Back to Journals » Drug Design, Development and Therapy » Volume 17
Si-Ni-San Reduces Hepatic Lipid Deposition in Rats with Metabolic Associated Fatty Liver Disease by AMPK/SIRT1 Pathway
Authors Zhang N, Liu T, Wang J, Xiao Y , Zhang Y, Dai J, Ma Z , Ma D
Received 28 April 2023
Accepted for publication 21 September 2023
Published 3 October 2023 Volume 2023:17 Pages 3047—3060
DOI https://doi.org/10.2147/DDDT.S417378
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Manfred Ogris
Ning Zhang,1,* Tong Liu,1,* Jianan Wang,2,* Yingying Xiao,1 Ying Zhang,1 Jun Dai,1 Zhihong Ma,1,3 Donglai Ma4
1School of Basic Medicine, Hebei University of Chinese Medicine, Shijiazhuang, Hebei, 050200, People’s Republic of China; 2Graduate School, Hebei University of Chinese Medicine, Shijiazhuang, Hebei, 050200, People’s Republic of China; 3Hebei Key Laboratory of Integrative Medicine on Liver-Kidney Patterns, Shijiazhuang, Hebei, 050200, People’s Republic of China; 4School of Pharmacy, Hebei University of Chinese Medicine, Shijiazhuang, Hebei, 050200, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Zhihong Ma; Donglai Ma, Hebei University of Chinese Medicine, Xingyuan Road 3, Shijiazhuang, Hebei, 050200, People’s Republic of China, Tel +86 31189926238, Email [email protected]; [email protected]
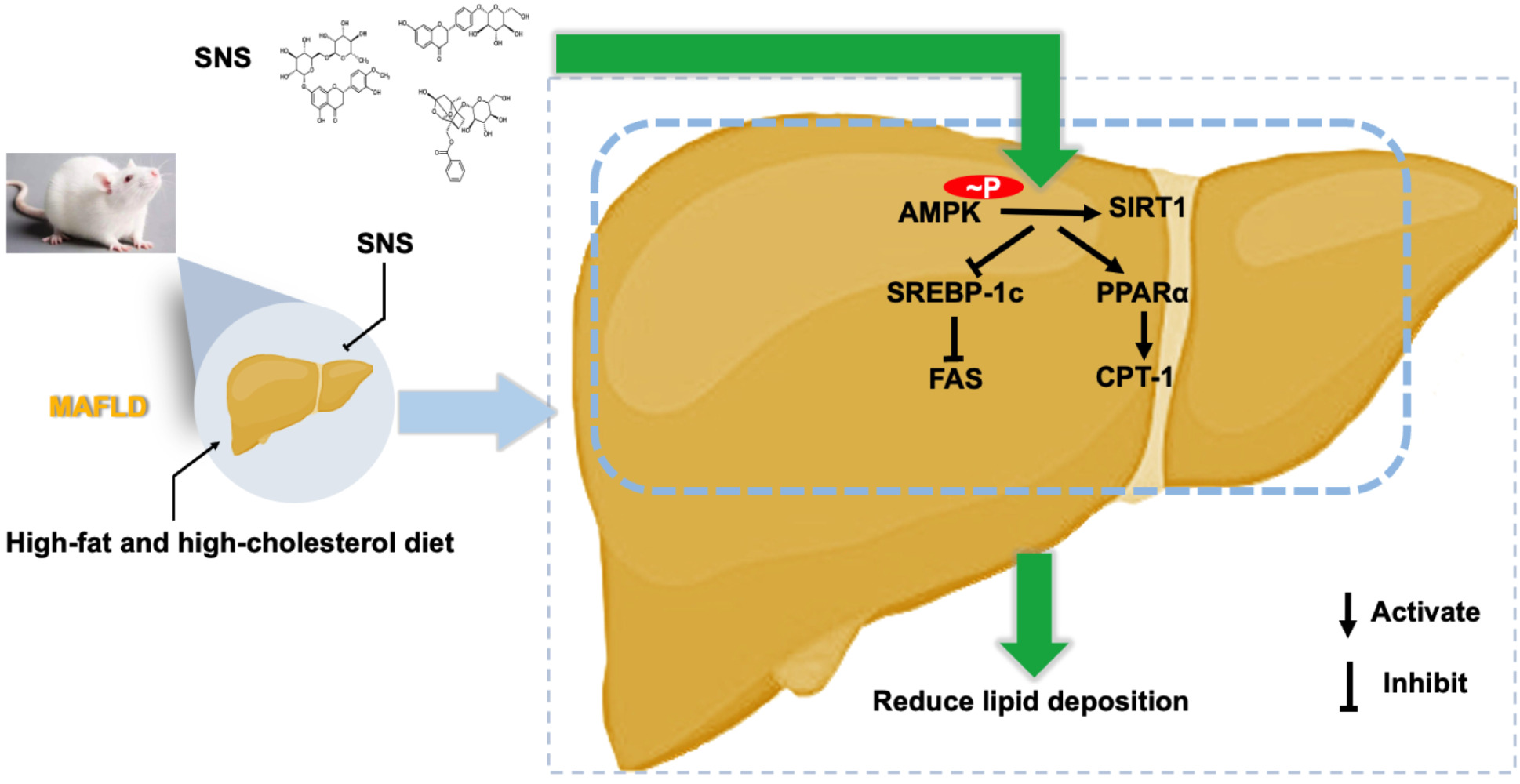
Background: Metabolic associated fatty liver disease (MAFLD) is a chronic disease characterized by excessive lipid deposition in the liver without alcohol or other clear liver-damaging factors. AMP-activated protein kinase (AMPK)/silencing information regulator (SIRT)1 signaling pathway plays an important role in MAFLD development. Si-Ni-San (SNS), a traditional Chinese medicine, has shown reducing hepatic lipid deposition in MAFLD rats, however, the underlying mechanisms of SNS are barely understood.
Purpose: The aim of this research was to investigate the mechanisms of SNS in reducing hepatic lipid deposition in MAFLD rats by regulating AMPK/SIRT1 signaling pathways.
Methods: The components of SNS were determined by high performance liquid chromatography with mass spectrometry (HPLC-MS) analysis. MAFLD rats were induced by high-fat and high-cholesterol diet (HFHCD), and treated by SNS. SNS-containing serum and Compound C (AMPK inhibitor) were used to treat palmitic acid (PA)-induced HepG2 cells. To elucidate the potential mechanism, lipid synthesis-related proteins (SREBP-1c and FAS), fatty acid oxidation (PPARα and CPT-1), and AMPK/SIRT1 signaling pathway (p-AMPK and SIRT1) were assessed by Western blot.
Results: SNS improved serum lipid levels, liver function and reduced hepatic lipid deposition in MAFLD rats. SNS-containing serum reduced lipid deposition in PA-induced HepG2 cells. SNS could up-regulate protein expressions of PPARα, CPT-1, p-AMPK and SIRT1, and down-regulate protein expressions of SREBP-1c and FAS. Similar effects of SNS-containing serum were observed in PA-induced HepG2 cells. Meanwhile, Compound C weakened the therapeutic effects of SNS-containing serum on lipid deposition.
Conclusion: SNS could reduce hepatic lipid deposition by inhibiting lipid synthesis and promoting fatty acid oxidation, which might be related with activating the AMPK/SIRT1 signaling pathway. This study could provide a theoretical basis for the clinical use of SNS to treat MAFLD.
Keywords: Si-Ni-San, MAFLD, lipid deposition, lipid synthesis, fatty acid oxidation, AMPK
Graphical Abstract:
Introduction
Non-alcoholic fatty liver disease (NAFLD) is a chronic disease characterized by excessive lipid deposition in the liver without alcohol or other clear liver-damaging factors.1 NAFLD ranges from simple steatosis to steatohepatitis, cirrhosis and hepatocarcinoma.2 Due to its association with insulin resistance and related disorders, NAFLD has been renamed metabolic associated fatty liver disease (MAFLD) in recent years.3 Based on recent data, the global prevalence of MAFLD is estimated at 25%, which is one of the most prevalent liver diseases worldwide.4 Although several drugs are in clinical trials, there is always a shortage of effective medicines recommended by the United States Food and Drug Administration (FDA).5 Therefore, effective medications for MAFLD are urgently needed.
MAFLD has a complex pathogenesis with multiple pathways involved, and lipid deposition plays an important role during the occurrence and progress of hepatic steatosis.6 Hepatic lipid deposition was usually regulated based on the following two pathways including lipid synthesis and fatty acid oxidation.7 Their imbalance leads to increased deposition of triglycerides (TG) and free fatty acids in the liver.8 Interestingly, activation and inhibition of these processes were associated with the AMP-activated protein kinase (AMPK)/silencing information regulator (SIRT)1 signaling pathway.9
AMPK is known as the energy balance sensor, and its downstream is SIRT1.10 Activating the AMPK/SIRT1 signaling pathway reduced lipid deposition by regulating lipid synthesis and fatty acid oxidation.11 Studies have revealed that metformin reduces lipid deposition by activating the AMPK/SIRT1 signaling pathway in patients with MAFLD.12 Therefore, much attention has been focused on activating the AMPK/SIRT1 signaling pathway to improve MAFLD.
Si-Ni-San (SNS), a well-known formula in traditional Chinese medicine, is used to promote the coordination of “liver and spleen” functions.This formula composes of the following four herbal medicines in equal proportions: Paeoniae Alba Radix (Bai-Shao), Bupleuri Radix (Chai-Hu), Aurantii Immaturus Fructus (Zhi-Shi), and Licorice Root (Gan-Cao).13 SNS has been widely applied to treat various liver diseases by protecting hepatocyte membranes, increasing nitric oxide release, and promoting apoptosis of liver-infiltrating cells.14 Network pharmacology-based analysis demonstrates that SNS significantly improves lipid accumulation in obesity through AMPK mediated lipolysis.15 Our previous study revealed that SNS could improve lipid deposition in MAFLD rats induced by a high-fat and high-cholesterol diet (HFHCD).16 However, whether this effect was through activating AMPK/SIRT1 signaling pathway or not remains unknown.
In this study, we hypothesized that SNS could reduce hepatic steatosis by activating the AMPK/SIRT1 signaling pathway. We evaluated the effects of SNS on reducing hepatic steatosis and explored its possible therapeutic mechanisms in HFHCD-induced MAFLD rats and palmitic acid (PA)-induced hepatocellular steatosis. This study will provide a theoretical basis for the clinical use of SNS to treat MAFLD.
Materials and Methods
Materials
Paeoniae Alba Radix (Cat: 20112301), Bupleuri Radix (Cat: 20112901), Aurantii Immaturus Fructus (Cat: 20111001), and Licorice Root (Cat: 20111301) were purchased from Shineway Pharmaceutical Co., Ltd. (Shijiazhuang, China). Plant voucher specimens identified by Professor Donglai Ma have been deposited at Hebei University of Chinese Medicine (Shijiazhuang, China). Metformin (Cat: C192010273) was purchased from China Shiyao Ouyi Pharmaceutical Co., Ltd. (Shijiazhuang, China). Paeoniflorin (Cat: C11860847, ≥98%) was purchased from Shanghai Macklin Biochemical Co., Ltd. (Shanghai, China). HFHCD (Cat: 1016712400381763584) was made by the Beijing Keao Xieli Feed Co., Ltd. (Beijing, China). Dulbecco’s modified Eagle’s medium (DMEM) (Cat: 11320033) and fetal bovine serum (FBS) (Cat: 2176377) were purchased from Thermo Fisher Scientific Co., Ltd. (Shanghai, China). Dorsomorphin (Compound C) (Cat: HY-13418A), liquiritin (Cat: HY-N0376, ≥99.68%) and hesperidin (Cat: HY-15337, ≥99.19%) were purchased from Medchemexpress Technology Co., Ltd. (Shanghai, China). Cell Count Kit-8 (CCK-8) (Cat: SB-CCK8) was purchased from Shanghai Share Biotechnology Co., Ltd. (Shanghai, China). Acetonitrile (Cat: A119010), phosphoric acid (Cat: P112025), and RIPA buffer (Cat: R301899) were purchased from Shanghai Aladdin Biochemical Technology Co., Ltd. (Shanghai, China). Kits for determining total cholesterol (TC) (Cat: A111-1-1), TG (Cat: A110-1-1), low-density lipoprotein (LDL) (Cat: A113-1-1), high-density lipoprotein (HDL) (Cat: A112-1-1), aspartate aminotransferase (AST) (Cat: C010-2-1), and alanine aminotransferase (ALT) (Cat: C009-2-1) were bought from Jian Cheng Biological Engineering Institute (Nanjing, China). Commercial kits for bicinchoninic acid (BCA) assay (Cat: CW0014), nuclear protein extract (Cat: P0027) and 5% fat-free milk (Cat: P0216) were purchased from Jiangsu CoWin Biotech Co., Ltd. (Jiangsu, China) and Beyotime Biotechnology Co., Ltd. (Shanghai, China). Antibodies against AMPK (Cat: CY5326), phosphorylated AMPK (p-AMPK) (Cat: CY6029), SIRT1 (Cat: CY5023), sterol regulatory element-binding protein (SREBP)-1c (Cat: AF4728), peroxisome proliferator-activated receptor (PPAR)α (Cat: AF5301), carnitine palmitoyl transferase (CPT)-1 (Cat: DF12150), fatty acid synthase (FAS) (Cat: CY6597), glyceraldehyde-phosphate dehydrogenase (GAPDH) (Cat: AB0036), and secondary antibodies (Cat: AB0102) were got from Shanghai Abways Biotechnology Co., Ltd. (Shanghai, China) and Jiangsu Affinity Biosciences Co., Ltd. (Jiangsu China), respectively. An enhanced chemiluminescence (ECL) kit (Cat: 36208ES60) was obtained from Yeasen Biotechnology Co., Ltd. (Shanghai, China).
Animal Ethics Statement
Male Sprague Dawley (SD) rats were purchased from Liaoning Changsheng Biotechnology Co., Ltd. [Certificate No. SCXK (Liao) 2020–0001] and were raised in the animal laboratory of the Experimental Animal Center of Hebei University of Chinese Medicine. The Animal Care and Ethical Committee of Hebei University of Chinese Medicine approved all animal protocols (approval number: DWLL2020001). All methods in this study were completed according to the 1996 National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Preparation and Qualitative Analysis of the SNS
SNS consists of four herbal medicines, including Paeoniae Alba Radix (6 g), Bupleuri Radix (6 g), Aurantii Immaturus Fructus (6 g), and Licorice Root (6 g). The preparation method of SNS was referenced from Shen et al.17 In short, these herbs were soaked in 10 times their volume of distilled water for 1 h, heated with high heat for 20 min, heated with low heat for 40 min, filtered with gauze, and concentrated to 1 g/mL using a rotary evaporator. To ensure the stability and conformity of SNS, we purchased the same batch of herbs and decocted them once a week under the same time and process, and conducted HPLC analysis on the 1st and 4th days, respectively.
The qualitative analysis of SNS was referenced to Jia et al and performed on a Shimadzu LC 20AD High Performance Liquid Chromatography (HPLC) system (Kyoto, Japan).18 The analytical column was a Thermo BDS Hypersil C18 column (250 mm × 4.6 mm, 5 μM) maintained at 40°C. The mobile phase was made of acetonitrile (solvent A) and water containing 0.1% phosphoric acid (solvent B), and the following linear gradient elution procedure was used: 0–8 min, 5–10% A, 95–90% B; 8–18 min, 10–20% A, 90–80% B; 18–30 min, 20–25% A, 80–75% B; 30–43 min, 25–30% A, 75–70% B; 43–50 min, 30–45% A, 70–55% B; 50–65 min, 45–50% A, 55–50% B; 65–75 min, 50–40% A, 50–60% B. This experiment was performed at 240 nM wavelength with a flow rate of 1 mL/min and an injection volume of 10 μL.
To further confirm the reliability of HPLC, we conducted HPLC-MS analysis. Mass spectrometry was performed on Thermo Orbitrap Exploris120 (New York, USA). The analytical column was the Hypersil Gold aQ (100 mm × 4.1 mm, 1.9 μM) maintained at 40°C. The electrospray ion (ESI) source adopted the positive ion and negative ion scanning modes. The conditions used for the ESI source were as follows: the positive and negative ion voltages are 3.5 kV and 2.5 kV, respectively. The temperature of the ion transfer tube is 325°C, the vaporization temperature is 350°C, the sheath gas flow rate is 45Arb, the auxiliary gas flow rate is 10Arb, and the purge gas flow rate is 0Arb.
Animal Modeling and Drug Administration
Forty male SD rats (200 ± 10 g) were randomly assigned to five groups (n=8): control group (CON), model group (MOD), low-dose SNS group (SNS-L), high-dose SNS group (SNS-H), and metformin group (MET). A standard diet was fed to the CON group, while HFHCD was fed to the other four groups for 8 weeks. The HFHCD consisted of a standard diet with 2% cholesterol, 10% lard oil, and 0.2% sodium cholate. The main component of lard is fat, which accounts for more than 99% of its contents.
From the 5th week of the experiment, the rats of the SNS-L, SNS-H, and MET groups were orally given SNS (2 or 4 g/kg) or metformin (150 mg/kg) for 4 weeks,15,19 and the rats in the CON and MOD groups received isovolumetric normal saline during this time, respectively. The low dose of SNS was determined by comparing the body surface area index between humans and rats. During the experiment, daily food intake and weekly body weight were recorded separately. At the end of the experiment, after fasting for 12 h, the rats were anesthetized by intraperitoneally injected with sodium pentobarbital (40 mg/kg). Blood was extracted from the abdominal aorta and centrifuged, and then the supernatant was collected and stored at −20°C for subsequent analysis. The liver was excised, weighed, and the liver index was calculated (liver weight/body weight × 100%). The same part of the liver was fixed in 4% paraformaldehyde, while the rest of the liver was stored at −80°C for further analysis.
Preparation of SNS-Containing Serum
Sixteen rats were randomly divided into two groups (n = 10/group): the normal serum group and the SNS-containing serum group. Rats were orally given isovolumetric normal saline or SNS (8 g/kg) once a day for 7 days, respectively. The blood samples were obtained from the abdominal aortas of rats at 2 h after the last administration, and centrifuged at 3000 rpm for 15 min. The supernatant was inactivated (56°C, 30 min), and the bacteria were removed by a 0.22 μM filtration membrane and then stored at −80°C for subsequent experiments.
Detection Cytotoxicity of SNS-Containing Serum
HepG2 cells were obtained from the Procell Life Science & Technology Co., Ltd. (Wuhan, China) and cultured in DMEM supplemented with 10% FBS in a humidified environment containing 5% CO2 and 95% O2 at 37°C. HepG2 cells were seeded into 96-well plates and treated with various concentrations of SNS-containing serum (0, 5%, 10%, 15%, 20%, 25%) for 12 h, and then 10 µL CCK-8 detection solution was mixed to each well, and the reaction time was 4 h at 37°C. The absorbance at 490 nm was measured by microplate reader. Finally, the viability of HepG2 cells was calculated according to the CCK-8 kit instructions.
Cell Culture and Treatment
HepG2 cells were divided into control group (CON), PA group (PA), PA + SNS-containing serum group (PA + SNS), PA + Compound C (PA + COM), and PA + Compound C + SNS-containing serum group (PA + COM + SNS). According to the cytotoxicity experiments, 10% SNS-containing serum was found to be nontoxic to HepG2 cells and used for subsequent experiments. The concentration of PA and Compound C (AMPK inhibitor) were chosen according to the reported experiments.20
In order to explore pharmacodynamic material basis of SNS, we compared the effects of SNS with the mixture of three active ingredients simultaneously on reducing lipid deposition in PA-induced HepG2 cells. The concentration of three active ingredients were chosen according to the result of HPLC-MS experiments.
Determination of Biochemistry Indexes in Serum
Serum total cholesterol (TC), triglycerides (TG), low-density lipoprotein (LDL), high-density lipoprotein (HDL), aspartate aminotransferase (AST), and alanine aminotransferase (ALT) levels were assayed according to the instructions of the commercially available kits.
Measurement of TC and TG Contents in Liver and HepG2 Cells
Liver tissues (50 mg) from each group were added to 500 μL of absolute ethanol and homogenized. After centrifugation at 2500 rpm for 10 min, the supernatant was obtained and stored at −20°C for testing. The HepG2 cells were harvested by gentle scraping and homogenized in 120 μL of the cell lysis buffer and stored at −20°C for testing. The TC and TG contents of the liver and HepG2 cells were assayed according to the specifications of the commercially available kits.
Observation of Lipid Deposition in Liver and HepG2 Cells
Liver tissue was placed in 4% paraformaldehyde, taken out for dehydration, and embedded in paraffin. The sections (4 µM) were excised and stained with hematoxylin-eosin (HE) and Oil red O staining. Finally, the slices were observed for histological changes using a microscope (Wetzlar, Germany) at 400× magnification. HepG2 cells were fixed with 4% paraformaldehyde for 1 h, and then stained with Oil red O solution for 30 min at room temperature. The cells were washed three times with deionized water and observed under a microscope. The histopathological score of HE was calculated according to the NAFLD activity scores, which were made by summation of single scores of steatosis (0–3), hepatocyte ballooning (0–2), and inflammatory cell infiltration (0–2). MAFLD was considered if the total score was greater than 3.21 The lipid droplets areas stained with Oil red O were analyzed by ImageJ software (Bethesda, USA).
Western Blot Analysis of Protein Expressions in Liver and HepG2 Cells
HepG2 cells and liver tissues were extracted in RIPA buffer and centrifuged for 30 min at 12,000 rpm. The supernatant was collected, and the protein concentration was detected using a commercial BCA protein kit. According to the instructions of the kit, the nuclear protein was extracted to detect the expressions of nuclear SREBP-1c and PPARα. An equal quantity of protein or nuclear protein from each sample was loaded and split using 10% or 8% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto polyvinylidene difluoride (PVDF) membranes. These membranes were closed using 5% skim milk for 2 h at 37°C and incubated overnight at 4°C with the following antibodies: AMPK, p-AMPK, SIRT1, SREBP-1c, CPT-1, PPARα, FAS, and GAPDH (1:1000). Next, the membranes were washed with Tris-buffered saline and Tween 20 (TBST) and incubated for 2 h at room temperature with secondary antibodies (1:5000). The immunoreactive bands were recognized using an enhanced chemiluminescence (ECL) kit and viewed using the Fusion FX5 Spectra system (Paris, France). The gray value of the Western blot band was analyzed using Vision Capt software (Kunming, China).
Statistical Analysis
All data are presented as the mean ± standard deviation (SD), and the differences among the groups were analyzed using one-way analysis of variance (ANOVA) followed by Tukey’s test using the GraphPad Prism 8.0.1 (San Diego, USA). P < 0.05 was considered statistically significant.
Results
Qualitative Analysis of the Major Bioactive Components of the SNS
The major components of SNS had been verified by HPLC and HPLC-MS. As illustrated in Figure 1A. Paeoniflorin, liquiritin, and hesperidin were determined to be the three major bioactive components of SNS and they were quantified by HPLC: paeoniflorin 3.14 mg/g, liquiritin 0.69 mg/g, and hesperidin 0.69 mg/g (Supplementary Table 1). Meanwhile, to further confirm the existence of three major bioactive components in SNS, we conducted qualitative analysis by HPLC-MS and confirmed the three major bioactive components in SNS (Figure 1B and C, Supplementary Table 2).
Effects of SNS on Body Weight and Food Intake
The body weight in the MOD group increased significantly compared to the CON group (P < 0.05), it was decreased after SNS and metformin treatment. In addition, there were no significant differences in food intake among the five groups. Thus, we excluded the anorexia effect of SNS on MAFLD rats (Supplementary Figure 1).
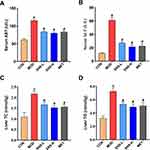
Effects of SNS on Serum Lipids Levels
Compared with the CON group, the serum TC, TG, and LDL levels were significantly increased, and the HDL level was significantly reduced in the MOD group. SNS or metformin treatment significantly reduced the serum TC, TG, and LDL levels, and increased the HDL level (all P < 0.05), with the high dose SNS and metformin being more effective (Figure 2).
Effects of SNS on Liver Function
The serum AST and ALT activities reflect liver function. The serum AST and ALT activities in the MOD group were significantly higher than those in the CON group (both P < 0.05), while they were decreased after SNS or metformin treatment (both P < 0.05). However, the serum AST and ALT activities in SNS and metformin treatment group were higher than those in the CON group, which suggested that SNS and metformin did not reverse liver damage caused by continuous feeding with a HFHCD (Figure 3A and B).
Effects of SNS on the Liver TC and TG Contents
The liver TC and TG contents of the MOD group were increased compared to the CON group (both P < 0.05), whereas they were significantly decreased by SNS or metformin treatment (both P < 0.05), with the high dose SNS being more effective (Figure 3C and D). These results indicated that SNS or metformin could reduce hepatic lipid deposition induced by the HFHCD.
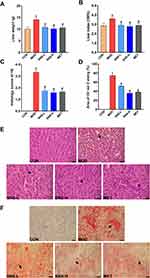
Effects of SNS on Liver Histological Changes
The liver weight and liver index were significantly increased in the MOD group compared to the CON group (both P < 0.05), but they were lowered by treatment with SNS or metformin (both P < 0.05) (Figure 4A and B).
HE and Oil red O staining showed that the livers in the CON group had distinct normal hepatocytes without obvious lipids deposits, whereas the livers in the MOD group had disordered hepatocytes and remarkable lipid droplets accumulation, but these changes were improved by SNS or metformin treatment. The histopathological scores of the livers and the quantitative analysis of the area of Oil red O staining exhibited similar trends (both P < 0.05) (Figure 4C–F).
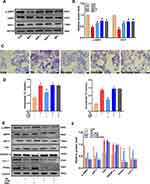
Effects of SNS on the Expression of Lipid Metabolism-Related Proteins
To investigate the mechanism by which SNS reduced lipid deposition, we detected hepatic protein expressions related to lipid metabolism, including CPT-1, FAS, nuclear and cytosolic SREBP-1c and PPARα by Western blot. As shown in Figure 5, in the way of lipid synthesis, the hepatic protein expressions of FAS and nuclear SREBP-1c were significantly up-regulated in the MOD group compared to the CON group (both P < 0.05), while they were down-regulated by SNS or metformin treatment (both P < 0.05). In the way of fatty acid oxidation, the protein expressions of CPT-1 and nuclear PPARα were significantly down-regulated in the MOD group compared to the CON group (both P < 0.05), but they were up-regulated by SNS or metformin (P < 0.05). In addition, the protein expressions of cytosolic SREBP-1c and PPARα showed opposite trends compared with those in the nucleus.
Effects of SNS on the AMPK/SIRT1 Signaling Pathway
To test whether SNS reduced lipid deposition by activating the AMPK/SIRT1 signaling pathway, we examined the protein expressions of p-AMPK and SIRT1 in liver tissue. Compared to the CON group, the protein expressions of p-AMPK and SIRT1 were significantly down-regulated in the MOD group (P < 0.05), while they were up-regulated by SNS or metformin treatment (P < 0.05) (Figure 6A and B).
To further investigate the role of AMPK activation in reducing lipid deposition effects of SNS, Compound C was used to block AMPK activation in HepG2 cells. According to the cytotoxicity experiments, 10% SNS-containing serum was found to be nontoxic to HepG2 cells (Supplementary Figure 2). As shown in Figure 6C–F, compared to the CON group, the lipid droplets and the contents of TC and TG were significantly increased in the PA group (P < 0.05), while they were reduced by SNS-containing serum (P < 0.05). Compared to the group PA + SNS, decreases in lipid droplet, TG, and TC contents of HepG2 cells induced by SNS were abolished in the PA+COM+SNS group. Interestingly, compared to the mix of paeoniflorin, liquiritin and hesperidin, SNS-containing serum has a better effect on reducing lipid deposition in HepG2 cell (Supplementary Figure 3). In addition, Compound C inhibited the activation of the AMPK/SIRT1 signaling pathway, up-regulated the protein expressions of SREBP-1c and FAS, and down-regulated the protein expressions of PPARα and CPT-1. However, the effects of SNS on the AMPK/SIRT1 signaling pathway and its downstream were blocked by co-incubation of compound C and SNS. The results suggested that AMPK/SIRT1 signaling pathway was crucial for the role of SNS in reducing hepatic lipid deposition.
Discussion
It has been reported that SNS could reduce lipid deposition in HFHCD-induced MAFLD rats, but the underlying mechanism was unclear. In this study, we investigated the mechanism of SNS in reducing hepatic lipid deposition in vitro and in vivo. We fed the rats with a HFHCD to induce MAFLD and cultured HepG2 cells with PA to induce hepatocellular steatosis. Our data suggested that SNS reduced hepatic lipid deposition by inhibiting lipid synthesis and promoting fatty acid oxidation, which might be associated with activation of the AMPK/SIRT1 signaling pathway in part.
NAFLD is a clinicopathologic syndrome characterized by hepatic lipid deposition and metabolic dysfunction.22 The risk factors for NAFLD include a high-fat high-calorie diet, a less active lifestyle, obesity, and other factors.23,24 As NAFLD is largely associated with insulin resistance and related disorders, it has been renamed metabolic associated fatty liver disease (MAFLD) in recent years.3 There are many methods for obtaining animal models of MAFLD, including chemical models, genetic models, and dietary models, such as a methionine and choline-deficient diet, atherogenic diet, and HFHCD.25 Because increased dietary intake of high fat and high cholesterol is a major risk factor for the development of MAFLD, the HFHCD is widely used to establish MAFLD model.26 Previous reports had shown that rats fed with a HFHCD for 8 weeks could serve as an experimental model of MAFLD, with typical hepatic lesions, including hepatomegaly, hepatocyte ballooning and steatosis, accompanied by elevated TC and TG in serum and liver compared with the rats fed a standard diet.27 In the present study, rats fed with the HFHCD developed similar pathological characteristics to those described above, indicating that we had successfully established MAFLD model.
HFHCD can cause dyslipidemia, while improving of dyslipidemia can alleviate MAFLD progression. Our results suggested that SNS and metformin could lower serum TC, TG, and LDL, in line with Cheng’s report in which SNS down-regulated the levels of serum lipids in MAFLD rats.13 This result further confirmed that SNS had the effect of improving dyslipidemia, but the involved mechanisms need further in-depth study. The important pathological change of MAFLD rat induced by HFHCD was hepatic lipid deposition accompanied by liver dysfunction.28 The hepatic lipid deposition was reflected by liver TC and TG contents, HE and Oil red O staining, and the degree of liver function damage was reflected by AST and ALT activity.29 In this study, the obvious hepatic lipid deposition and the liver function damage were seen in the MAFLD rats. Interestingly, SNS and metformin could improve these changes, as evidenced by improved liver tissue morphology, as well as decrease in serum AST and ALT activities and liver TC and TG contents. The results agreed with the study conducted by Xu et al,30 and further confirmed that SNS could reduce hepatic lipid deposition and alleviate liver damage. However, during continuous feeding a HFHCD, SNS could not reversed these changes to normal, which suggested the importance of diet control.
Many factors affect hepatic lipid deposition, including increased lipid synthesis and impaired fatty acid oxidation.3 SREBP-1c and FAS play critical roles in hepatic lipid synthesis, and overexpressed SREBP-1c and FAS cause hepatic lipid deposition, and then lead to MAFLD.31 In addition, PPARα promotes fatty acid oxidation by up-regulating the transcription of CPT-1.32 It has been shown that the activity of the PPARα/CPT-1 pathway is obviously decreased, which contributes to the progression of hepatic steatosis.33,34 In this study, the protein expressions of SREBP-1c and FAS were significantly increased in HFHCD-fed rats, whereas the protein expressions of both PPARα and CPT-1 were significantly decreased. SNS and metformin could decrease the expressions of SREBP-1c and FAS, while increase the expressions of PPARα and CPT-1. Taken together, our results suggested that SNS could reduce lipid deposition by reducing lipid synthesis and promoting fatty acid oxidation.
As an important intracellular energy sensor, the AMPK/SITR1 signaling pathway plays a key role in lipid synthesis and metabolism.12 Growing evidence suggests that the AMPK/SIRT1 signaling pathway participates in the hepatic lipid metabolism in MAFLD.35 As reported in previous studies, the activation of AMPK increases intracellular NAD+ levels and activates SIRT1, and then inhibits fatty synthesis through SREBP-1c and FAS.36 Likewise, activation of the AMPK/SIRT1 signaling pathway up-regulates PPARα and CPT-1 expression and then promotes fatty acid oxidation.37 In this study, the AMPK/SIRT1 signaling pathway was blocked in HFHCD fed MAFLD rats and PA-induced HepG2 cells, and SNS could activated the AMPK/SIRT1 signaling pathway. However, when AMPK/SIRT1 signaling pathway was blocked by compound C, this effect of SNS was suppressed. This implied that SNS could be involved in lipid synthesis and fatty acid oxidation by activating the AMPK/SIRT1 signaling pathway, which in turn effectively reduces hepatic lipid deposition.
As a formula for treating MAFLD, SNS has the advantages of being nontoxic and inexpensive.38 The modern pharmacological research reveals that SNS has multiple ingredients, such as paeoniflorin, hesperidin, liquiritin, and quercetin, and has multiple targets to exert synergistic therapeutic effects.39 Previous studies had shown that paeoniflorin exhibits hepatoprotective effects acting as an endogenous agonist of liver X receptor alpha (LXRα). Hesperidin could reduce hepatic lipid deposition though activating PPARγ.40 Liquiritin has been reported to alleviate liver injury by regulating farnesoid X receptor (FXR) in liver.41 We further confirmed that SNS contains these bioactive components through HPLC and HPLC-MS, compared the effects of SNS-containing serum and the mixture of three bioactive components simultaneously on reducing lipid deposition in PA-induced HepG2 cells. The results showed that SNS-containing serum had a better effect on reducing lipid deposition than the mixture of three active ingredients, which implied that SNS not only relied on the combination of these three components, but other components also play a role, however, more experiments need to determine the relationships among the different components of SNS.
Conclusion
Our results showed that SNS could reduce lipid deposition in MAFLD rats possibly by activating the AMPK/SIRT1 signaling pathway. Therefore, SNS may be viewed as a potential therapeutic medication for MAFLD. However, more experiments need to investigate the precise molecular mechanism of SNS on the AMPK/SIRT1 signaling pathway and clarify the pharmacodynamic material basis of SNS in the future.
Acknowledgments
This work was supported by the Hebei Administration of Traditional Chinese Medicine (NO. 2022084), the Hebei Key Laboratory of Integrative Medicine on Liver-Kidney Patterns (NO. A202107), the Hebei Province Innovation Ability Promotion Plan (NO. 20567624H), and the College Students Innovative Training Project of Hebei University of Chinese Medicine (NO. 202214432009).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Friedman SL, Neuschwander-Tetri BA, Rinella M, Sanyal AJ. Mechanisms of NAFLD development and therapeutic strategies. Nat Med. 2018;24(7):908–922. doi:10.1038/s41591-018-0104-9
2. Cobbina E, Akhlaghi F. Non-alcoholic fatty liver disease (NAFLD) - pathogenesis, classification, and effect on drug metabolizing enzymes and transporters. Drug Metab Rev. 2017;49(2):197–211. doi:10.1080/03602532.2017.1293683
3. Samuel VT, Shulman GI. Nonalcoholic fatty liver disease as a nexus of metabolic and hepatic diseases. Cell Metab. 2018;27(1):22–41. doi:10.1016/j.cmet.2017.08.002
4. Bugianesi E, Petta S. NAFLD/NASH. J Hepatol. 2022;77(2):549–550. doi:10.1016/j.jhep.2022.02.006
5. Shen B, Lu LG. Efficacy and safety of drugs for nonalcoholic steatohepatitis. J Dig Dis. 2021;22(2):72–82. doi:10.1111/1751-2980.12967
6. Powell EE, Wong VW, Rinella M. Non-alcoholic fatty liver disease. Lancet. 2021;397(10290):2212–2224. doi:10.1016/s0140-6736(20)32511-3
7. Ipsen DH, Lykkesfeldt J, Tveden-Nyborg P. Molecular mechanisms of hepatic lipid accumulation in non-alcoholic fatty liver disease. Cell Mol Life Sci. 2018;75(18):3313–3327. doi:10.1007/s00018-018-2860-6
8. Geng Y, Faber KN, de Meijer VE, Blokzijl H, Moshage H. How does hepatic lipid accumulation lead to lipotoxicity in non-alcoholic fatty liver disease? Hepatol Int. 2021;15(1):21–35. doi:10.1007/s12072-020-10121-2
9. Anggreini P, Kuncoro H, Sumiwi SA, Levita J. Role of the AMPK/SIRT1 pathway in non‑alcoholic fatty liver disease (Review). Mol Med Rep. 2023;27(2):35. doi:10.3892/mmr.2022.12922
10. Day EA, Ford RJ, Steinberg GR. AMPK as a therapeutic target for treating metabolic diseases. Trends Endocrinol Metab. 2017;28(8):545–560. doi:10.1016/j.tem.2017.05.004
11. Chen XY, Cai CZ, Yu ML, et al. LB100 ameliorates nonalcoholic fatty liver disease via the AMPK/Sirt1 pathway. World J Gastroenterol. 2019;25(45):6607–6618. doi:10.3748/wjg.v25.i45.6607
12. Fang C, Pan J, Qu N, et al. The AMPK pathway in fatty liver disease. Front Physiol. 2022;13:970292. doi:10.3389/fphys.2022.970292
13. Cheng F, Ma C, Wang X, et al. Effect of traditional Chinese medicine formula Sinisan on chronic restraint stress-induced nonalcoholic fatty liver disease: a rat study. BMC Complement Altern Med. 2017;17(1):203–213. doi:10.1186/s12906-017-1707-2
14. Zhu F, Li YM, Feng TT, et al. Freeze-dried Si-Ni-San powder can ameliorate high fat diet-induced non-alcoholic fatty liver disease. World J Gastroenterol. 2019;25(24):3056–3068. doi:10.3748/wjg.v25.i24.3056
15. Li J, Wu K, Zhong Y, et al. Si-Ni-SAN ameliorates obesity through AKT/AMPK/HSL pathway-mediated lipolysis: network pharmacology and experimental validation. J Ethnopharmacol. 2023;302(Pt A):115892. doi:10.1016/j.jep.2022.115892
16. Song L, Chen C, Li CX, Yang Y. Hepatoprotection of Sini Powder on rat with non-alcoholic steatohepatitis. Hebei Univer Chin Med. 2019;34(03):1–4. doi:10.16370/j.cnki.13-1214/r.2019.03.001
17. Shen C, Cao K, Cui S, et al. SiNiSan ameliorates depression-like behavior in rats by enhancing synaptic plasticity via the CaSR-PKC-ERK signaling pathway. Biomed Pharmacother. 2020;124:109787. doi:10.1016/j.biopha.2019.109787
18. Jia KK, Chen SS, Xu MJ, et al. Simultaneous determination of eight constituents in sini powder by HPLC. Chin Tradit Patent Med. 2022;44(2):372–375. doi:10.3969/j.issn.1001-1528.2022.02006
19. El-Din SH S, Salem MB, El-Lakkany NM, et al. Early intervention with probiotics and metformin alleviates liver injury in NAFLD rats via targeting gut microbiota dysbiosis and p-AKT/mTOR/LC-3II pathways. Hum Exp Toxicol. 2021;40(9):1496–1509. doi:10.1177/0960327121999445
20. Xiao Q, Zhang S, Yang C, et al. Ginsenoside Rg1 ameliorates palmitic acid-induced hepatic steatosis and Inflammation in HepG2 Cells via the AMPK/NF-κB Pathway. Int J Endocrinol. 2019:7514802. doi:10.1155/2019/7514802
21. El-Sherbiny M, Eldosoky M, El-Shafey M, et al. Vitamin D nanoemulsion enhances hepatoprotective effect of conventional vitamin D in rats fed with a high-fat diet. Chem Biol Interact. 2018;288:65–75. doi:10.1016/j.cbi.2018.04.010
22. Kuchay MS, Choudhary NS, Mishra SK. Pathophysiological mechanisms underlying MAFLD. Diabetes Obes Metab. 2020;14(6):1875–1887. doi:10.1016/j.dsx.2020.09.026
23. Kanuri G, Bergheim I. In vitro and in vivo models of non-alcoholic fatty liver disease (NAFLD). Int J Mol Sci. 2013;14(6):11963–11980. doi:10.3390/ijms140611963
24. Younossi Z, Anstee QM, Marietti M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15(1):11–20. doi:10.1038/nrgastro.2017.109
25. Van Herck MA, Vonghia L, Francque SM. Animal models of nonalcoholic fatty liver disease-A starter’s guide. Nutrients. 2017;9(10):1072. doi:10.3390/nu9101072
26. Jahn D, Kircher S, Hermanns HM, Geier A. Animal models of NAFLD from a hepatologist’s point of view. Biochim Biophys Acta Mol Basis Dis. 2019;1865(5):943–953. doi:10.1016/j.bbadis.2018.06.023
27. Nie H, Deng Y, Zheng C, et al. A network pharmacology-based approach to explore the effects of Chaihu Shugan powder on a non-alcoholic fatty liver rat model through nuclear receptors. J Cell Mol Med. 2020;24(9):5168–5184. doi:10.1111/jcmm.15166
28. Suzuki S, Sato Y, Umegaki K, Chiba T. Influence of high-fat and high-cholesterol diet on major CYP activities in the liver. Yakugaku Zasshi. 2016;136(9):1297–1305. doi:10.1248/yakushi.16-00034
29. Yin Y, Liu H, Zheng Z, Lu R, Jiang Z. Genistein can ameliorate hepatic inflammatory reaction in nonalcoholic steatohepatitis rats. Biomed Pharmacother. 2019;111:1290–1296. doi:10.1016/j.biopha.2019.01.004
30. Xu W, Du X, Li J, et al. SiNiSan alleviates liver injury by promoting hepatic stem cell differentiation via Wnt/β-catenin signaling pathway. Phytomedicine. 2022;99:153969. doi:10.1016/j.phymed.2022.153969
31. Fang K, Wu F, Chen G, et al. Diosgenin ameliorates palmitic acid-induced lipid accumulation via AMPK/ACC/CPT-1A and SREBP-1c/FAS signaling pathways in LO2 cells. BMC Complement Altern Med. 2019;19:255–267. doi:10.1186/s12906-019-2671-9
32. Kim B, Woo MJ, Park CS, et al. Hovenia dulcis extract reduces lipid accumulation in oleic acid-induced steatosis of hep G2 Cells via Activation of AMPK and PPARα/CPT-1 pathway and in acute hyperlipidemia mouse model. Phytother Res. 2017;31(1):132–139. doi:10.1002/ptr.5741
33. Zheng F, Cai Y. Concurrent exercise improves insulin resistance and nonalcoholic fatty liver disease by upregulating PPAR-γ and genes involved in the beta-oxidation of fatty acids in ApoE-KO mice fed a high-fat diet. Lipids Health Dis. 2019;18(1):6. doi:10.1186/s12944-018-0933-z
34. Yang H, Deng Q, Ni T, et al. Targeted Inhibition of LPL/FABP4/CPT1 fatty acid metabolic axis can effectively prevent the progression of nonalcoholic steatohepatitis to liver cancer. Int J Biol Sci. 2021;17(15):4207–4222. doi:10.7150/ijbs.64714
35. Chang YH, Chen YL, Huang WC, Liou CJ. Fucoxanthin attenuates fatty acid-induced lipid accumulation in FL83B hepatocytes through regulated Sirt1/AMPK signaling pathway. Biochem Biophys Res Commun. 2018;495(1):197–203. doi:10.1016/j.bbrc.2017.11.022
36. Chyau CC, Wang HF, Zhang WJ, et al. Antrodan alleviates high-fat and high-fructose diet-induced fatty liver disease in C57BL/6 Mice Model via AMPK/Sirt1/SREBP-1c/PPARγ Pathway. Int J Mol Sci. 2020;21(1):350–359. doi:10.3390/ijms21010360
37. Diniz TA, de Lima Junior EA, Teixeira AA, et al. Aerobic training improves NAFLD markers and insulin resistance through AMPK-PPAR-α signaling in obese mice. Life Sci. 2021;266:118868. doi:10.1016/j.lfs.2020.118868
38. Shu Z, He W, Shahen M, et al. Clarifying of the potential mechanism of Sinisan formula for treatment of chronic hepatitis by systems pharmacology method. Biomed Pharmacother. 2018;100:532–550. doi:10.1016/j.biopha.2018.02.047
39. Wei X, Hou W, Liang J, et al. Network pharmacology-based analysis on the potential biological mechanisms of sinisan against non-alcoholic fatty liver disease. Front Pharmacol. 2021;12:693701. doi:10.3389/fphar.2021.693701
40. Meng HW, You HM, Yang Y, et al. 4-Methylcoumarin-[5,6-g]-hesperetin attenuates inflammatory responses in alcoholic hepatitis through PPAR-γ activation. Toxicology. 2019;421:9–21. doi:10.1016/j.tox.2019.04.004
41. Yan M, Guo L, Ma J, et al. Liquiritin alleviates alpha-naphthylisothiocyanate-induced intrahepatic cholestasis through the Sirt1/FXR/Nrf2 pathway. J Appl Toxicol. 2023;43(3):350–359. doi:10.1002/jat.4385
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.