2023;166:115289. doi:10.1016/j.biopha.2023.115289
101. Abdollahi E, Keyhanfar F, Delbandi AA, Falak R, Hajimiresmaiel SJ, Shafiei M. Dapagliflozin exerts anti-inflammatory effects via inhibition of LPS-induced TLR-4 overexpression and NF-κB activation in human endothelial cells and differentiated macrophages. Eur J Pharmacol. 2022;918:174715. doi:10.1016/j.ejphar.2021.174715
102. Lin F, Song C, Zeng Y, et al. Canagliflozin alleviates LPS-induced acute lung injury by modulating alveolar macrophage polarization. Int Immunopharmacol. 2020;88:106969. doi:10.1016/j.intimp.2020.106969
103. Ganbaatar B, Fukuda D, Shinohara M, et al. Empagliflozin ameliorates endothelial dysfunction and suppresses atherogenesis in diabetic apolipoprotein E-deficient mice. Eur J Pharmacol. 2020;875:173040. doi:10.1016/j.ejphar.2020.173040
104. Tsigalou C, Vallianou N, Dalamaga M. Autoantibody production in obesity: is there evidence for a link between obesity and autoimmunity? Curr Obes Rep. 2020;9(3):245–254. doi:10.1007/s13679-020-00397-8
105. Brandes RP. Endothelial dysfunction and hypertension. Hypertension. 2014;64(5):924–928. doi:10.1161/HYPERTENSIONAHA.114.03575
106. Cheng CK, Bakar HA, Gollasch M, Huang Y. Perivascular adipose tissue: the sixth man of the cardiovascular system. Cardiovasc Drugs Ther. 2018;32(5):481–502. doi:10.1007/s10557-018-6820-z
107. Lian X, Gollasch M. A clinical perspective: contribution of dysfunctional Perivascular Adipose Tissue (PVAT) to cardiovascular risk. Curr Hypertens Rep. 2016;18(11):82. doi:10.1007/s11906-016-0692-z
108. Lee I, Kim S, Nagar H, et al. CR6-interacting factor 1 deficiency reduces endothelial nitric oxide synthase activity by inhibiting biosynthesis of tetrahydrobiopterin. Sci Rep. 2020;10(1):842. doi:10.1038/s41598-020-57673-9
109. Daiber A, Xia N, Steven S, et al. New therapeutic implications of Endothelial Nitric Oxide Synthase (eNOS) function/dysfunction in cardiovascular disease. Int J Mol Sci. 2019;20(1). doi:10.3390/ijms20010187
110. Striepe K, Jumar A, Ott C, et al. Effects of the selective sodium-glucose cotransporter 2 inhibitor empagliflozin on vascular function and central hemodynamics in patients with type 2 diabetes mellitus. Circulation. 2017;136(12):1167–1169. doi:10.1161/CIRCULATIONAHA.117.029529
111. Solini A, Giannini L, Seghieri M, et al. Dapagliflozin acutely improves endothelial dysfunction, reduces aortic stiffness and renal resistive index in type 2 diabetic patients: a pilot study. Cardiovasc Diabetol. 2017;16(1):138. doi:10.1186/s12933-017-0621-8
112. Li C, Zhang J, Xue M, et al. SGLT2 inhibition with empagliflozin attenuates myocardial oxidative stress and fibrosis in diabetic mice heart. Cardiovasc Diabetol. 2019;18(1):15. doi:10.1186/s12933-019-0816-2
113. Chen YY, Wu TT, Ho CY, et al. Dapagliflozin prevents NOX- and SGLT2-dependent oxidative stress in lens cells exposed to fructose-induced diabetes mellitus. Int J Mol Sci. 2019;20(18):1.
114. Khemais-Benkhiat S, Belcastro E, Idris-Khodja N, et al. Angiotensin II-induced redox-sensitive SGLT1 and 2 expression promotes high glucose-induced endothelial cell senescence. J Cell Mol Med. 2020;24(3):2109–2122. doi:10.1111/jcmm.14233
115. Zhou Y, Tai S, Zhang N, Fu L, Wang Y. Dapagliflozin prevents oxidative stress-induced endothelial dysfunction via sirtuin 1 activation. Biomed Pharmacothe. 2023;165:115213. doi:10.1016/j.biopha.2023.115213
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.