Back to Journals » Nature and Science of Sleep » Volume 8
Slow sleep spindle and procedural memory consolidation in patients with major depressive disorder
Authors Nishida M, Nakashima Y, Nishikawa T
Received 11 November 2015
Accepted for publication 23 December 2015
Published 28 January 2016 Volume 2016:8 Pages 63—72
DOI https://doi.org/10.2147/NSS.S100337
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Steven A Shea
Masaki Nishida,1 Yusaku Nakashima,2 Toru Nishikawa1
1Department of Psychiatry and Behavioral Sciences, Graduate School of Medical and Dental Sciences, Tokyo Medical and Dental University, Yushima, Bunkyo, 2Medical Technology Research Laboratory, Research and Development Division, Medical Business Unit, Sony Corporation, Tokyo, Japan
Introduction: Evidence has accumulated, which indicates that, in healthy individuals, sleep enhances procedural memory consolidation, and that sleep spindle activity modulates this process. However, whether sleep-dependent procedural memory consolidation occurs in patients medicated for major depressive disorder remains unclear, as are the pharmacological and physiological mechanisms that underlie this process.
Methods: Healthy control participants (n=17) and patients medicated for major depressive disorder (n=11) were recruited and subjected to a finger-tapping motor sequence test (MST; nondominant hand) paradigm to compare the averaged scores of different learning phases (presleep, postsleep, and overnight improvement). Participants' brain activity was recorded during sleep with 16 electroencephalography channels (between MSTs). Sleep scoring and frequency analyses were performed on the electroencephalography data. Additionally, we evaluated sleep spindle activity, which divided the spindles into fast-frequency spindle activity (12.5–16 Hz) and slow-frequency spindle activity (10.5–12.5 Hz).
Result: Sleep-dependent motor memory consolidation in patients with depression was impaired in comparison with that in control participants. In patients with depression, age correlated negatively with overnight improvement. The duration of slow-wave sleep correlated with the magnitude of motor memory consolidation in patients with depression, but not in healthy controls. Slow-frequency spindle activity was associated with reduction in the magnitude of motor memory consolidation in both groups.
Conclusion: Because the changes in slow-frequency spindle activity affected the thalamocortical network dysfunction in patients medicated for depression, dysregulated spindle generation may impair sleep-dependent memory consolidation. Our findings may help to elucidate the cognitive deficits that occur in patients with major depression both in the waking state and during sleep.
Keywords: depression, memory consolidation, motor skill, polysomnography, sleep spindle, thalamocortical network
Introduction
Research shows that sleep is important for memory consolidation and that following learning, additional “offline” memory improvements develop during sleep.1 In terms of procedural motor memory, previous reports have indicated that posttraining sleep facilitates offline enhancement,2,3 whereas sleep disturbances can lead to impaired offline consolidation in humans.4 Several studies have demonstrated that the extent of initial learning, as well as the subsequent offline enhancement, is commonly correlated with non-rapid eye movement (NREM) sleep and some of its neurophysiological characteristics, including the sleep spindle.5,6
Sleep spindles represent stage 2 of NREM sleep, and spindle activity and offline motor memory improvement may be associated. Several previous studies have suggested that the beneficial effects of spindles on memory stabilization extend beyond healthy individuals to patients with neuropsychiatric disorders, including schizophrenia7 and depression.8–10 Dresler et al8 demonstrated impaired offline motor memory consolidation in middle-aged-to-elderly patients with depression. Furthermore, a recent neuroimaging study11 showed increased basal ganglia activity and decreased deactivation of the default mode network in patients with depression, suggesting spindle involvement in hippocampal–prefrontal connectivity. However, few studies have performed quantitative neurophysiological evaluations using multichannel, all-night polysomnography recordings.
Numerous studies have shown that alterations in electroencephalograms (EEGs) occur not only in drug-naïve patients but also in patients medicated for depression.12,13 Patients taking antidepressant medications for major depression exhibit considerable alterations in their sleep architecture, including REM suppression.14 Adjunctive benzodiazepine administration also yields sleep spindles, a hallmark of stage 2 NREM sleep.15 Collectively, these results imply that the impairments in sleep-dependent memory processing that are observed in patients medicated for major depression involve neurophysiological alterations in sleep spindle generation.
Evidence supports the existence of two types of spindles, categorized by frequency: fast spindles (~14 Hz) and slow spindles (~12 Hz).16,17 Past studies have demonstrated that fast spindles ameliorate motor memory consolidation in healthy individuals;18,19 however, the functions of fast and slow spindles in memory processing and the neuropsychiatric mechanisms underlying major depression with pharmacological modulations remain unclear.
We hypothesized that motor memory consolidation would be facilitated by fast spindles and deteriorated by slow-frequency spindle activity. Herein, similar to other studies,6,8 we used a finger-tapping motor sequence task (MST), which is often used to assess procedural learning and to test motor procedural learning. Unlike previous studies, we also performed multichannel polysomnography on all participants during intervening, all-night sleep. Polysomnographic recordings can reveal sleep architecture and other parameters and hence can be quantitatively analyzed to elucidate how the two spindle types are propagated. We investigated the relationship between sleep spindle propensities and overnight memory consolidation in both control participants and patients with major depression and thereafter present both the behavioral differences and the differences in the topographical distribution between the two groups.
Methods
Participants
Seventeen healthy control participants (15 males, two females; mean ± standard error of the mean [SEM] age: 33.76±1.38 years) and eleven inpatients with major depressive disorder (seven males, four females; mean age: 30.00±2.89 years) were included in this study. The participants were volunteers who responded to an advertisement for this study; no financial reward or incentive was offered to participants. All participants were between 22 years and 52 years of age and were right-handed; participants who were experienced in professional key typing or piano playing were excluded from this study. Each participant underwent clinical screening assessments, including the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV) Axis I Disorders (SCID-I), which were performed by Masters Level Clinicians with experience in psychiatric diagnosis. Additionally, the SCID-I results of each participant were compared with the results of psychiatric interviews performed by a research psychiatrist; discrepancies between the two assessments were resolved by discussion before inclusion in the study. All participants were also assessed using the Hamilton Depression Rating Scale (HAM-D) and were asked to rate their level of sleepiness based on the Epworth Sleepiness Scale (ESS) and Pittsburgh Sleep Quality Index (PSQI) prior to the experiment.
The protocols in this study were approved by the Ethics Committee of Tokyo Medical and Dental University (project identification code: number 581), and written informed consent was obtained from each participant prior to the study. The trial has been registered with trial number (project identification code) 581 at the “Research for mood and sleep disorders” registry.
Control participants
Exclusion criteria for the control participants were substance abuse/dependence within the previous year, identifiable neurological or psychiatric disorders requiring concurrent medical treatment, and sleep disorders diagnosed by both clinical evaluation and polysomnography.
Patients with major depressive disorder
Patients with major depressive disorder were all confirmed to have a history of current major depressive episodes and met the criteria for the disorder in the International Statistical Classification of Diseases, 10th Revision, and DSM-IV–Text Revision. All depressed participants met the DSM-IV criteria for major depressive disorder if a current major depressive episode had persisted for >1 month. This was the first episode of major depression in all patients, and the mean duration of illness was 3.65±1.52 years. Severely depressed patients who exhibited suicide ideation or suffered from delusion were not recruited into this study, and participating patients did not have any comorbidities. Depression severity was assessed using the 17-item HAM-D. Patients were treated on an inpatient basis, and the experiment was conducted at the initiation of the treatment.
Nine of the patients with depression were receiving zolpidem. Three were receiving selective serotonin-reuptake inhibitors, four were on selective serotonin- and norepinephrine-reuptake inhibitors (SNRIs), and three were receiving trazodone. Additionally, four patients were receiving mood stabilizers, and five were receiving second-generation antipsychotic medication.
Procedures
Finger-tapping MST
The MST required subjects to press four numeric keys on a standard computer keyboard with the fingers of their left (nondominant) hand, repeating the five-element sequence, 4-1-3-2-4, “as quickly and as accurately as possible” for a period of 30 seconds (details can be found in the study by Walker et al2). Participants were tested on two consecutive days at 2.00 pm (8 hours after awakening) with a sequential finger-tapping MST. Trials were automatically scored for both performance speed (number of correctly typed sequences per trial) and accuracy (error rate: number of errors per sequence). The training session on the first day lasted a total of 12 minutes and consisted of twelve 30-second trials with 30-second rest periods between trials. After a night of sleep, the participants again performed the twelve 30-second trials of the same sequence separated by 30-second rest periods.
Behavioral data analysis
We averaged data from sequential groups of four trials in each session (first and second days) of the MST, providing us with six averages of four trials each (Figure 1). The average of the third group of four trials, subtracted from the average of the first group of four trials, was defined as the “presleep” training performance. After a night of sleep, the average of the sixth group of trials subtracted from the average of the fourth group of trials was defined as the “postsleep” performance. Offline memory consolidation (practice-independent, ie, overnight sleep) improvement was defined as the difference between the averages of the third and fourth groups of trials.
For statistical analysis, the averaged scores were compared using analyses of variance (ANOVAs), with additional post hoc tests for multiple comparisons using Benjamini–Hochberg’s method. To avoid the confounding effect of age, we applied multivariate regression analysis, adjusting for age, to determine the association between the score of overnight improvement and the duration of sleep stages.
Polysomnography
The participants were asked to come to the overnight EEG Laboratory at Tokyo Medical and Dental University Hospital at 8.30 pm and were prepared for polysomnography. The lights were turned off at 9.00 pm, and the participants were awakened at 6.00 am. An adaptation night was allowed in order to control for the first-night effect. Brain activity was recorded in all participants during sleep using 16 EEG electrodes, two electrooculogram electrodes, and two chin electromyogram electrodes. The 16 electrodes were placed according to the International 10–20 system (C3, C4, F3, F4, F7, F8, Fp1, Fp2, O1, O2, P3, P4, T3, T4, T5, and T6). For the recordings, scalp electrodes were referenced against the contralateral mastoid. EEG data were recorded with a Neurofax EEG-1214 system (Nihon Koden Corp, Tokyo, Japan) and digitalized at 200 Hz. The stage of sleep was visually scored from the EEG data by two experts, individually, in accordance with standard criteria.20 The sleep stages (1, 2, 3, 4, and REM sleep), awake time, and movement artifacts were scored offline at 30-second intervals. Slow-wave sleep (SWS) was defined as the amount of stage 3 and 4 sleep. The epochs that were affected by artifacts were excluded from further quantitative EEG analysis after visual inspection.
Data analysis (spindle evaluation and statistical analyses)
The power of spindles was calculated with an automatic algorithm as follows: 1) perform Fourier spectral analysis on the EEG data in each epoch from all channels; 2) average the EEG spectrogram power of each epoch in the sleep stage for all channels; 3) calculate the integrals of the 12.5–16 Hz and 10.5–12.5 Hz bands in the EEG spectrogram for fast- and slow-frequency spindle activities, respectively, for all channel types, according to approaches used in previous studies.16–18 Spindle calculation was performed in MATLAB (The MathWorks, Natick, MA, USA).
For statistical analyses, the powers of the slow- and fast-frequency spindle activities at different electrodes, both within and between the two groups, were examined by ANOVAs, with additional post hoc tests for multiple comparisons using Benjamini–Hochberg’s method.
We investigated the association between the overnight change in motor memory performance and spindle propensities, incorporating the fast- and the slow-frequency spindle activities during NREM stages 2–4. Topographical differences in the sleep spindles were also evaluated to determine the regional alterations during sleep that are related to the motor area of the brain.
Sleep parameters, including the total sleep time, sleep efficiency, sleep onset latency, powers of the fast- and slow- frequency spindle activities, and magnitude of overnight improvement were evaluated by correlation analysis. Under the assumption of a normal distribution, the Pearson product–moment correlation test was applied for correlation analysis. Spindle power correlations were evaluated for each brain region. The topographical distribution of Pearson r-values for the correlation between spindle power and the extent of overnight improvement was obtained.
Results
Demographics
The participants’ demographic characteristics and sleep architecture variables are shown in Table 1. The patients with major depressive disorder had significantly higher HAM-D scores (range: 8–24) than did the control participants (Student’s t-test, P<0.001). The cutoff point for HAM-D scores was set as 8.0, and participants with scores less than the cutoff point were excluded from this study. The average PSQI score (±SEM) of the control participants (5.65±0.42) was significantly lower than the average score for patients with major depressive disorder (11.73±4.14, P<0.001), indicating that the subjective sleep quality of the patients with depression was worse than that of the control participants. The ESS scores of the two groups were not statistically different.
The total sleep time of patients with depression was not different from that of control participants, although patients with depression spent significantly more time in stage 2 NREM sleep than did control participants (P<0.005). The REM sleep durations of the two groups were not significantly different. Patients with depression spent less time in SWS (P<0.005), and the duration of stage 4 NREM sleep of patients with depression was significantly shorter than that of control participants (P<0.01).
Overnight improvement on the MST
Figure 2A shows the learning curves in both groups and demonstrates practice-dependent learning on the first day; the averaged scores are also plotted (Figure 2B) and differed significantly between the two groups (P<0.001). A two-way ANOVA for MST score improvement via practice or sleep showed that score improvements gained via sleep were significantly better than the improvements gained via practice (P=0.0001), but there were no significant differences in MST score improvement between controls and patients, and no interaction effect (P=0.522 and P=0.134).
Despite the lack of an interaction effect, we separated the controls and patients. In controls, differences in memory scores (presleep, overnight, and postsleep) were 1.18±0.55, 3.42±0.33, 1.38±0.75, respectively. Control participants showed significant overnight improvement effects between the third group of averaged scores and the fourth group of averaged scores as compared with presleep practice (95% simultaneous confidence interval [CI]: −4.3 to −0.16) and postsleep practice effects (95% CI: 2.4–6.6) (Figure 3). In depressed patients, the differences in the memory scores (presleep, overnight, and postsleep) were 2.43±1.40, 2.80±0.99, 3.46±0.45, respectively. In contrast to the controls, the patients with major depressive disorder failed to show significant practice-dependent learning after sleep (95% CI: −4.3 to 3.6), indicating reduced overnight improvement (95% CI: −1.9 to 5.9) (Figure 3).
Although normal controls showed no significant correlation between age and overnight improvement, age was negatively correlated with overnight improvement in patients with depression (r=0.64, P=0.031) (Figure 4A). The magnitude of the overnight improvement demonstrated a weak positive correlation with the average MST score (r=0.50, P=0.118). Multivariate regression analysis for the effect of age between overnight improvement and sleep stages (stage 2 NREM sleep and SWS) revealed a moderately significant multiple correlation coefficient (R=0.551).
]Relationships among overnight MST improvement, sleep parameters, and sleep spindles
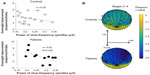
In control participants, the sleep parameters, including total sleep time, sleep efficiency, and sleep onset latency, were not significantly correlated with overnight improvement. In both groups, the extent of overnight improvement was not significantly correlated with the amount of NREM stage 2 sleep (Figure 4B). Patients with major depressive disorder showed a positive correlation between overnight improvement and the duration of SWS (r=0.62, P=0.042) (Figure 4C).
Multiple comparisons tests for the powers of slow- and fast-frequency spindles at different electrodes did not show statistical differences between the two groups. With regard to the slow-frequency spindle activity within each group, the EEG data at F7, F8, and T3 were significantly different between control participants and patients (P=0.092 at FP1, P=0.133 at FP2, P=0.439 at F3, P=0.487 at F4, P=0.493 at C3, P=0.492 at C4, P=0.619 at P3, P=0.490 at P4, P=0.502 at O1, P=0.520 at O2, P=0.045 at F7, P=0.047 at F8, P=0.005 at T3, P=0.131 at T4, P=0.360 at T5, and P=0.491 at T6).
Fronto-centro-parietal slow-frequency spindle activities were negatively correlated with overnight improvement on the MST in both groups. The extent of the reduction in overnight improvement was marked in patients as compared with that in control participants (Figure 5). Fast-frequency spindle activity was not significantly correlated with overnight improvement in either group. In depressive patients, r-values were negatively correlated with the overnight improvement (r=−0.451 at FP1, r=−0.530 at FP2, r=−0.598 at F3, r=−0.498 at F4, r=−0.620 at C3, r=−0.499 at C4, r=−0.529 at P3, r=−0.480 at P4, r=−0.596 at O1, r=−0.391 at O2, r=−0.507 at F7, r=−0.571 at F8, r=−0.381 at T3, r=−0.383 at T4, r=−0.566 at T5, and r=−0.355 at T6).
Discussion
This study investigated whether disturbed sleep spindle regulation in major depression leads to a negative association between slow-frequency spindle activity and procedural memory consolidation. The observed association was specific solely to overnight consolidation or was present for baseline memory performance. Past studies21,22 have shown that the amount of stage 2 NREM sleep is associated with overnight enhancement; however, we did not observe beneficial effects of stage 2 NREM sleep on motor memory consolidation. These discrepant findings may stem from MST timing differences, as the MST in our study was performed in the afternoon, which is different from the performance times in other studies. For example, a similar study6 had participants perform the MST after a daytime nap. Additionally, SWS was only associated with motor memory consolidation in patients with depression. Although previous studies proposed that SWS has beneficial effects on motor memory consolidation,23 the duration and amplitude were substantially suppressed in patients medicated for depression.14,24 We speculate that SWS activity may play a lesser role than sleep spindles, as suggested by the prominent electrophysiological features observed during both light and deep NREM sleep using pharmacological EEG.25
The possibility that sleep spindle alterations contribute to cognitive dysfunction in major depression is consistent with established evidence regarding spindle function.26 Thalamocortical spindles are candidate physiological oscillations for promoting the synaptic plasticity crucial for memory consolidation during sleep in healthy individuals.27 Two different spindle types, slow-frequency prefrontal spindles and fast-frequency centroparietal spindles, have been proposed. Fast-frequency spindles driven by slow (<1 Hz) oscillations may be important for sleep-dependent memory processing, enforcing the cycling between spindles and slow oscillations.28 Because previous research13 found significant increases in frontal and parietal spindle densities in patients medicated for major depressive disorder, the impaired motor memory enhancement may be attributed to dysregulation of this cycle. Considering that an indirect connection between slow-frequency spindle activity and memory processing has been indicated, slow-frequency spindle activity may deteriorate the effect of procedural memory consolidation more substantially in the medicated depressive patients than in healthy controls.
In addition, age was negatively correlated with the magnitude of enhancement. We used multivariate regression analysis, adjusting for age, to avoid the effect of age on both memory and sleep. Dresler et al8 demonstrated that patients with depression who were over the age of 30 years failed to show overnight improvement on the MST, while motor memory enhancement was not affected in younger patients (<30 years).8 A potential reason for the impaired offline memory consolidation observed in our study may be that the mean age of the participants was slightly higher than the threshold set by Dresler et al,8 as we did not include adolescents or children in our study. Considering the age-related decline in sleep-dependent procedural consolidation, increased age may be detrimental to offline consolidation.29,30
Our study did not successfully clarify the precise regional involvement of fast- and slow-frequency sleep spindles, due to the limited spatial resolution of the approach used. Intracranial EEG recordings show that sleep spindles occur and propagate locally in different brain regions.31 Indeed, the supplementary motor area contralateral to the trained hand is a core brain region for sleep-dependent MST consolidation. Despite detecting regionally enhanced oscillations in this area with the spindle array, consolidation may occur in a larger cortical network involving regions outside of the motor area, including prefrontal and parietal regions, which are affected in major depression.
The limitations of our exploratory study include the low severity of depression in our subjects, inevitable pharmacological effects, small sample size, and different participant numbers between the groups. The recruitment of mild/moderately depressed patients may have contributed to the lack of observed differences between the groups in terms of overnight consolidation. More specifically, the small number of patients with depression and the low number of female controls are considerable limitations. We also performed a substantial number of analyses, based on a comparably small sample size, which may have yielded both false-positive and false-negative results. Furthermore, pharmacological effects cannot be excluded, as the patients were treated with a variety of drugs, including antidepressants, benzodiazepines, and adjunctive agents. Due to the REM-suppressing effects of antidepressants,12 the REM sleep duration in patients with depression was significantly shorter than that in control participants. Although Rasch et al32 showed that REM sleep suppression via SNRI administration after training enhanced motor memory consolidation using the MST paradigm, other studies33,34 failed to demonstrate REM sleep involvement in procedural memory consolidation. We speculate that stage 2 NREM sleep, rather than REM sleep, is associated with motor memory stabilization.
Benzodiazepines are known to slightly suppress REM sleep and to increase sleep spindles.35 Specifically, benzodiazepines induce a preferential increase in slow-frequency spindle activities, especially prefrontal slow-frequency spindle activity, at the expense of fast-frequency spindle activity.15,36 Regarding the association between pharmacological effects and memory consolidation, Morgan et al37 demonstrated that the motor memory consolidation observed in humans was worsened by administering triazolam, but not by administering zolpidem, a short-acting GABAA agonist hypnotic.37 In our study, hypnotics may have had less of an effect on sleep-dependent procedural memory consolidation, although it may cause it to deteriorate over time in patients medicated for major depression.
In summary, alterations in the spindles generated by pharmacologic medications probably have an inverse effect on the cognitive function of depression, probably altering brain plasticity throughout the thalamocortical network and hippocampus. Altered spindles may affect memory consolidation by interfering with normal coordinated memory reactivation processes. Therefore, we propose that abnormal sleep spindles be considered biomarkers of underlying cognitive deficits in patients medicated for major depression. Additional studies are needed to elucidate the neural network underlying offline cognitive function in these patients, including synaptic and molecular interactions.
Acknowledgments
We thank Takashi Tomita, Haruhiko Soma, Seiji Wada, Takuro Yamamoto, and Akio Yasuda for their technical support. We also thank Matthew P Walker for providing the MST program tools. This work was supported by a research grant from the Sony Corporation to the Department of Psychiatry and Behavioral Sciences, Graduate School of Medical and Dental Sciences, Tokyo Medical and Dental University.
Author contributions
Masaki Nishida was the principal psychiatrist involved in managing the study. Additionally, he was responsible for the review of all literature, medical data collection, and the writing of the manuscript. Masaki Nishida is also the corresponding author and was involved in management of the participants, responsible for the literature review associated with the report, responsible for obtaining participants’ consent, and participated in the writing of the manuscript. Yusaku Nakashima was responsible for assisting with data analysis. Toru Nishikawa was responsible for supervising the management of the participants and was involved in writing the manuscript. All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Stickgold R. Parsing the role of sleep in memory processing. Curr Opin Neurobiol. 2013;23(5):847–853. | |
Walker MP, Brakefield T, Morgan A, Hobson JA, Stickgold R. Practice with sleep makes perfect: sleep-dependent motor skill learning. Neuron. 2002;35(1):205–211. | |
Albouy G, Fogel S, Pottiez H, et al. Daytime sleep enhances consolidation of the spatial but not motoric representation of motor sequence memory. PLoS One. 2013;8(1):e52805. | |
Djonlagic I, Saboisky J, Carusona A, Stickgold R, Malhotra A. Increased sleep fragmentation leads to impaired off-line consolidation of motor memories in humans. PLoS One. 2012;7(3):e34106. | |
Smith C, MacNeill C. Impaired motor memory for a pursuit rotor task following Stage 2 sleep loss in college students. J Sleep Res. 1994;3(4):206–213. | |
Nishida M, Walker MP. Daytime naps, motor memory consolidation and regionally specific sleep spindles. PLoS One. 2007;2(4):e341. | |
Manoach DS, Cain MS, Vangel MG, Khurana A, Goff DC, Stickgold R. A failure of sleep-dependent procedural learning in chronic, medicated schizophrenia. Biol Psychiatry. 2004;56(12):951–956. | |
Dresler M, Kluge M, Genzel L, Schussler P, Steiger A. Impaired off-line memory consolidation in depression. Eur Neuropsychopharmacol. 2010;20(8):553–561. | |
Dresler M, Kluge M, Pawlowski M, Schussler P, Steiger A, Genzel L. A double dissociation of memory impairments in major depression. J Psychiatr Res. 2011;45(12):1593–1599. | |
Goder R, Seeck-Hirschner M, Stingele K, et al. Sleep and cognition at baseline and the effects of REM sleep diminution after 1 week of antidepressive treatment in patients with depression. J Sleep Res. 2011;20(4):544–551. | |
Genzel L, Dresler M, Cornu M, et al. Medial prefrontal–hippocampal connectivity and motor memory consolidation in depression and schizophrenia. Biol Psychiatry. 2015;77(2):177–186. | |
Wilson S, Argyropoulos S. Antidepressants and sleep: a qualitative review of the literature. Drugs. 2005;65(7):927–947. | |
Plante DT, Goldstein MR, Landsness EC, et al. Topographic and sex-related differences in sleep spindles in major depressive disorder: a high-density EEG investigation. J Affect Disord. 2013;146(1):120–125. | |
Armitage R. The effects of antidepressants on sleep in patients with depression. Can J Psychiatry. 2000;45(9):803–809. | |
Aeschbach D, Dijk DJ, Trachsel L, Brunner DP, Borbely AA. Dynamics of slow-wave activity and spindle frequency activity in the human sleep EEG: effect of midazolam and zopiclone. Neuropsychopharmacology. 1994;11(4):237–244. | |
De Gennaro L, Ferrara M, Bertini M. Topographical distribution of spindles: variations between and within nrem sleep cycles. Sleep Res Online. 2000;3(4):155–160. | |
Anderer P, Klosch G, Gruber G, et al. Low-resolution brain electromagnetic tomography revealed simultaneously active frontal and parietal sleep spindle sources in the human cortex. Neuroscience. 2001;103(3):581–592. | |
Tamaki M, Matsuoka T, Nittono H, Hori T. Fast sleep spindle (13–15 hz) activity correlates with sleep-dependent improvement in visuomotor performance. Sleep. 2008;31(2):204–211. | |
Barakat M, Doyon J, Debas K, et al. Fast and slow spindle involvement in the consolidation of a new motor sequence. Behav Brain Res. 2011;217(1):117–121. | |
Rechtschaffen A, Kales A; University of California LABIS, Network NNI. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. Bethesda, MD: US Dept. of Health, Education, and Welfare; 1968. | |
Walker MP, Stickgold R, Alsop D, Gaab N, Schlaug G. Sleep-dependent motor memory plasticity in the human brain. Neuroscience. 2005;133(4):911–917. | |
Fogel SM, Smith CT. Learning-dependent changes in sleep spindles and stage 2 sleep. J Sleep Res. 2006;15(3):250–255. | |
Huber R, Ghilardi MF, Massimini M, Tononi G. Local sleep and learning. Nature. 2004;430(6995):78–81. | |
Benca RM, Obermeyer WH, Thisted RA, Gillin JC. Sleep and psychiatric disorders. A meta-analysis. Arch Gen Psychiatry. 1992;49(8):651–668; discussion 669–670. | |
Nishida M, Nakashima Y, Nishikawa T. Topographical distribution of fast and slow sleep spindles in medicated depressive patients. J Clin Neurophysiol. 2014;31(5):402–408. | |
Fogel SM, Smith CT. The function of the sleep spindle: a physiological index of intelligence and a mechanism for sleep-dependent memory consolidation. Neurosci Biobehav Rev. 2011;35(5):1154–1165. | |
Rosanova M, Ulrich D. Pattern-specific associative long-term potentiation induced by a sleep spindle-related spike train. J Neurosci. 2005;25(41):9398–9405. | |
Molle M, Bergmann TO, Marshall L, Born J. Fast and slow spindles during the sleep slow oscillation: disparate coalescence and engagement in memory processing. Sleep. 2011;34(10):1411–1421. | |
Spencer RM, Gouw AM, Ivry RB. Age-related decline of sleep-dependent consolidation. Learn Mem. 2007;14(7):480–484. | |
Brown RM, Robertson EM, Press DZ. Sequence skill acquisition and off-line learning in normal aging. PLoS One. 2009;4(8):e6683. | |
Nir Y, Staba RJ, Andrillon T, et al. Regional slow waves and spindles in human sleep. Neuron. 2011;70(1):153–169. | |
Rasch B, Pommer J, Diekelmann S, Born J. Pharmacological REM sleep suppression paradoxically improves rather than impairs skill memory. Nat Neurosci. 2009;12(4):396–397. | |
Genzel L, Dresler M, Wehrle R, Grozinger M, Steiger A. Slow wave sleep and REM sleep awakenings do not affect sleep dependent memory consolidation. Sleep. 2009;32(3):302–310. | |
Hornung OP, Regen F, Danker-Hopfe H, Schredl M, Heuser I. The relationship between REM sleep and memory consolidation in old age and effects of cholinergic medication. Biol Psychiatry. 2007;61(6):750–757. | |
Kubicki S, Herrmann WM, Holler L, Haag C. On the distribution of REM and NREM sleep under two benzodiazepines with comparable receptor affinity but different kinetic properties. Pharmacopsychiatry. 1987;20(6):270–277. | |
Plante DT, Goldstein MR, Cook JD, et al. Effects of oral temazepam on sleep spindles during non-rapid eye movement sleep: a high-density EEG investigation. Eur Neuropsychopharmacol. 2015;25(10):1600–1610. | |
Morgan PT, Kehne JH, Sprenger KJ, Malison RT. Retrograde effects of triazolam and zolpidem on sleep-dependent motor learning in humans. J Sleep Res. 2010;19(1 pt 2):157–164. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.