Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 19
Shifting Responsibilities: Developing a Pan-European Service Model for an eHealth Technology Supporting Self-Management of People with Chronic Obstructive Pulmonary Disease and Comorbidities
Authors te Braake E , Grünloh C , Tabak M
Received 5 September 2023
Accepted for publication 15 December 2023
Published 17 January 2024 Volume 2024:19 Pages 175—192
DOI https://doi.org/10.2147/COPD.S432568
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Eline te Braake,1,2 Christiane Grünloh,1,2 Monique Tabak2
1Roessingh Research and Development, Enschede, the Netherlands; 2University of Twente, Biomedical Signals and System Group, Faculty of Electrical Engineering, Mathematics, and Computer Science, Enschede, the Netherlands
Correspondence: Eline te Braake, Roessingh Research and Development, Roessinghsbleekweg 33b, Enschede, 7522AH, the Netherlands, Tel +31 88 087 5734, Email [email protected]
Introduction: Active participation of patients in their care via self-management is an important pillar to manage chronic conditions. Self-management education and continuous support are needed to improve patients’ confidence to take such active role. One way to do this is through eHealth technologies. However, those technologies can only be successful when actively used in daily practice and when integrated in overall care. Therefore, this study investigated how a self-management eHealth technology could be implemented that emphasises the active role of patients in their care.
Methods: The service modelling method was utilized as implementation strategy. The design process consisted of five phases with salient stakeholders and consortium members of a European project to develop the service model. Studies with salient stakeholders were carried out in three different countries (Italy, Estonia, the Netherlands). A combination between face-to-face and online methods facilitated the participatory design process.
Results: Due to the pan-European context, different stakeholders in the three countries were identified. Research nurses and case managers were not yet established in practice but once implemented, expected to contribute to optimal implementation. During service modelling, a crucial step was revealed: providing self-management training before technology use to let patient familiarise with the concept of taking an active role. As HCPs felt that they were not necessarily equipped to guide patients in terms of self-management, they also should have access to such self-management training.
Conclusion: By demonstrating a way for implementation while emphasising patients’ active role, we also showed the complexity of the method in two ways. First, by demonstrating the fine line between the descriptive and prescriptive model. Thus, showcasing the need to recognize that prescriptive models may be hampered by the delay in changing work practices. Second, by highlighting the importance of identifying country-specific differences in the pan-European context, revealing that service modelling is not a one-size-fits-all approach.
Keywords: service model, self-management, patient empowerment, responsibility, eHealth, COPD
Introduction
Chronic Obstructive Pulmonary Disease (COPD) is a heterogeneous lung condition characterized by chronic respiratory symptoms (dyspnea, cough, expectoration and/or exacerbations) due to abnormalities of the airways (bronchitis, bronchiolitis) and/or alveoli (emphysema) that cause persistent, often progressive, airflow obstructions.1,2 COPD is one of the major problems of public health and is steadily increasing in prevalence, morbidity, and mortality.1,3,4 Because of this increase, COPD was listed as third leading cause of death in 2019.5
The management of COPD is complex. One reason for this complexity is that COPD often coexists with other diseases, impacting the disease course.1 These comorbidities worsen patients’ outcomes and increase the economic burden and mortality.6–8 Because of these comorbidities, multiple different healthcare professionals (HCPs) are involved and ideally, need to work together to provide a multidisciplinary treatment strategy.9 However, this may not always be the case in practice, making it challenging to align the different advices. Additionally, the chronic nature of the disease implies that the largest part of care is originated at the home of the patient without HCPs having the possibility to monitor or support them. The complexity also shows in that patients may experience acute exacerbations: “A episode of sustained symptom worsening that might require specialised treatment”.10 Exacerbations negatively affect patients’ health status and wellbeing, making early diagnosis and prevention essential.11 Therefore, to manage COPD, quite some effort is required from both HCPs and patients. An active role of the patient within their disease management is here essential. This is often referred to as self-management.
Self-management can be defined as:
The ability of the individual, in conjunction with family, community, and HCPs, to manage symptoms, treatments, lifestyle changes, and psychosocial, cultural, and spiritual consequences of health conditions.12
It challenges patients to take more responsibility and ownership towards their own health and well-being. With taking more responsibility, patients may take charge of their illness and become active participants in care.13
As recently concluded by Wang et al,14 patient empowerment may improve confidence and adherence to self-management with self-efficacy mediating this relationship. Self-efficacy refers to the level of confidence in one’s ability to perform self-managements activities.12 Patient empowerment can be conceptualised as a process that: “enhances the patient’s feeling of control, self-efficacy, coping abilities, and ability to achieve change over their condition”.15 In addition, it is increasingly advocated that clinical decision-making takes part in a collaboration between patient and clinician (ie, shared decision-making16,17). For a patient to be able to engage in shared decision-making in the first place, it can be assumed that some level of empowerment has to be reached already. Therefore, both mechanisms can be considered to be important in the process of self-management.
One way to empower patients and support them in their self-management is through eHealth technologies: “The actual technological instrument via which health, well-being and healthcare are supported, often information or communication technology”.18 Many benefits are expected; it has the potential to reduce healthcare costs, to improve accessibility of healthcare, and to empower patients in their self-management.19–23 To eventually achieve these expected benefits for patients, it is important to involve them early on in the different stages of the development process, eg, during requirement elicitation, prototype development, and usability testing. Such co-design approaches may enhance learning and empower patients.24 In this way, group specific challenges (such as low (e) Health literacy and low motivation25) can be taking into consideration early on and the technology can be fitted towards the specific needs and capabilities of the patients. In recent years, many self-management eHealth technologies have been developed, aiming to support patients with chronic diseases and positively change healthcare (eg,26–28).
An example of such an eHealth technology aiming to improve healthcare and COPD self-management is RE-SAMPLE. RE-SAMPLE is a research and innovation project (Horizon 2020 Grant Agreement No. 965315) that aims to develop an adaptive virtual companionship programme that supports people with COPD and comorbidities in their self-management.29 In addition, a clinical dashboard will be developed to support HCPs monitoring their patients and making decisions. RE-SAMPLE collects real-world data (RWD) from cohorts in 3 clinical pilot sites: Medisch Spectrum Twente (MST) in the Netherlands (NL), Gemelli hospital in Italy (IT), and Tartu hospital in Estonia (EST). The monitoring function of RE-SAMPLE will collect data from a Garmin smartwatch (activity, sleep, heart rate, oxygen saturation), data from several questionnaires, and from a Electronic Patient Reported Outcome measure (ePROMs). Such monitoring functionalities, ePROMS in particular, have been increasingly used in practice and shown to be beneficial for eg shared decision-making and supporting self-management.30,31 This data, among others, will be used to develop prediction models through artificial intelligence (AI).29 The virtual companionship programme includes self-management support by means of coaching. Thus, all aspects of the technology aim to engage and support people with COPD and comorbidities in their care.
These services can only be successful when actively used in daily practice. However, not much is known about the roles and responsibilities that stakeholders, especially patients and HCPs should take when RE-SAMPLE is implemented in daily practice. To shape this new way of providing care, an implementation strategy should be prepared as early as possible during the development phase of the eHealth technology.32 This strategy should focus on identifying important stakeholders, structures, and processes within daily practice. Implementation models may guide this strategy and should be a central part of the eHealth technology development. To eventually improve care while using this eHealth technology, its implementation should be feasible and realistic, while empowering patients.33,34
There are various methods available for developing an implementation model.32,35–38 This study utilized the service modelling method (also referred to as service blueprinting) as it provides a complete picture of implementation processes and is clearly demonstrated by means of a service model. A service model is a schematic representation of how an eHealth technology should be implemented in practice39 and entails components such as: end-user actions, stakeholder actions, and support services.38 It describes all the activities and responsibilities of the stakeholders of a specific eHealth technology. An individual or an organization can be considered stakeholder when they affect or are affected by the project.40 Because the activities of stakeholders are part of the model, and they will eventually use the service or support its implementation, it is critical to understand their needs, concerns, and expectations.41 By engaging stakeholders, their interests and needs can be identified and accounted for in the service model, by which support and motivation for future implementation of an eHealth technology may be created.39
This paper investigates how a newly developed eHealth technology could be implemented in practice that emphasises the active role of people with COPD and comorbidities in their care. To support successful implementation, the service model was iteratively developed using the service modelling method38 with key stakeholders in three European countries. The paper contributes by detailing the process of the service model design and by describing a pan-European service model for a COPD eHealth technology that stresses the importance of taking responsibility within self-management.
Methods
This study is part of the RE-SAMPLE project. During the time of the current study, the project was in its development phase. To develop the service model, this study pursues the iterative and gradual design process as proposed by Broekhuis et al.39 In this process, salient stakeholders are continuously involved to incorporate their perspectives and needs and to gather their feedback on the different iterations of the service model, to ensure that their perspectives have been correctly understood and accounted for. Salient stakeholders are those stakeholders that are giving priority to within the project.42 Components of a service model as defined by Bitner et al38 (eg, end-user actions, support processes) were taken into consideration during the design process, and project-specific components, such as the components of the RE-SAMPLE technology, were added to tailor the service model. In the empirical studies conducted at the start of the project (eg, co-creating patient journeys, eliciting user needs, understanding the context), patients from all three countries participated in creating the basis for the service modelling. The insights from these studies (obtained from patients’ needs, wishes, and experiences), together with the proposed vision within the RE-SAMPLE project, were used as input for the building blocks of the RE-SAMPLE eHealth technology. These building blocks served as starting point of the service model design, consisting of: peer-to-peer contact (the possibility to be in contact with other people with COPD and CCC), data collection (data collected from the Garmin smartwatch, questionnaire data, and ePROMS), self-management and coaching (self-management support and coaching on different topics relevant for the patient), risk alert (alert from the system when unusual symptoms are detected), data review (process in which a HCP reviews the collected data of the patient when unusual symptoms are detected and a data review is requested by the patient), exacerbation (worsening of symptoms), and hospitalization. Figure 1 shows a simplified version of these building blocks. A more detailed version of this figure was used as starting point to facilitate the first iterations.
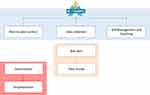 |
Figure 1 Building blocks of the RE-SAMPLE eHealth technology. |
Design and Setting
Table 1 shows the service model design process consisting of 5 phases with salient stakeholders and consortium members to develop the service model involving three iterations of the model. Studies with salient stakeholders were carried out in the three different pilot sites of the RE-SAMPLE project. In the studies where consortium members were invited, the different pilot sites and different perspectives were discussed together in break-out and plenary sessions. The studies were carried out by the consortium partner leading the task “Service model development” (anonymised for submission) between January and August 2022. A combination between face-to-face and online studies facilitated the design process. All participants received information about the study and gave their (digital) informed consent before participating. The nature of this study does not require formal medical ethical approval. This was assessed by the Medical Research Ethics Committee (MREC) Oost-Nederland in terms of the Medical Research Involving Human Subjects Act (WMO) obligation (File number: 2021–13,319). All procedures were in accordance with the Declaration of Helsinki and the Good Clinical Practice (GCP) guidelines.
 |
Table 1 Overview Design Activities of the RE-SAMPLE Service Model |
Round 1. Stakeholder Identification (Clinical Partners of the Consortium)
As the first step in the service model development, potential important stakeholders were identified in all three pilot sites, by means of an online workshop conducted with the clinical partners within the project. This workshop aimed to identify stakeholders that might be important to put the eHealth solution into practice. Clinical partners from the RE-SAMPLE consortium were asked as domain experts43 to identify relevant stakeholders for the stakeholder identification. These members were first asked to create a general list of important stakeholders for their respective country and later created a top 15 of the most important stakeholders. Discussions during the workshop were audio recorded, and stakeholder identification exercises were documented within Mural,44 an online platform for visual collaboration. Results from the Mural board were used as input for round 2.
Round 2. Stakeholder Saliency Analysis (Consortium Members and Potential Stakeholders)
Using the outcomes from the stakeholder identification workshop, an online stakeholder saliency survey (Supplementary Material 1) was designed in Qualtrics45 applying the constructs: power, legitimacy, and urgency.42 The goal was to identify the salient stakeholders of the RE-SAMPLE project by help of direct stakeholders in each pilot country. The survey was designed in three parts representing the three constructs. For each construct, participants could rate on a 5-point Likert scale whether a certain stakeholder was salient (1: not at all - 5: very much). The survey was distributed via e-mail and shared with both HCPs and people with COPD. Outcomes were analyzed using descriptive statistics. For each pilot site in RE-SAMPLE, the top 5 (the highest mean) were identified to be the most salient.
Round 3. Identification of Current Practices (Salient Stakeholders)
The results of the stakeholder salience survey were used to invite salient stakeholders to the first workshop. Three (online) workshops were carried out in the different pilot sites to collect input for the development of the initial service model (Version A). This workshop aimed to collect information about current COPD care practices and the desired roles and responsibilities within the eHealth technology in each pilot site. To achieve the workshop goals, an inventory of the current situation and identification of weak and strong point in care was made. A patient journey, developed in the early phase of the project and based on interviews with both patients and HCPs in all 3 countries, was used during these activities to support the inclusion of the patient perspective.46 In line with the user-centred design process,47 a persona- and scenario-based approach was utilized to understand and discuss the current care practices, which facilitates the design of the new situation (ie, using the eHealth technology in practice). The desired roles and responsibilities of salient stakeholders and potential strengths and weaknesses of current practice of care were discussed. All workshops were audio-recorded, and workshop outcomes were documented within Mural or with the use of post-its (depending on whether the workshop was online or offline). Both documented and audio-recorded data collected during the workshops were combined and used as input for the initial service model development (Version A).
Round 4. Feedback (Partners Within the Consortium)
The goal of this workshop with consortium partners was to show the initial service model (Version A), receive feedback and gather specific points for improvement. The workshop was designed in a funnel manner: starting with general impressions, and gradually zooming in to the different activities and roles and responsibilities within the initial service model. Meaning that the workshop started with gathering broad first impressions. Thereafter, detailed feedback was provided by looking into specific strong and weak points, the different roles and responsibilities, and ways to improve the service model. All exercises were facilitated using Mural. The workshop was audio recorded and analysed using the Mural results. These served as input for another round of optimizing the service model (version B). This version would later be used during the subsequent workshops with the salient stakeholders.
Round 5. Feedback (Salient Stakeholders)
Three (online) workshops with stakeholders in each pilot site were held during the final round. Both stakeholders who were present and who could not be present during the first workshop were invited to participate in the second and final feedback workshop. Through the process of feedback, new roles were identified to be affected by the eHealth technology. Therefore, these other additional stakeholders were also invited (such as GP nurses in Estonia). This workshop aimed to show the second version of the service model (Version B) and to receive feedback from the salient stakeholders. The same workshop design and its activities used during round 4 were applied to round 5. Data was analyzed per pilot site using the audio recordings and documentation of Mural. General improvements to the model were combined, and pilot site-specific differences were identified and incorporated to finalize the service model (Version C).
Results
As results from different study rounds in the three different pilot sites were collected, key findings of the studies are presented in the sections below.
Participants
A total of N=50 unique stakeholders helped facilitate the design process of the RE-SAMPLE service model. N=29 stakeholders filled in the survey anonymously, of which some might have joined the workshops. Participants had different backgrounds: People with COPD, (clinical, senior) researchers, pulmonologist(s), healthcare specialist(s), nurse practitioner, specialized nurse, rehabilitation physician, GP, EU Affairs officer, clinical specialist, assistant professor, PhD candidate(s), social worker, head of institute at a university, program manager, pulmonary residents, research nurse, physiotherapist, postdoc student, EU Affairs Officer, epidemiologist, physician assistants, and participants from the ICT department (technical data consultant, and IT cloud and infrastructure-architect). Four participants only mentioned their workplace: city government, laboratory, ministry of social affairs, and health insurance fund. Some participants participated in multiple studies. An overview of participants per study can be seen in Table 2.
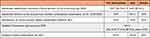 |
Table 2 Overview of Participants in Each Study Conducted in the Respective Country |
The Service Model Design Process
Round 1. Stakeholder Identification (Clinical Partners of the Consortium)
Table 3 shows an overview of the stakeholder identification for all three countries. For Estonia, 33 potential stakeholders were initially identified and narrowed down to the following list of 15 most relevant stakeholders (these are randomly ordered): People with COPD, General Practitioner (GP), pulmonologist, pulmonary nurse, physiotherapist, caregiver (family member), cardiologist, information specialist, social ministry, management of laboratory, insurance company, Information and Communication Technology (ICT) department, legal department, head of pulmonary outpatient department, innovation manager. For Italy, a total of 40 potential stakeholders were mentioned in the first identification. As for Estonia, the list was narrowed down to the 15 most relevant stakeholders, namely: People with COPD, GP, pulmonologist, pulmonary nurse, physiotherapist, caregiver (family members or close people), cardiologist, diabetologist, nephrologist, radiologist, nutritionist, ICT department, hospital board, region/health institution, Specialized Continued Assistance Units (USCA). This list was randomly ordered. For the Netherlands, 37 potential stakeholders were mentioned in the first identification. Followed by the 15 most important stakeholders (these are randomly ordered): People with COPD, GP, pulmonologist, specialized nurses at outpatient department (eg, self-management), physiotherapist primary [care], caregiver/family member, cardiologist, diabetologist (other specialist in general), psychologist/psychiatrist, pharmacist primary [care], insurance companies, ICT department, hospital board, hospital purchasing department, innovation manager.
 |
Table 3 Overview of the Stakeholder Identification in Three Countries (Estonia, Italy, the Netherlands) |
Round 2. Stakeholder Saliency Analysis (Consortium Members and Potential Stakeholders)
The results from the different constructs of the stakeholder saliency analysis are shown in Table 4. The saliency analysis revealed several similarities of salient stakeholders between pilot sites. Nevertheless, some important differences were identified. First, there were differences when it comes to certain HCPs. Physiotherapists were mentioned in both Italy and Estonia to be legit and justifiable stakeholders to include in the process. However, they were not stated to be most salient in the Netherlands. The GP, pulmonary nurse, and pulmonologist were identified in almost all categories in all the three pilot sites. Only the Netherlands did not rate the GP to have power in the process. There were also some differences in the rating of people with COPD. In Italy, people with COPD were rated to have power, but they were not rated to have power in Estonia nor the Netherlands. In the Netherlands and Italy, the needs and wishes of people with COPD were rated to be most urgent. In Estonia, people with COPD were also stated as urgent but others (eg, pulmonary nurse) were rated to be more urgent. In Italy, people with COPD were not identified in the legitimacy category, although this is in contrast with their urgency rating. There were also some differences regarding other stakeholders or organisations. The insurance company was mentioned in the Netherlands to have power and in Estonia to be urgent but not identified in any of the categories for Italy. The innovation manager was mentioned in Italy and Estonia to have power but not mentioned at all for the Netherlands. The hospital board was identified in the Netherlands and Estonia, but not in Italy. Finally, the Social Ministry appeared to be important in Estonia but was not identified in any of the categories in the other countries.
 |
Table 4 Stakeholder Saliency Analysis (EST, IT, NL) |
Round 3. Identification of Current Practice (Salient Stakeholders)
The results showed some similarities between the pilot sites in ways to implement the technology in the future. In all pilot sites, participants indicated that some sort of HCP could play a role in introducing the eHealth technology to the patient and help with the onboarding and data review. However, the type of HCP differed between pilot sites. For example, in Italy, the physician or GP would fulfil most of the roles and responsibilities in the new situation. The GP is the first contact point of the patients and therefore very important to involve. The research nurse might help in the onboarding, but this is a new role within Italy that is not fully operating yet. In Estonia, most of the responsibilities regarding the onboarding will be for the pulmonologist, the nurse, or even a trained specialist. In the Netherlands, it appeared that this is the responsibility for the specialized nurses and the pulmonary nurse who apparently play a crucial part in the disease management of the patient with COPD. It also appeared that in Estonia, the ministry of social affairs, city government, and social welfare could possibly play some roles in terms of introducing the eHealth technology to the people with COPD. However, these stakeholders and roles were not mentioned in the other pilot sites.
The results also revealed challenges regarding how care is organized in the different pilot sites. In Italy, for example, there is no technical system available with which both the GP and the clinicians in the hospital can communicate results. Additional tests can be performed in or outside the hospital and the patient needs to bring the results themselves to a GP or physician for them to analyze it. In the Netherlands, the communication between the hospital and the GP could be seen as a strength of current care, given that it is digital. Another difference is that in Estonia, patients are facing long waiting lists of specialized care, while in the Netherlands there are short waiting times. These results demonstrated the need to differentiate between the different pilot sites within the service model.
Round 4. Feedback (Partners Within Consortium)
The results revealed that some important steps within the service model were missing. It became clear that the service model is currently heavily focused on people with COPD. While this reflects the patient-centered approach of the project, clinical partners emphasized that HCPs also have an important role within the project, which in their view was underrepresented in Version A of the service model. For example, in Version A, the clinical dashboard for HCPs was not included and neither was the potential value it could provide for professionals. Furthermore, it was critically discussed that the patient is notified that their symptoms worsen, and they are asked whether they want to have a data review from an HCP. The motivation behind this was that patients are aware of their context and might be able to assess, whether they need an assessment. Thus, in terms of patient empowerment, they are responsible to take action instead of an automatic request that add to the workload of professionals. However, this was critically discussed by the clinical partners in the consortium, given that some patients might ignore this. Some first suggested that this should go directly to the HCP, but after consideration about the workload, agreed that a notification could also be provided to the HCP after the patient has repeatedly ignored their notification. Thus, in the HCP dashboard, the possibility for the professionals to see the patient’s exacerbation risk should be described. Although the responsibility to act still lies with the patient, the professional should be able to see any changes related to their exacerbation risk. Furthermore, it was also mentioned that there should be an eligibility screening added before the onboarding. Although multiple HCPs could inform the patient about the technology, the pulmonary department should be responsible for the eligibility screening. In this way, the responsibility lies within one specialty.
Another process that was missing in Version A was a self-management training before patients start using the application. The clinical partners reported that a lot of patients may not be familiar with self-managing their disease. Therefore, they should receive training about how to self-manage their disease, receive information about how the eHealth technology can support that, and create a possibility for patients to ask follow-up questions. In this way, the patient knows what to expect and is supported before starting the eHealth technology.
Finally, the partners stated that during an exacerbation, it is also possible that the patient receives home treatment. Not all patients need to be hospitalized when going through an exacerbation. This should therefore be added to the model. There were also some stakeholders missing in the initial version of the service model. These were mainly clinicians who are specialized in comorbidities. However, it was also mentioned that the outpatient clinic for Italy should be removed as a responsible stakeholder for the onboarding, and that some (new) roles (eg, research nurse) might be added, even though this is not a fully operating role yet. This means that the next version of the service model needed to include those processes and stakeholders that were currently missing.
Round 5. Feedback (Salient Stakeholders)
During the final feedback round, some general improvements were suggested by the stakeholders in the respective pilot site. First, the clinical dashboard should be separated from the application for the patients. It was not clearly shown how the clinical dashboard is something meant for HCPs and that should therefore be changed. Furthermore, comorbidities were not represented in the model. It was not clear where actions or activities need to take place for the comorbidities, and who needs to be involved in this. These should also be visualized and described in the model. This version of the model is too much focused-on COPD.
Regarding the self-management training that should take place before the start of the eHealth technology, it was explicitly mentioned that there should also be a training for the HCPs responsible for training patients. This was mainly the case in Italy and Estonia where it is sort of a new concept, but in the Netherlands, participants also said that there should be a clear manual with instructions. As regards to the self-management training itself, it should contain both face-to-face contact and technology supported training. It was suggested that, for example, in the beginning, there should be more face-to-face contact to educate patients on how to self-manage their disease with a more controlling role from the HCPs. In this way patients can gradually learn about self-management.
For the assignment of data reviews, it was discussed that the responsible HCP should have a wider clinical view. Because there are comorbidities involved, it may be the case that pulmonary nurses are not the ones who can answer all questions. Considering that case managers do not yet exist in Italy, Estonia, or the Netherlands, it was concluded not to be realistic for now of adding them with the full responsibility in the service model. However, it was mentioned to be a good alternative for the future when such roles have been introduced in practice.
Analyzing the workshops showed that several differences between the pilot sites exist. In Estonia, there should be some sort of case manager (eg, nurse) who will also help patients with technical support. However, in the Netherlands, it was explicitly stated that HCPs should not help with technical support. They should only be responsible for the symptoms and content-related questions; technical questions should be answered by some sort of help desk. Furthermore, in Italy, it was explicitly mentioned that caregivers should also be included as a stakeholder. Since it is the case that the caregiver accompanies the patient at every appointment, it was discussed that they should also be involved with the self-management training. However, this was not at all discussed in Estonia and the Netherlands.
Finally, it was discussed in Italy, that the ways of communicating exacerbation risks to the patients would require some serious attention for the eHealth technology development as a whole. They stressed the importance of not worrying patients too much if this is not necessary. Thus, they were cautious about showing the risk alert to patients in the first place. Participants in Italy indicated that it would be better to lie this responsibility at the HCPs. Thereby letting HCPs receive the risk alert and letting them be responsible for the subsequent action to take. Others agreed that if showing the risk, it should be thoroughly investigated how this can be best shown so that chances are reduced that patients would worry unnecessarily after seeing such an alert.
The RE-SAMPLE Service Model
The various phases of the service modelling process including the feedback provided by salient stakeholders and clinical partners within the consortium enabled the development of the final service model (Version C) (Figure 2). This model shows the different activities, roles, and responsibilities within the three pilot sites, and is described below:
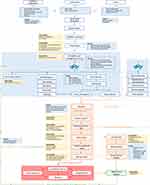 |
Figure 2 Final service model of RE-SAMPLE. |
Introducing the RE-SAMPLE Application and Garmin Smartwatch
There are different groups involved in the current RE-SAMPLE technology; HCPs, the patient with COPD, and others. The patients may be informed about the technology (consisting of an application and smartwatch) in two ways: 1) The HCPs or others introduce it to the patient, or 2) The patient gets to know about the technology from their own search or by flyers in the waiting room from different departments and professionals. The patient then learns about the technology and decides whether or not he/she wants to join. If the patient wants to join, the eligibility screening can take place. During this screening, the pulmonary department (in case of the current project) checks if the patient is eligible for participation based on certain parameters. If the patient is eligible, onboarding can take place.
Onboarding
During the onboarding, the patient receives a wearable (which is set up during the appointment), gets information about the application, and fills in baseline questionnaires together with the one responsible, which differs per country. In the Netherlands, the pulmonary nurse, the research nurse, or the ICT helpdesk will be responsible. The onboarding itself is the responsibility of the pulmonary nurse or research nurse. In case of questions, the patient can ask content-related questions to these professionals. For technical support, or when experiencing technical issues, the patient can contact the ICT help desk from the hospital. In this way, HCPs save time and can answer more specific questions. In Italy, the onboarding is the responsibility of the physician or nurses. Since nurses see the patients quite often, they can do the onboarding with the patient. However, depending on the time and setting, physicians can also perform this activity. For Estonia, it is still unclear whether GPs will be involved. Depending on the decision that GPs are going to be actively involved in the implementation of the eHealth technology, GP nurses can perform the onboarding with the patients. Otherwise, physicians (eg, pulmonologists) will be responsible for this step. In case research nurses will become a sustainable role in Italy in the future, this activity can then become their responsibility. However, this role is currently not fully operating in practice, but they are planning on doing so in the future.
Self-Management Training
After the onboarding, the patient will receive self-management training on their disease and comorbidities. It is also explained what the eHealth technology does to support them in their self-management, and patients can ask follow-up questions. This will be the responsibility of the pulmonary nurses in all three pilot sites. The training is provided in a hybrid way. Meaning that the first appointment will probably take place face-to-face, and the HCP will have a more controlling role in the beginning. Depending on the patients’ health literacy, it can be decided to increase or decrease the number of face-to-face appointments and the level of control the pulmonary nurse will have. However, before the ones responsible will provide the self-management training to patients, they should first receive a training themselves on how to educate patients and on how to support them in their self-management. In this way, all responsible pulmonary nurses can support the patient and learn self-management in the same way.
The RE-SAMPLE Technology
After the self-management training, the patient can start using the application. This consists of peer-to-peer contact, self-management, and data collection. During the usage of the eHealth technology, the patient wears the wearable, fills in questionnaires and the symptom diary, and uses the self-management module. There is a separate application for HCPs which is called “clinical dashboard”.
Peer-to-Peer Contact
The patient can make use of the peer-to-peer contact feature, by which (s)he can chat with other patients with the same disease. If the patient decides to not use the data collection part of the eHealth technology, (s)he can still use the chat. The peer-to-peer contact is the full responsibility of the patient. (S)he can also decide to not use this feature at all. The HCP can join the peer-to-peer contact. Details on in what exact way HCPs can join were not elaborated upon yet.
Self-Management
The self-management component consists of different activities. The patient can make use of goal setting, education, action plan, and coaching. With goal setting, the patient can define specific goals which might be set up during the shared decision-making in the consult or might be set up by the patient him/herself. Using the educational feature, the patient can learn more about specific topics (eg, the technology, their disease, self-management, medication use, benefits of physical activity, etc.). Some of these modules might be useful for patients shortly after starting with the programme. Others may be useful after an exacerbation or other events. A patient’s action plan can also be viewed in the technology. By including the action plan, the patient can follow certain steps themselves when a worsening of symptoms is happening before asking for a data review request. Finally, coaching will also be available. Being coached on a specific topic might be motivated by different sources, for example, they might be intrinsically motivated, be made aware by an HCP or an interest was sparked when looking at trends and progresses. As with the education feature, coaching will be provided for different topics. It is important to note that if the patient decides not to use the data collection, he/she can still use the self-management feature within the technology.
Data Collection
Data collection needs to be performed to support the monitoring feature and risk prediction. Data is collected by wearing a Garmin smartwatch (physical activity, sleep, heart rate, oxygen saturation) and filling in questionnaires and ePROMS. Based on these RWD, trends and progress can be visualised, and risk predictions can be made. Both the trends and progress and the risk predictions are input for the risk alert. Here, the patients receive a notification when there is something out of the ordinary that indicates a higher risk of developing an exacerbation. The system then offers a data review performed by a HCP, which the patient can accept or decline. The control whether a data review is requested or not is the full responsibility of the patient. When a review is requested, it would be ideal to have an algorithm to assign the request to the appropriate HCPs, this is currently not included in RE-SAMPLE. Therefore, the case manager could have the responsibility to assign the review to an HCP. However, the case manager is currently not operating in practice in Italy and Estonia, but something they want to include in the future. The Netherlands does not have such a case manager to perform those tasks, nor is it planned to establish this role. Therefore, the specialised nurse or the physician needs to be responsible for assigning the review to HCPs. For Italy, this will be the responsibility of the physician or the GP. For Estonia, this will be the responsibility for the GP or the specialised nurses. The responsible HCP then reviews the data and risk predictions made and decides whether or not additional tests are needed. In case of no additional tests, the patient is informed and continues using the eHealth technology as usual. In case of additional tests, the patient is asked to have these tests performed. In the Netherlands, these additional tests are either the responsibility of the Shared Care Facility (SCF) or the hospital. For Italy, it will be the responsibility of the GP or the hospital to perform the additional tests. For Estonia, it will be the responsibility of the clinic. After performing these tests, the results need to be shared with the patient. This will be the responsibility of the pulmonary nurse or physician in the Netherlands, and the GP or specialised nurses in Estonia. For Italy, sharing the results are the responsibility of the physician (eg, pulmonologist) or GP, although in some cases the patient also has some responsibility to transfer results between HCPs, because in Italy systems are not connected. Without patients taking responsibility, it might be that results are not shared; therefore, patients have a role in this activity. It may be possible that when sharing the results, a consult is necessary for additional explanation and follow-up. Here, the risk prediction will be discussed and through shared decision-making, next steps are decided upon. For example, it could be that it will be discussed that certain medication or treatment needs to be changed, other goals need to be set, or that the coaching plan will be changed. The same HCPs who are responsible for sharing results are also responsible for this step. After the consult, the patient is invited by the technology to reflect on their patterns to look what happened the days before the worsening of the symptoms. This is the full responsibility of the patient. The patient can use the reflection so that over time, one might be able to identify their personal patterns (for example, too much exercise). They can thereafter use the application as usual.
Clinical Dashboard
HCPs are supposed to use the clinical dashboard. This dashboard has different features: multidisciplinary communication, patient contact, decision support, and risk overview. With multidisciplinary communication, HCPs can easily communicate with other care departments or specialists. Furthermore, the HCP can contact their patients, see the risk prediction, and trends and progress of the patients. When the patient him/herself does not undertake any action upon the risk alert, the professional receives a notification about the elevated risk. In this way, the responsibility still lies by the patient, but the HCP can see risk in case the patient is not responding to their notifications. The clinical dashboard will contain decision support which can then facilitate shared decision-making.
Worsening of Symptoms
The patient might experience an acute exacerbation or flare-up at some point, which may even lead to hospitalization or can be treated at home. After the hospitalization or at home treatment, the patient will hopefully recover. After the recovery, the patient is invited to have a reflection on patterns by using the symptom diary to look at the days before the worsening of the symptoms. Here, the patient will pay attention to potential symptoms that were experienced that could trigger a worsen of the symptoms. This reflection is the full responsibility of the patient. Thereafter, the patient can continue using the application as usual.
Discussion
The results outline the process and results of a service modelling process that also illustrate the complexities of implementing an innovative eHealth technology in practice. In the following, we will discuss the results related to (1) the role of patients within a service model for a COPD self-management eHealth technology and (2) the iterative method of service modelling for an innovative eHealth technology still under development.
Shifting Responsibilities: Empowering Patients to Actively Engage in COPD Self-Management
This study revealed that according to clinical partners and HCPs, not all patients are ready to take an active role and responsibility that was envisioned with this eHealth technology for self-management. It became clear that some patients need to be familiarised with the concept of patient empowerment and their new role before using the technology. This is especially important in countries where self-management is a relatively new concept. Empowerment will not happen overnight but can rather be seen as a process for “identifying needs, taking action, and gaining mastery over issues that are self-identified as important”.48 It provides patients with knowledge, skills, and responsibility to effectively change their behaviour.49 In this way, patients will gradually develop a better sense of self-efficacy, and this may lead to a better management of one’s disease.50 In the final service model, we try to empower and prepare patients by providing self-management training before starting to use the technology which may have beneficial effects during the eHealth use. Thus, instead of solely looking at implementation of the technology, this study also revealed a crucial step before starting the actual technology use: preparing and empowering patients to reflect on their role and to take responsibility as active partner in their care, also often referred to as patient activation. When highlighting all aspects of patient activation, it can be effectively used to improve self-management and clinical outcomes.51 As low levels of activation are common in patients with COPD,52 patient activation is in our opinion, a crucial step in every (e)Health intervention for this, and other, target population(s). One might think of this as a rather simple step, but practice shows that this step of “activating patients” is often neglected.53 Therefore, this paper acknowledges this importance by adding self-management training before starting to use the intervention to gradually familiarise patients with taking (some of the) responsibilities.
Besides patients in some countries not being used to take responsibility, results also revealed that not all HCPs can let go of their responsibility and their need to monitor and control. Some would rather receive notifications and take responsibility in an attempt to protect the patient from getting notifications, seeing their data and maybe getting worried. Similar results were found by Grünloh et al,54 in which this paternalistic approach was also found to be present, even though HCPs did not seem to be aware of this. While this approach is likely based on good intentions, it defeats at the same time the purpose of self-management. Part of this aim is that patients take responsibility for their disease management, eg, taking responsibility of the risk alerts they receive. Undoubtedly, not all patients are immediate capable of fully taking this responsibility, and this should be guided by the HCP. Therefore, responsibility from HCP may still be required at times (eg, receiving an alert if the patient does not take any action). This is in line with one of the conditions of Redman55 for successful empowerment: giving responsibility should be gradually. Hence, patients should be prepared for taking their own responsibility. To truly increase the self-management of patients in such technologies, the responsibility to act should eventually lie with the patient. For this reason, this service model highlights the possibility of self-managing one’s disease by taking their own, but prepared, responsibility within the eHealth technology.
Methodological Reflection on Service Modelling for Implementing the eHealth Technology
This study illustrated the importance of developing a service model from an early stage in the project. Through the process of modelling, new requirements were identified that impacted both the project and the technology design. For example, as mentioned before, it became clear that HCPs should receive an alert when no action is taken from patients. As a consequence, the technology should be able to send those notification to the clinical dashboard of the HCPs, and further studies in the project should investigate the patient perspective of notifying the HCP of their inactivity and the effect of taking away a degree of responsibility from the patients. This was not uncovered during early requirements elicitation activities and showcases the necessity of early-stage service modelling.
Furthermore, this study also showed the importance of identifying context- and country-specific differences to increase the chances of successful implementation. The RE-SAMPLE project aims to implement its technology in three different countries. Thus, its implementation should also take place locally. Therefore, the service model had to take into account their context to fit to existing structures and possibilities within the healthcare systems of the respective countries. Revealing that service modelling is not a one-size-fits-all approach, not even within one project.
Finally, as previous research also revealed, the implementation of eHealth in general, and the use of implementation models and their related methods is complex.56–58 This study demonstrated similar results by illustrating the complexity of the service modelling method. In particular, we encountered the challenge between developing a more descriptive versus a very prescriptive service model. On the one hand, it is important to develop a service model that aligns with current practice to facilitate the implementation process. If the service model is too far away from current practices, the implementation might fail and/or HCPs might refuse to use the new technology. On the other hand, a new technology is also supposed to change current practices, making them more efficient for example. So, while there are some descriptive elements (ie, current practices, roles, responsibilities), there are of course also several prescriptive elements in the service model that describe the new ways of working. It is a delicate balance how far one can go with prescribing new ways of providing care, even with the support of stakeholders who share new visions or plans to change provision of care. For example, in this study, research nurses in Italy were supposed to get more responsibilities in the future. However, this has not been established yet in current practice. Some roles (such as the case manager) are not yet operating, making it too risky to assign them with certain tasks or responsibilities in the service model. However, this may change over time and therefore a service model might be adapted even after implementation.
Limitations
A limitation of this study is the relatively low number of participants. This may also have affected the stakeholder identification, and stakeholder saliency analysis at the beginning. For example, in Estonia, the GP nurse was omitted from the initial stakeholder saliency analysis based on the identification ranking. During subsequent studies, in which more participants were included, GP nurses seemed to be important to include in the process. This is a methodological issue in which results from a low number of participants served as the basis of the development process, marking its consequences during subsequent studies. However, because the salient stakeholders list was not fixed, we were able to adapt accordingly, and invited stakeholders identified later in the process.
Another limitation of this study is that due to the complex nature of the eHealth technology in this study, the actual service model design sessions were performed within the internal project team based on input provided by stakeholders. Stakeholders were not actively involved in the creation, for example, through co-creation. One may question the level of stakeholder engagement when not participating in the design session of the model. However, by adding several feedback rounds in the design process and focussing on specific points for improvements, we tried to collect useful input for the design. Future studies may include stakeholder co-creation sessions in which the different versions of the service model are designed.
Finally, engagement of patients in the different phases was limited as explaining the concept and importance of service modelling was insufficient to motivate them to participate. In addition, we had some concerns in terms of safety for people with COPD to join the different sessions with other stakeholders (COVID, potential power imbalances59). However, everything we did during this study to develop the service model was based on earlier input provided by people with COPD (such as interviews, patient journeys). This is also reflected in the critique during one of the feedback sessions that the service model was too patient-centred. Nevertheless, we recommend future studies to include additional feedback rounds for patients or if safe (when taken care of power imbalances), let people with COPD join the sessions with the different stakeholders.
Conclusion
This paper described how an innovative eHealth technology to support self-management for people with COPD could be implemented in practice that emphasises the active role of patients in their care. It aimed to elaborate on an iterative service model development approach trying to include the perspectives of stakeholders and highlights the self-management component throughout the implementation process.
We showed how an eHealth self-management technology for people with COPD can be implemented in three different countries, in which self-management plays a role in more parts than only within the eHealth technology itself. By illustrating this process, we invite eHealth researchers and practitioners to think beyond the scope of an eHealth solution and to consider the processes of changing roles and empowering patients in the service design.
Service modelling in an early stage can elicit new requirements that need to be addressed in the design and ensures that the implementation process is considered early on together with key stakeholders. As we aim to positively change work practice with the use of technology, a service model needs to consider both current practice (to enable feasible implementation), and future practice (to improve quality of care). However, as this study demonstrates, there is a fine line between the two worlds. This complexity stresses the importance of continuous stakeholders’ engagement, and alignment of their needs, wishes, and current possibilities. Service modelling is therefore, not set in stone but shapes along with the dynamics of the changing daily practice.
Acknowledgments
The research is supported by the European project RE-SAMPLE. This project is funded by the European Union’s Horizon 2020 research and innovation program under Grant Agreement No 965315. The authors of this paper would like to thank all stakeholders for their participation and valuable input during the different study phases. In addition, we would also like to thank Tartu hospital in Estonia, Gemili hospital in Italy, and Medisch Spectrum Twente (MST) in the Netherlands for their support.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. GOLD. Global initiative for chronic obstructive lung disease: global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (2023 report); 2022. Available from: www.goldcopd.org.
2. Agustí A, Celli BR, Criner GJ, et al. Global initiative for chronic obstructive lung disease 2023 report: GOLD executive summary. Eur Respir J. 2023;61(4):2300239. doi:10.1183/13993003.00239-2023
3. Adeloye D, Song P, Zhu Y, Campbell H, Sheikh A, Rudan I. Global, regional, and national prevalence of, and risk factors for, chronic obstructive pulmonary disease (COPD) in 2019: a systematic review and modelling analysis. Lancet Respir Med. 2022;10(5):447–458. doi:10.1016/S2213-2600(21)00511-7
4. Jankowska-Polańska B, Kasprzyk M, Chudiak A, Uchmanowicz I. Relation between illness acceptance and quality of life in patients with chronic obstructive pulmonary disease (COPD). Adv Respir Med. 2016;85(1):3–10. doi:10.5603/PiAP.a2015.0079
5. Quaderi SA, Hurst JR. The unmet global burden of COPD. Glob Health Epidemiol Genom. 2018;3. doi:10.1017/gheg.2018.1
6. Shah CH, Onukwugha E, Zafari Z, Villalonga-Olives E, Park J, Slejko JF. Economic burden of comorbidities among COPD patients hospitalized for acute exacerbations: an analysis of a commercially insured population. Expert Rev Pharmacoecon Outcomes Res. 2022;22(4):683–690. doi:10.1080/14737167.2021.1981291
7. Iheanacho I, Zhang S, King D, Rizzo M, Ismaila AS. Economic burden of chronic obstructive pulmonary disease (COPD): a systematic literature review. Int J COPD. 2020;15:439–460. doi:10.2147/COPD.S234942
8. Sethi S, Make BJ, Robinson SB, et al. Relationship of COPD exacerbation severity and frequency on risks for future events and economic burden in the medicare fee-for-service population. Int J COPD. 2022;17:593–608. doi:10.2147/COPD.S350248
9. Corlateanu A. COPD and comorbidities in the Republic of Moldova. Eurasian J Pulmonol. 2022. doi:10.14744/ejop_78_21
10. Chaudhary MFA, Hoffman EA, Guo J, et al. Predicting severe chronic obstructive pulmonary disease exacerbations using quantitative CT: a retrospective model development and external validation study. Lancet Digit Health. 2023;5(2):e83–e92. doi:10.1016/s2589-7500(22)00232-1
11. MacLeod M, Papi A, Contoli M, et al. Chronic obstructive pulmonary disease exacerbation fundamentals: diagnosis, treatment, prevention and disease impact. Respirology. 2021;26(6):532–551. doi:10.1111/resp.14041
12. Richard AA, Shea K. Delineation of self-care and associated concepts. J Nurs Scholarsh. 2011;43(3):255–264. doi:10.1111/j.1547-5069.2011.01404.x
13. Audulv Å, Asplund K, Norbergh KG. Who’s in charge? The role of responsibility attribution in self-management among people with chronic illness. Patient Educ Couns. 2010;81(1):94–100. doi:10.1016/j.pec.2009.12.007
14. Wang R, Zhou C, Wu Y, et al. Patient empowerment and self-management behaviour of chronic disease patients: a moderated mediation model of self-efficacy and health locus of control. J Adv Nurs. 2022;78(4):1055–1065. doi:10.1111/jan.15077
15. Small N, Bower P, Chew-Graham CA, Whalley D, Protheroe J. Patient empowerment in long-term conditions: development and preliminary testing of a new measure. BMC Health Serv Res. 2013;13(1). doi:10.1186/1472-6963-13-263
16. Charles C, Gafnv A, Whelan T. Shared decision-making in the medical encounter: what does it mean? (or it takes at least two to tango). Soc Sci Med. 1997;44(5):681–692. doi:10.1016/s0277-9536(96)00221-3
17. Elwyn G, Frosch D, Thomson R, et al. Shared decision making: a model for clinical practice. J Gen Intern Med. 2012;27(10):1361–1367. doi:10.1007/s11606-012-2077-6
18. van Gemert-Pijnen L, Kip H, Kelders SM, Sanderman R. Introducing eHealth. In: EHealth Research, Theory and Development: A Multidisciplinary Approach.
19. Kermelly SB, Bourbeau J. eHealth in self-managing at a distance patients with COPD. Life. 2022;12(6):773. doi:10.3390/life12060773
20. Limna P. The digital transformation of healthcare in the digital economy: a systematic review. Int J Adv Health Sci Technol. 2023;3(2):127–132. doi:10.35882/ijahst.v3i2.244
21. Stefanicka-Wojtas D, Kurpas D. eHealth and mHealth in chronic diseases—identification of barriers, existing solutions, and promoters based on a survey of EU stakeholders involved in Regions4PerMed (H2020). J Pers Med. 2022;12(3):467. doi:10.3390/jpm12030467
22. Ahmed RE, Bdair IA, AL-Mugheed K, et al. Empowering self-efficacy by using patient empowerment among chronic obstructive pulmonary disease: pre–post-test study. Healthcare. 2023;11(3):430. doi:10.3390/healthcare11030430
23. Ossebaard HC, van Gemert-Pijnen L. EHealth and quality in health care: implementation time. Int J Qual Health Care. 2016;28(3):415–419. doi:10.1093/intqhc/mzw032
24. Cole AC, Adapa K, Khasawneh A, Richardson DR, Mazur L. Codesign approaches involving older adults in the development of electronic healthcare tools: a systematic review. BMJ Open. 2022;12(7):e058390. doi:10.1136/bmjopen-2021-058390
25. Nyberg A, Wadell K, Lindgren H, Tistad M. Internet-based support for self-management strategies for people with COPD-protocol for a controlled pragmatic pilot trial of effectiveness and a process evaluation in primary healthcare. BMJ Open. 2017;7(7):e016851. doi:10.1136/bmjopen-2017-016851
26. Hallensleben C, van Luenen S, Rolink E, Ossebaard HC, Chavannes NH. eHealth for people with COPD in the Netherlands: a scoping review. Int J COPD. 2019;14:1681–1690. doi:10.2147/COPD.S207187
27. Kelly M, Fullen B, Martin D, Mcmahon S, Mcveigh JG. eHealth interventions to support self-management in people with musculoskeletal disorders, “eHealth: it’s TIME” - a scoping review. Phys Ther. 2022;102(4). doi:10.1093/ptj/pzab307
28. Shen H, Van Der Kleij RMJJ, Van Der Boog PJM, Chang X, Chavannes NH. Electronic health self-management interventions for patients with chronic kidney disease: systematic review of quantitative and qualitative evidence. J Med Internet Res. 2019;21(11):e12384. doi:10.2196/12384
29. RE-SAMPLE. Available from: https://www.re-sample.eu/.
30. Meirte J, Hellemans N, Anthonissen M, et al. Benefits and disadvantages of electronic patient-reported outcome measures: systematic review. JMIR Perioper Med. 2020;3(1):e15588. doi:10.2196/15588
31. Holmes MM, Stanescu S, Bishop FL. The use of measurement systems to support patient self-management of long-term conditions: an overview of opportunities and challenges. Patient Relat Outcome Meas. 2019;10:385–394. doi:10.2147/prom.s178488
32. van Limburg M, van Gemert-Pijnen JEWC, Nijland N, Ossebaard HC, Hendrix RMG, Seydel ER. Why business modeling is crucial in the development of eHealth technologies. J Med Internet Res. 2011;13(4):e124. doi:10.2196/jmir.1674
33. Varsi C, Nes LS, Kristjansdottir OB, et al. Implementation strategies to enhance the implementation of eHealth programs for patients with chronic illnesses: realist systematic review. J Med Internet Res. 2019;21(9):e14255. doi:10.2196/14255
34. Ross J, Stevenson F, Lau R, Murray E. Factors that influence the implementation of e-health: a systematic review of systematic reviews (an update). Implement Sci. 2016;11(1). doi:10.1186/s13012-016-0510-7
35. Ammenwerth E, Iller C, Mahler C. IT-adoption and the interaction of task, technology and individuals: a fit framework and a case study. BMC Med Inform Decis Mak. 2006;6. doi:10.1186/1472-6947-6-3
36. van Gemert-Pijnen JEWC, Nijland N, van Limburg M, et al. A holistic framework to improve the uptake and impact of eHealth technologies. J Med Internet Res. 2011;13(4):e111. doi:10.2196/jmir.1672
37. Greenhalgh T, Wherton J, Papoutsi C, et al. Beyond adoption: a new framework for theorizing and evaluating nonadoption, abandonment, and challenges to the scale-up, spread, and sustainability of health and care technologies. J Med Internet Res. 2017;19(11):e367. doi:10.2196/jmir.8775
38. Bitner M, Ostrom A, Morgan F. Service blueprinting: a practical technique for service innovation. Calif Manage Rev. 2008;50(3):66–94. doi:10.2307/41166446
39. Broekhuis M, van Weering MD, Schuit C, Schürz S, van Velsen L. Designing a stakeholder-inclusive service model for an eHealth service to support older adults in an active and social life. BMC Health Serv Res. 2021;21(1). doi:10.1186/s12913-021-06597-9
40. Freeman R. The stakeholder approach revisited. Zeitschrift für Wirtschafts- und Unternehmensethik. 2004;5. doi:10.5771/1439-880X-2004-3-228
41. Carr D, Howells A, Chang M, Hirji N, English A. An integrated approach to stakeholder engagement. Healthc Q. 2009;12:62–70. doi:10.12927/hcq.2009.20754
42. Mitchell RK, Agle BR, Wood DJ. Toward a theory of stakeholder identification and salience: defining the principle of who and what really counts. Acad Manage Rev. 1997;22(4):853–886. doi:10.2307/259247
43. Sharp H, Finkelstein A, Galal G. Stakeholder Identification in the Requirements Engineering Process. IEEE; 1999. doi:10.1109/DEXA.1999.795198
44. Mural. Available from: https://www.mural.co/.
45. Qualtrics. Available from: https://www.qualtrics.com/.
46. Simonse L, Albayrak A, Starre S. Patient journey method for integrated service design. Des Health. 2019;3(1):82–97. doi:10.1080/24735132.2019.1582741
47. Idoughi D, Seffah A, Kolski C. Adding user experience into the interactive service design loop: a persona-based approach. Behaviour Inf Technol. 2012;31(3):287–303. doi:10.1080/0144929X.2011.563799
48. Funnell MM. Patient empowerment: what does it really mean? Patient Educ Couns. 2016;99(12):1921–1922. doi:10.1016/j.pec.2016.10.010
49. Funnell MM, Anderson RM, Arnold MS, et al. Empowerment: an idea whose time has come in diabetes education. Diabetes Educ. 1991;17(1):37–41. doi:10.1177/014572179101700108
50. Raina RS, Thawani V. The zest for patient empowerment. J Clin Diagn Res. 2016;10(6):FE01–FE03. doi:10.7860/JCDR/2016/16816.7902
51. Shnaigat M, Downie S, Hosseinzadeh H. Effectiveness of patient activation interventions on chronic obstructive pulmonary disease self-management outcomes: a systematic review. Aust J Rural Health. 2022;30(1):8–21. doi:10.1111/ajr.12828
52. Peters JB, Antons JC, Koolen EH, et al. Patient activation is a treatable trait in patients with chronic airway diseases: an observational study. Front Psychol. 2022;13. doi:10.3389/fpsyg.2022.947402
53. Yadav UN, Hosseinzadeh H, Baral KP. Self-management and patient activation in COPD patients: an evidence summary of randomized controlled trials. Clin Epidemiol Glob Health. 2018;6(3):148–154. doi:10.1016/j.cegh.2017.10.004
54. Grönloh C, Myreteg G, Cajander A, Rexhepi H. “why do they need to check me?” Patient participation through eHealth and the doctor-patient relationship: qualitative study. J Med Internet Res. 2018;20(1). doi:10.2196/jmir.8444
55. Redman BK. Responsibility for control; ethics of patient preparation for self-management of chronic disease. Bioethics. 2007;21(5):243–250. doi:10.1111/j.1467-8519.2007.00550.x
56. Gemert-Pijnen J, Kelders S, Kip H, Sanderman R. EHealth Research, Theory and Development: A Multi-Disciplinary Approach. Routledge; 2018.
57. Pieterse M, Kip H, Cruz-Martínez RR. The complexity of eHealth implementation: a theoretical and practical perspective. In: EHealth Research, Theory and Development. Routledge; 2018:247–270.
58. Nilsen ER, Stendal K, Gullslett MK. Implementation of eHealth technology in community health care: the complexity of stakeholder involvement. BMC Health Serv Res. 2020;20(1). doi:10.1186/s12913-020-05287-2
59. Cvetanovska N, Jessup RL, Wong Shee A, Rogers S, Beauchamp A. Patients’ perspectives of factors influencing active participation in healthcare interactions: a qualitative study. Patient Educ Couns. 2023;114:107808. doi:10.1016/j.pec.2023.107808
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
