Back to Journals » Veterinary Medicine: Research and Reports » Volume 12
Situation Analysis of Varroosis and Tropilaelaps Infestation of Honeybees in Thailand, 2017–2018
Authors Thongsawang T, Rueangsom P , Boonyo K, Wongphruksasoong V, Suphanchaimat R
Received 17 February 2021
Accepted for publication 26 April 2021
Published 14 June 2021 Volume 2021:12 Pages 169—176
DOI https://doi.org/10.2147/VMRR.S306658
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Young Lyoo
Tawan Thongsawang,1 Putthipanya Rueangsom,2 Khemmapat Boonyo,1 Vilaiporn Wongphruksasoong,1 Rapeepong Suphanchaimat2,3
1Bureau of Disease Control and Veterinary Services, Department of Livestock Development, Ministry of Agriculture and Cooperatives, Bangkok, Thailand; 2International Health Policy Program, Ministry of Public Health, Nonthaburi, Thailand; 3Division of Epidemiology, Department of Disease Control, Ministry of Public Health, Nonthaburi, Thailand
Correspondence: Tawan Thongsawang
Bureau of Disease Control and Veterinary Services, Department of Livestock Development, 69/1 Phayathai Road, Ratchathewi District, Bangkok, 10400, Thailand
Email [email protected]
Background/Aim: To explore the prevalence of Varroa destructor and Tropilaelaps infestation in honeybees in Thailand and investigate factors associated with those diseases.
Methods: A quantitative cross-sectional design was employed during 2017– 2018. We sampled 144 apiaries in 13 provinces from the surveillance database of the Department of Livestock Development. In total, 1,152 bee samples were collected. A microscopic exam was performed to assess if each sample was infested with Varroa destructor mites and tropilaelaps mites. A chi-square test and multivariable logistic regression were conducted.
Results: The prevalence of Varroa destructor and Tropilaelaps infestation at the apiary level was 50.69% and 32.64%, respectively. At the beehive level, we found that the prevalence of Varroa destructor infestation was 22.74% while that of Tropilaelaps infestation was 6.94%. The northern region saw the highest prevalence of Varroa destructor and Tropilaelaps infestation. Apiaries that received a “Good Agricultural Practice” (GAP) certificate from the Bureau of Livestock Standards and Certification, demonstrated a 42% lower chance of contracting both parasitic infestations; however, no statistically significant difference was reported. Apiaries that had a history of chemical use showed approximately 2.7 times greater odds of Tropilaelaps infestation (adjusted odds ratio [AOR] = 2.69; 95% confidence interval [CI] = 1.16– 6.21) with statistical significance (p = 0.02). The probability of Varroa destructor infestation amongst apiaries with apiary movement was approximately 60% lower than amongst those without apiary movement (AOR = 0.40, 95% CI = 0.20– 0.80, p = 0.01).
Conclusion: Varroa destructor and Tropilaelaps infestations are a critical concern for beekeeping in Thailand. Apiary movement tended to lower the risk of Varroa destructor infestation while chemical use tended to enhance the risk of Tropilaelaps infestation. Further studies that allow a more comprehensive collection of determinants of parasitic infestation in honeybees, for instance, apiary cleaning frequency and farm environments (such as temperature and rainfall), are recommended.
Keywords: Tropilaelaps, Varroa destructor, honeybee, Thailand
Introduction
The apiculture business in Thailand started in 1953 and has grown rapidly since then with support from both government and private sectors.1 The development of beekeeping in Thailand is based on the intention to meet international standards. At present, the quality of Thai honey and bee products is well-accepted in wider international markets around the world. In 2015, Thailand exported over 19,000 tonnes of honey products and produced revenue of over 1.6 million Thai Baht (US$ 51,364).2
The rapid expansion of the apiculture market and beekeeping industries comes alongside high-density bee farming practices, which is likely to cause disease transmission among beehives/apiaries. Varroa destructor and Tropilaelaps infestations are amongst many parasitic diseases in beehives/apiaries that potentially undermine honey production and have a detrimental effect on bees’ health, and may eventually compromise the honey market. Both diseases were also listed as harmful diseases as specified by the World Organisation for Animal Health (OIE) and Thailand Animal Epidemics Act, B.E. 2558 (2015).3,4 Varroa spp. is also a carrier of deformed wing virus (DWV) that causes abnormality of the wings in adult bees,5 subsequently leading to colony collapse disorder (CCD).6
In addition, a decline in the stock of bees negatively affects the whole agricultural cycle as over 70% of food crops require pollination from bees.7 Therefore, bees are an essential component of food security for humans and biodiversity of the ecosystem.8
For the above reasons, the Department of Livestock Development (DLD) within the Ministry of Agriculture and Cooperatives initiated a survey on diseases, chemicals, and drug residuals in bees during 2002–2003. The survey findings revealed that Varroa destructor and Tropilaelaps infestations, and European foulbrood were commonly found in many regions of Thailand, particularly the North, the Northeast, and the South.9
During 2017–2018, the DLD launched an active surveillance system for bee diseases using bee-apiary data in 13 sentinel provinces in the Thai Good Agricultural Practices (GAP) database. However, data from the survey have not been analysed and presented in a systematic manner until now. Furthermore, apiary-related factors that may contribute to parasitic diseases in bees have also not been scientifically explored.
Thus, the purpose of this study is to describe the prevalence of parasitic mite diseases in Thailand and the correlation between potential risk factors and the presence of these diseases. The results of this study should help provide recommendations for effective surveillance and better management of parasitic diseases in the apiculture areas in Thailand.
Materials and Methods
Study Design
Cross-sectional quantitative study.
Participants and Study Sites
The study target was bee apiaries in 13 provinces registered in the database of the active surveillance system of the DLD. These provinces consisted of Chaiyaphum, Chiang Mai, Chiang Rai, Chon Buri, Chumphon, Lamphun, Loei, Nakhon Sawan, Nan, Phayao, Phitsanulok, Phrae and Uttaradit.
Sampling and Sample Size Calculation
We used a two-stage cluster sampling technique for sample acquisition. The primary sampling unit was apiaries, and the elementary sampling unit was bees. Sample size estimation was based on the prevalence estimation formula: n = Z2α/2 P(1-P)/d2, where Zα/2 = 1.96; P denoted expected prevalence and d was an acceptable error. We assumed that the expected prevalence of parasitic disease in bee samples was 0.2,9 and we presumed an acceptable error of 0.05. As we applied the two-stage cluster sampling, the sample size needed to be adjusted for design effect. In this respect, we assumed a design effect of 2.10 Based on these assumptions, the estimated number of samples amounted to 922 samples. However, to account for the possibility of incomplete information due to errors from sample collection, we increased this sample size by 20% based on the concept of probability proportional to size (PPS) for a final sample size estimate of 1,160 samples.
Initially, we planned to select 145 apiaries from a pool of 318 apiaries in the 13 provinces to obtain the 1,160 bee samples – with eight bee samples randomly collected from each apiary. However, since there was one sampled apiary that we could not approach during fieldwork, we ultimately collected 1,152 bee samples, which still outnumbered the required sample size of 922 as shown in Table 1.
 |
Table 1 Number of Bee Samples and Apiaries Selected in Each Province |
To collect the samples, we firstly collected approximately 100–200 bees in a clear bottle and covered it with a plastic lid. Secondly, we submersed the bees with 70% alcohol and waited for 30–60 minutes. Finally, we filtered the bees with a mesh strainer and collected the liquid to examine the mites under a stereomicroscope. This sample collection procedure was adapted from the OIE and the Thai National Institute of Animal Health.
Data Analysis
Both descriptive statistics and inferential statistics were applied. For descriptive statistics, frequency and percentage were used to assess the prevalence of Varroa destructor and Tropilaelaps infestations. Prevalence was analysed at two levels: (i) the beehive level and (ii) the apiary level. An apiary was classified as having contracted a disease if at least one of the bee samples tested positive for Varroa spp. or Tropilaelaps spp. from the microscopic examination. For inferential statistics, we used a Chi-square test for univariable analysis and a multivariable logistic regression for multivariable analysis; the multivariable logistic regression analysis was conducted at the apiary level only. The outcome variable of interest was the presence of Varroa destructor and Tropilaelaps infestations in the apiary, regardless of the number of infested bees. The independent variables were (i) the acquisition of a GAP certificate, (ii) history of apiary movement within 30 days before sample collection, and (iii) the use of chemicals (repellents and acaricides) within 30 days prior to the fieldwork. The measures of association were presented in terms of crude odds ratio (COR) and adjusted odds ratio (AOR) with a 95% confidence interval (95% CI).
Ethics Consideration
This study was approved by the Research Committee of the Bureau of Disease Control and Veterinary Services, Department of Livestock Development, Thailand (Permit number: 0610.04/272).
Results
Descriptive Analysis
This study found that the prevalence of Varroa destructor and Tropilaelaps infestations at the apiary level was 50.69% and 32.64%, respectively. The top three provinces with the greatest prevalence of Tropilaelaps infestation were Chon Buri, Phrae, and Uttaradit. For Varroa destructor infestation, the top three provinces were Loei, Nan, and Phrae.
At the beehive level, the overall prevalence of Tropilaelaps and Varroa destructor infestation was 6.94% and 22.74%, respectively. For Tropilaelaps infestation the province with the greatest prevalence was Uttaradit, followed by Phrae and Chon Buri. For Varroa destructor infestation, Loei had the highest prevalence, followed by Uttaradit and Nan. Chiyaphum, Phayao, Lumphun, and Nakorn Sawan saw no positive bee samples for either Tropilaelaps or Varroa destructor infestations. More details are presented in Table 2.
 |
Table 2 Prevalence of Honeybee Parasitic Diseases by Provinces |
Figure 1 provides information about the characteristics of the apiaries analysed in the study. Of the 144 apiaries participating in the survey, 86 received a GAP certificate (59.72%). Just over half of the apiaries had moved within a month prior to the survey (56.20%). Approximately a quarter of the apiaries had used chemicals at some point prior to the survey (24.82%). Note that, for the history of apiary movement and chemical usage, we had complete information from only 137 apiaries.
 |
Figure 1 Characteristics of the participating apiaries. |
Inferential Analysis
For Tropilaelaps infestations, a univariable analysis by the Chi square test revealed that 61.86% of disease-free apiaries had a GAP certificate; on the other hand, 55.32% of infested apiaries also had a GAP certificate. However, this percentage difference did not show any statistical significance. Likewise, there was no statistical significance found in apiary movement. The only variable that showed a statistically significant difference was the use of chemicals, with a COR of 2.27 and 95% CI of 1.02–5.04, Table 3.
 |
Table 3 Association of Apiary Characteristics and Tropilaelaps infestation from Univariable Analysis |
In terms of Varroa destructor infestations, apiary movement was more concentrated among disease-free apiaries than infested apiaries (67.16% versus 45.71%). This difference exhibited a statistical significance, as evidenced by the P-value of 0.01. Apiaries with a history of movement were about 59% less likely to experience Varroa destructor infestation relative to those with static domiciles. Having a GAP certificate and the use of chemicals were also more common among disease-free apiaries compared with infested apiaries; both factors did not show a statistically significant difference, Table 4.
 |
Table 4 Association of Apiary Characteristics and Varroa destructor Infestation from Univariable Analysis |
The multivariable logistic regression demonstrated that chemical usage was positively correlated with Tropilaelaps infestations (AOR = 2.69 [95% CI = 1.16–6.21]) with statistical significance (P-value = 0.02). There was no statistically significant difference between apiary movement and Tropilaelaps infestations (AOR = 0.59 [95% CI = 0.28–1.25]). A similar result was found for GAP certificate (AOR = 0.58 [95% CI = 0.27–1.25]). For Varroa destructor, apiary movement showed a negative association with developing the disease as evidenced by an AOR of 0.40 (95% CI = 0.20–0.80) with a P-value of 0.01. GAP certificate and chemical usage exhibited the same direction of association with apiary movement. Statistical significance was not found in GAP certificate and chemical usage variables (P-value = 0.14 and 0.85, respectively) as shown in Tables 5 and 6.
 |
Table 5 Association of Apiary Characteristics and Tropilaelaps Infestation from Multivariable Analysis |
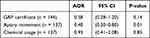 |
Table 6 Association of Apiary Characteristics and Varroa destructor Infestation from Multivariable Analysis |
Discussion
Overall, we found that most of the study samples were collected from the northern region of Thailand. The prevalence of Tropilaelaps infestations at the apiary level and at the beehive level was 32.64% and 6.94%, respectively, while the corresponding prevalence of Varroa destructor infestation was 50.69% and 22.74%, respectively. Considering the prevalence in each province, the highest apiary prevalence of Tropilaelaps infestation was in Chon Buri (100%), followed by Phrae (58.82%), and Uttaradit (50%). For Varroa destructor infestation, the provincial prevalence was greatest in Loei (100%), followed by Nan (65.38%), and Phrae (52.94%).
It appears the prevalence of Tropilaelaps and Varroa destructor infestations was quite high in most provinces in the northern region (such as Nan, Chiang Rai, and Phrae). This might be due to the region being an area where honeybee food sources are abundant (for instance, longan and lychee orchards). Phankaew also points to some specific behaviours of most orchard owners that put beehives under stress, such as the use of pesticides on orchards and prohibiting the movement of apiaries into orchards. Some longan-orchard owners do not allow beekeepers to move honeybee colonies into the orchards due to their belief that honeybees may cause longan flowers to drop and reduce the amount of longans grown.11 This practice causes stress to the honeybees as a result of food shortage in the orchards and ultimately makes honeybees more vulnerable to diseases.
The possession of a GAP certificate appears to be a protective factor for parasitic diseases, although the strength of association did not show a statistical significance. It is possible that the possession of a GAP certificate ensures beekeepers follow good beekeeping practices by introducing prevention and control programmes against honeybee diseases and parasites in every stage of production. The requirements consist of strict beekeeping hygiene, regular cleaning of apiary and beekeeping equipment, and elimination of beehives suspected of being at risk of outbreak.12 A study in Argentina by Giacobino et al supports this notion; it demonstrated that honeybee apiaries that do not follow standard management guidelines (like GAP) face a 3% greater risk of Varroa destructor infestation, compared with those following standard guidelines.13
In addition, apiary movement showed a favourable impact against Varroa destructor infestations, and this association presented a statistical significance. A possible explanation for this is that during the apiary movement process, the beekeepers usually extract honey from the honeycomb with a centrifuge machine. In this process, honeybee parasites are eliminated from beehives. The movement process also allows beekeepers to observe parasites and clean beehives. Moreover, the majority of beekeepers who move apiaries regularly are a group of commercial beekeepers that pay careful attention to the cleanliness of the beehives prior to movement.
Chemical use also demonstrated a statistically significant association with Tropilaelaps infestations, with the odds of infestation greater than one. A previous study by Chantawannakul et al reported that Tropilaelaps spp. has a short life cycle and rapid propagation. The use of chemicals over a long duration may increase the resistance of Tropilaelaps spp. towards these chemicals.14 However, chemical use seems to have some protective effect against Varroa destructor infestations, although without any statistical significance. Pettis suggested that many chemicals used by beekeepers (such as Amitraz) are likely to offer better protection against Varroa destructor infestations than Tropilaelaps infestations. However, because of the nature of Tropilaelaps spp. and Varroa spp. where they tend to remain in the combs and or in brood cells, some chemicals may not be sufficient to get rid of honeybee parasites.15 On the other hand, there is literature that supports the use of chemicals to protect beehives against parasitic diseases. A study by Haber et al suggested that varroacides, especially Amitraz, help reduce the loss of honey production during winter (winter losses).16
In terms of recommendations, we recommend that all apiaries in Thailand should follow the standards stipulated by the GAP certificate. This is supported by our studies where meeting the standards of a GAP certificate is likely to provide protective effects against parasitic diseases, although statistical significance was not shown at the 95% confidence level. However, if the confidence level is further relaxed (for instance, at the 80–85% confidence level), the protective effect of GAP certificate will be more obvious. In addition, beekeepers should be crucially aware of the risks and benefits of apiary movement and chemical use. In theory, chemicals may protect against parasites in honeybees but may create the risk of chemical resistance at the same time. Apiary movement enables the beekeepers to clean the beehives but it may cause further spread of disease to a new area in parallel.
This study contains both strengths and weaknesses. One of the strengths is the use of primary data collection on selected apiaries in all regions of Thailand. However, there are also some limitations. Firstly, the nature of a cross sectional study does not allow us to draw out strong evidence of a causal relationship between the factors of interest and parasitic diseases in honeybees. Regular data collection in the future that allows time-series analysis will be of great value. Secondly, identifying the presence of disease is largely subject to the competency of examiners; in this study, we employed more than one examiner but we provided a briefing session to all examiners prior to the fieldwork in order to ensure similar examination protocol in all apiaries. Lastly, as this study was the first national surveillance of honeybee diseases in Thailand, many variables that might be related to Varroa destructor and Tropilaelaps infestations were not collected from the outset such as apiary location, the cleaning process for each apiary, beekeeping practices, source of queen bees, the reason of chemical use (parasitic protection or elimination), density of beehive per harvest area, and the density of honeybee per beehive. This issue warrants further research to extend the academic value of beehive research in Thailand.
Conclusion
The prevalence of Varroa destructor and Tropilaelaps infestation at the apiary level was 50.69% and 32.64%, respectively. At the beehive level, the prevalence of Varroa destructor infestation was 22.74% while the prevalence of Tropilaelaps infestation was much smaller (6.94%). The northern region presented the highest prevalence of Varroa destructor and Tropilaelaps infestation. An apiary that acquired a GAP certificate was less likely to face Varroa destructor and Tropilaelaps infestations, although a statistically significant difference was not exhibited. Apiaries that had a history of chemical use showed approximately three times greater odds of Tropilaelaps infestation. The odds of encountering Varroa destructor infestation amongst apiaries with a history apiary movement was about 60% lower than those without apiary movement. We recommend that all apiaries in Thailand follow the standards stipulated by the GAP certificate. In addition, beekeepers should be crucially aware of the risk and benefits of apiary movement and chemical use. Further studies to collect comprehensive information on apiaries, including information about cleaning processes, source of queen bees, and density of beehive per harvest area would be of great academic value.
Acknowledgments
This study was supported by the Department of Livestock Development under the Ministry of Agriculture and Cooperatives (Thailand). The authors would like to thank Mr Nopporn Tohmee from the National Institute of Animal Health (NIAH) and Mr Chairoj Pocharoen from the Livestock Administrative Region 5 for extremely helpful advice and immense support on this manuscript.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The authors received funding for fieldwork from the Department of Livestock Development, Ministry of Agriculture and Cooperatives, Thailand. The publication fee was supported by the International Health Policy Program (IHPP), Ministry of Public Health, Thailand. IHPP received funding support from the Thailand Science Research and Innovation (TSRI) under the Senior Research Scholar on Health Policy and System Research [Contract No. RTA6280007].
Disclosure
The authors declare that there are no conflicts of interest.
References
1. Chantawannakul P, Wongsiri S, Chakpitak N. Beekeeping Guideline. Vol. 1. Chantawannakul P, editor. Chiang Mai: Chotana Print Company Limited; 2018.
2. Phanphum P. Report of honey production of Thailand 2014–2016. 2016. Available from: http://www.agriman.doae.go.th/home/news/year.2017/043_breed.bees.pdf.
3. World Organisation for Animal Health. Recommendations applicable to OIE Listed diseases and other diseases of importance to international trade. 2019. Available from: https://www.oie.int/en/standard-setting/terrestrial-code/access-online/.
4. Ministry of Agriculture and Cooperatives of Thailand. Notification of the Ministry of agriculture and cooperatives on the prescribing of additional animal epidemics under animal epidemic Act B.E.2558 (2015). R Thai Gov Gaz. 2015;132(special part 347 Ngor):10–15.
5. Shimanuki H, Knox DA. Diagnosis of Honey Bee Diseases. Beltsville, MD: Agricultural Research Service U.S. Department of Agriculture; 1991.
6. Ryabov EV, Childers AK, Chen Y, et al. Recent spread of Varroa destructor virus-1, a honey bee pathogen, in the United States. Sci Rep. 2017;7(1). doi:10.1038/s41598-017-17802-3
7. Center of EIA Burapha University. What happens if bees disappear from the earth? 2019. Available from: https://www.eiaburapha.com/site/2019/04/26/.
8. Food and Agriculture Organization of United Nations. Declining bee populations pose threat to global food security and nutrition. 2019. Available from: http://www.fao.org/news/story/en/item/1194910/icode/.
9. Ratananakorn L, Neramitmansook W, Wongpakorn M. Survey of bee diseases, chemical and drug residues in honey and bee products in Thailand. Khon Kaen Agric J. 2008;36:123–130.
10. Shackman G Sample size and design effect. 2001. Available from: http://faculty.smu.edu/slstokes/stat6380/deff.doc.pdf.
11. Phankaew C. Apiculture and pollinator industry survey in Thailand. Int J Agric Ext. 2016;04(02):95–103.
12. Ministry of Agriculture and Cooperatives of Thailand. Notification of the Ministry of agriculture and cooperatives on the standardization of agricultural products: good agricultural practices for bee farms under Agricultural standards act B.E. 2551 (2008). R Thai Gov Gaz. 2016;133(special part 106 Ngor):12.
13. Giacobino A, Cagnolo NB, Merke J, et al. Risk factors associated with the presence of Varroa destructor in honey bee colonies from east-central Argentina. Prev Vet Med. 2014;115(3–4):280–287. doi:10.1016/j.prevetmed.2014.04.002
14. Chantawannakul P, Natapot W, Khongpinitbunjong K, Buawangpong N, Chaimanee V, Sirisa-ard P. Parasitic mites and nosemosis in bees. Chiang Mai: Max Printing; 2011.
15. Pettis JS, Rose R, Chaimanee V, Hong XY. Chemical and cultural control of Tropilaelaps mercedesae mites in honeybee (Apis mellifera) colonies in Northern Thailand. PLoS One. 2017;12(11):e0188063. doi:10.1371/journal.pone.0188063
16. Haber AI, Steinhauer NA, vanEngelsdorp D. Use of chemical and nonchemical methods for the control of Varroa destructor (Acari: varroidae) and associated winter colony losses in U.S. beekeeping operations. J Econ Entomol. 2019;112(4):1509–1525. doi:10.1093/jee/toz088
17. Office of Agricultural Economics of Thailand. Land utilization for agriculture by province in Thailand, 2017. 2017. Available from: http://www.oae.go.th/assets/portals/1/files/Land.Utilization2560.pdf.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
