Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 16
Sex Difference in the Association Between Serum Versican and Albuminuria in Patients with Type 2 Diabetes Mellitus
Authors Li S, Li N , Li L, Zhan J
Received 13 September 2023
Accepted for publication 1 November 2023
Published 11 November 2023 Volume 2023:16 Pages 3631—3639
DOI https://doi.org/10.2147/DMSO.S434287
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Konstantinos Tziomalos
Shuang Li, Niman Li, Linsen Li, Junkun Zhan
Department of Geriatrics, The Second Xiangya Hospital, Institute of Aging and Age-Related Disease Research, Central South University, Changsha, Hunan, 410011, People’s Republic of China
Correspondence: Junkun Zhan, Department of Geriatrics, The Second Xiangya Hospital, Institute of Aging and Age-Related Disease Research, Central South University, Changsha, Hunan, 410011, People’s Republic of China, Email [email protected]
Introduction: Diabetic kidney disease (DKD) is one of the major microvascular complications of diabetes. DKD is associated with oxidative stress and inflammation. Versican (VCAN), a chondroitin sulphate proteoglycan, has been proven to participate in oxidative stress and inflammation. This study aimed to explore the overall and sex-based relationship between serum VCAN levels and albuminuria in patients with type 2 diabetes mellitus (T2DM).
Methods: 428 patients with T2DM and 84 healthy individuals were enrolled. Patients with diabetes were separated into normal albuminuria, microalbuminuria, and macroalbuminuria groups, according to their urinary albumin/creatinine ratio (UACR). Serum VCAN levels were tested using an enzyme-linked immunosorbent assay.
Results: Compared with males, female patients were older, and had higher total cholesterol and high-density lipoprotein cholesterol, but lower body mass index, diastolic blood pressure, glycated hemoglobin A1, alanine aminotransferase, urinary albumin (UA), and serum creatinine (SCr) (P < 0.05). The VCAN levels in male patients with T2DM were significantly higher than those in the healthy individuals. Male patients with T2DM with albuminuria (micro and macro) had higher levels of VCAN than in patients with normal albuminuria; the highest level was seen in patients with macroalbuminuria (P < 0.05). In male patients with T2DM, serum VCAN correlated positively with systolic blood pressure, blood urea nitrogen, UA, SCr, and UACR, but correlated negatively with the estimated glomerular filtration rate. The area under the receiver operating characteristic curve of serum VCAN to diagnose albuminuria was 0.702, with a corresponding cut-off value of 0.399 ng/mL (P < 0.001). However, the association between serum VCAN and UACR was not observed in female patients with T2DM.
Conclusion: Serum VCAN levels correlated positively with the severity of albuminuria in male patients with T2DM.
Keywords: diabetic kidney disease, type 2 diabetes mellitus, versican, biomarker
Introduction
Diabetes mellitus (DM) is a group of metabolic diseases characterized by hyperglycemia. Type 2 diabetes mellitus (T2DM) is the most common type of DM, accounting for 90% of all cases.1
Long-term chronic hyperglycemia could lead to several diabetic complications. Diabetic kidney disease (DKD), as one of the most common diabetic microvascular complications, has become the leading cause of end-stage renal disease (ESRD).2 DKD has a complex pathogenesis, including changes in renal hemodynamics, oxidative stress, mitochondrial dysfunction, inflammation, an overactive renin-angiotensin-aldosterone system (RAAS), and heredity.3 Considering that the kidney is a mitochondria-rich organ and the regular function of mitochondria is essential for normal kidney physiology, the contribution of mitochondrial dysfunction to DKD has attracted more research attention recently.
Versican (VCAN), a chondroitin sulphate proteoglycan, is a component of the extracellular matrix (ECM), playing a role in regulating cell adhesion, migration, and ECM assembly.4 VCAM is also involved in tubulointerstitial injury through its interaction with immune chemokines.5 Overexpression of VCAN in tubular cells induced renal interstitial fibrosis in vivo.6 In addition, our previous study showed that VCAN could regulate hyperglycemia-induced vascular calcification and aging by modulating mitochondrial function.7 These data suggest a potential association between VCAN and DKD. However, the exact role of VCAN in DKD remains unclear.
Albuminuria is the first clinical symptom of DKD, and uncontrolled albuminuria could evolve into renal damage, and ultimately, ESRD. Therefore, we aimed to assess the serum VCAN levels in patients with T2DM with different albuminuria stages and explore the relationship between them, as well as the sex difference in this association.
Methods
Study Design and Population
Patients hospitalized with T2DM in the Department of Geriatrics of the Second Xiangya Hospital of Central South University (Changsha, China), from January 2020 to January 2022, were recruited initially. The T2DM inclusion criteria were: (1) Diagnosis of T2DM, which included fasting blood glucose (FBG) ≥ 7.0 mmol/l and/or 2-h plasma glucose ≥ 11.0 mmol/l, according to the World Health Organization criteria,8 or having self-reported, doctor-diagnosed T2DM. Exclusion criteria were as follows: (1) Those under 18 years old; (2) Diabetic acute complications, acute inflammatory disease, or malignant tumors; and (3) Other systemic diseases that can induce proteinuria; and (4) Missing data. Finally, a total of 428 patients with T2DM were included in this retrospective study. During the same period, 84 healthy non-T2DM individuals were eligible and participated in this study. The patients with T2DM were further classified into subgroup comprising the normal albuminuria group [urinary albumin/creatinine ratio (UACR) < 30 mg/g], microalbuminuria group (UACR 30–300 mg/g), and macroalbuminuria group (UACR ≥ 300 mg/g). Patients in the microalbuminuria and macroalbuminuria groups were defined as having DKD (UACR ≥ 30 mg/g) in this study.9 This study was approved by the Ethics Committees of the Second Xiangya Hospital of Central South University, Changsha, Hunan Province, China (No: 2022–163). Written informed consent was obtained from all participants.
Measurements
Medical history and clinical characteristics [age, sex, diabetic duration, weight, height, systolic blood pressure (SBP), and diastolic blood pressure (DBP)] were collected from the inpatient medical record system of the Second Xiangya Hospital of Central South University. Body mass index (BMI) was calculated as weight (kg) divided by height squared (m2), while the estimated glomerular filtration rate (eGFR) was calculated by the formula: eGFR = (104 × cystatin C (CysC)–1.019 × 0.996 age (years)) – 8 (male); eGFR = (104 × CysC–1.019 × 0.996 age (years) × 0.929)–8 (female).10
Blood samples were collected in the morning after overnight fasting. An automatic biochemical analyzer was used to detect the following parameters: FBG, glycated hemoglobin (HbA1c), blood urea nitrogen (BUN), serum creatinine (SCr), uric acid (UA), CysC, alanine aminotransferase (ALT), aspartate aminotransferase (AST), total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C). Urine samples were collected in the morning. Urinary albumin and creatinine concentrations were measured using a turbidimetric immunoassay and enzymatic assay, respectively. UACR was calculated to estimate the levels of urinary proteins. Serum VCAN levels were quantified using an enzyme-linked immunosorbent assay (ELISA) kits (ZC-32878, ZCIBIO Technology Co., Ltd., Shanghai, China).
Statistical Analysis
Normally distributed quantitative variables were shown as mean ± standard deviation (SD). Non-normally distributed quantitative variables were shown as the median (interquartile range). Differences between the UACR groups were analyzed using one-way analysis of variance (ANOVA) and sex groups were analyzed using Student’s t-test for normally distributed values and by the Kruskal–Wallis H-test for non-normally distributed values. After logarithmic transformation was carried out for non-normally distributed values, the correlation between VCAN and other indices was assessed using Pearson’s correlation test. The diagnostic potential of VCAN for albuminuria was analyzed by plotting a receiver operating characteristic (ROC) curve. A two-sided P value < 0.05 was considered significant. All statistical analyses were performed using SPSS, version 26.0 (IBM Corp., Armonk, NY, USA).
Results
As shown in Table 1, the mean age of the males was 58.65 ± 10.20 years (n = 267) and that of the females was 60.98 ± 9.47 years (n = 161). We observed significant differences in age, BMI, DBP, HbA1c, ALT, UA, SCr, TC, and HDL-C between the two groups according to sex (P < 0.05, Table 1). The female patients were older and had significantly higher levels of TC and HDL-C (P < 0.05), but lower levels of BMI, DBP, HbA1c, ALT, UA, and SCr than the male patients (P < 0.05) (Table 1). However, no significant difference was observed in other parameters between the different sexes (P > 0.05).
 |
Table 1 Levels of Serum VCAN and Clinical Characteristic of All T2DM Patients |
Based on the UACR, male patients with T2DM were divided into the normal albuminuria group (n = 160), microalbuminuria group (n = 70), and macroalbuminuria group (n = 37, Table 2). Compared with the normal healthy group, diabetic duration, FBG, HbA1c, and VCAN in male patients with T2DM were significantly increased (P < 0.0001 for all). No significant differences in age, BMI, ALT, AST, and LDL-C were found among the four groups. The levels of SCr and VCAN increased significantly in the normal albuminuria-macroalbuminuria groups, while eGFR decreased significantly (P < 0.0001 for all).
 |
Table 2 Levels of Serum VCAN and Clinical Characteristic in Male T2DM Groups with Different UACR |
Based on the UACR, female patients with T2DM were divided into the normal albuminuria group (n = 88), the microalbuminuria group (n = 44), and the macroalbuminuria group (n = 29, Table 3). Compared with the normal healthy group, diabetic duration, FBG, and HbA1c in female patients with T2DM were significantly increased (Table 3, P < 0.0001 for all). No significant differences in age, ALT, AST, BUN, UA, TG, LDL-C, HDL-C, and VCAN were observed between healthy individuals and female patients with T2DM (P > 0.05). The levels of UACR increased significantly in the normal albuminuria-macroalbuminuria groups, while eGFR decreased significantly (P < 0.0001 for all).
 |
Table 3 Levels of Serum VCAN and Clinical Characteristic in Female T2DM Groups with Different UACR |
Table 4 shows the correlation analysis in all groups of patients with T2DM. In the overall group, serum VCAN levels correlated positively with diabetic duration, SBP, UA, SCr, and UACR and correlated negatively with eGFR (P < 0.05 for all). In male patients with T2DM, serum VCAN levels correlated positively with SBP, BUN, UA, SCr, and UACR and correlated negatively with eGFR (P < 0.05 for all). However, no significant correlation was observed between serum VCAN levels and UACR in female patients with T2DM.
 |
Table 4 Correlation Between VCAN and Clinical Characteristic in Male and Female T2DM Patients |
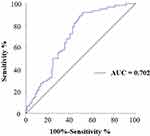
We further explored the predictive efficiency of VCAN for DKD in male patients with T2DM by plotting a ROC curve. The results showed that the area under the curve (AUC) of VCAN in male patients with T2DM was 0.702 (95% CI 0.628–0.776, P < 0.001), with a cutoff value 0.399 ng/mL (Figure 1). This suggested VCAN as a promising biomarker to diagnose DKD in male patients with T2DM.
Discussion
In the present study, we explored the overall and sex-specific association between serum VCAN levels and UACR, and examined the sex difference in this association. First, our data showed that VCAN levels correlated independently and positively with UACR in all and male patients with T2DM. Second, high serum VCAN levels predicted a high risk of DKD in male patients with T2DM.
With rapid economic development and lifestyle changes, the incidence of T2DM has increased significantly worldwide, and chronic hyperglycemia eventually leads to the occurrence of DKD. UACR is recommended as an assessment for DKD by the American Diabetes Association (ADA).9 Similarly, the Kidney Disease Improving Global Outcomes (KDIGO) also recommends UACR as an early indicator of DKD.11 Moreover, increased UACR is strongly associated with adverse events, such as mortality and cardiovascular disease (CVD), even after adjustment for DM, hypertension, and kidney disease.12,13 Therefore, it is important to identify people at high-risk of albuminuria.
To the best of our knowledge, this is the first study of the serum level of VCAN and its relationship with UACR in patients with T2DM. VCAN is a large chondroitin sulphate proteoglycan that is a major component of the ECM. In recent years, studies have highlighted the effect of the ECM on diabetic kidney injury. Rodent models revealed that suppression of enhanced ECM gene expression resulted in decreased albuminuria.14 We found that serum VCAN levels correlated positively with UACR and negatively with eGFR in the overall and male patients with T2DM. This is similar to the results of previous studies.5,15 Rudnicki et al15 identified renal expression of VCAN as a marker for impaired renal function. Using an integrated bioinformatics, Xu et al identified VCAN as a hub gene in DKD tubulointerstitial injury.5 All the above findings indicate that serum VCAN might be a useful marker for the detection of albuminuria in male patients with T2DM.
Multiple biological mechanisms might mediate the association between VCAN and albuminuria. VCAN is produced by several cell types and its expression increases markedly when tissues become inflamed.16–18 It has been reported that VCAN might affect inflammatory responses by interacting with inflammatory cells or multiple cytokines involved in regulating inflammation.16 Accumulating in vitro and in vivo evidence suggests that mitochondrial dysfunction contributes to the development and progression of DKD.19,20 Our previous study reported that VCAN might participate in hyperglycemia-induced vascular calcification and aging via modulation of mitochondrial function.7 Thus, mitochondrial dysfunction might also play a key mechanistic role in explaining the association between VCAN and DKD. Moreover, kidney fibrosis is a major hallmark of DKD.21 Existing evidence based on animal studies suggests that excess VCAN might induce kidney fibrosis.6
The present study revealed that male patients with T2DM were more vulnerable to kidney damage in relation to VCAN exposure than female patients. A previous study also reported sex differences in diabetic vascular complications.22 One of the possible reasons is the differences in sex hormones between male and female patients. Estrogen might exert a protective effect against kidney injury through its anti-inflammation,23 antifibrotic,24 and antioxidant effects,25 whereas testosterone has deleterious pro-inflammation and pro-oxidant effects.26 A population study in patients with T2DM found that male patients had a higher risk of microalbuminuria than female patients.27 Furthermore, other factors could affect the UACR value, including muscle mass, and the amount of food and salt intake. SCr is also a reliable biomarker for muscle mass status. Our results indicated that female patients had lower SCr levels than male patients. However, whether or not these mechanisms are involved in the potential sex difference in the association between serum VCAN and UACR deserves further research.
Notably, the relationship between serum VCAN levels and UACR was not observed in female patients with T2DM. An animal model study showed that the expression of VCAN in the mouse uterus could be regulated by circulating levels of estradiol.28 Moreover, a recent proteomic analysis revealed an inverse correlation between VCAN expression and estradiol levels in atherosclerotic plaques.29 Therefore, we hypothesized that serum VCAN levels in female patients might be affected by estrogen. However, our study did not measure estrogen levels; therefore, we were unable to further investigate the association between estrogen and VCAN levels. This could be an interesting topic for further research.
In the standard clinical setting, eGFR and UACR can be used to evaluate the kidney damage in patients with T2DM. Actually, serum VCAN levels were significantly correlated with both eGFR (r = –0.207, P < 0.01) and UACR (r = 0.238, P < 0.0001). In our study, we chose UACR, rather than eGFR, as a biomarker of DKD, because it has been shown that elevated albuminuria alone appears to be a greater predictor of progression to ESRD, in addition to the risk of CVD and mortality, as compared with a decline in eGFR alone.30–32 Furthermore, increasing UACR, but not eGFR, has been reported to reflect the early structural changes observed on kidney biopsy in T2DM patients.33
The serum VCAN levels correlated with SBP, BUN, UA, SCr, and eGFR in male patients with T2DM. Most of these risk factors are modifiable, which is particularly important for DKD prevention. A recent study demonstrated that a traditional Chinese medicine, Gua Lou Er Chen decoction, could lower VCAN levels and attenuate atherosclerosis in both in vivo animal experiment and in vitro cell experiments.34 Therefore, based on our results and previous studies, VCAN might be used as a potential target for albuminuria therapy.
Our study has some advantages. First, there have been no previous studies regarding the relationship between serum VCAN levels and UACR. Second, this study was performed in a department that has a standard examination process. However, this study also has some limitations. Firstly, its cross-sectional nature does not allow for causality inference. Secondly, the analysis did not include some lifestyle factors, such as smoking status, alcohol consumption, and dietary salt intake, which might be potential contributors to albuminuria. Thirdly, this was a single-center study, and thus the sample size was limited. Therefore, further well-designed studies based on larger populations are needed to confirm our results.
In summary, our study showed that serum VCAN levels increase as UACR increases in male patients with T2DM. Furthermore, we found that VCAN might be a promising biomarker to predict DKD in male patients with T2DM. However, the specific mechanisms of VCAN remain unknown and present a great challenge in medical research.
Data Sharing Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval
This study was approved by the ethics committees of the Second Xiangya Hospital of Central South University, Changsha, Hunan Province, China (No: 2022-163) and was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Informed Consent
Written informed consent was obtained from all participants.
Acknowledgments
We thank all participants in this study.
Funding
This work was supported by the National Natural Science Foundation of China [grant number 82101663] and the Natural Science Foundation of Hunan Province [grant number 2023JJ60080].
Disclosure
The authors declare no competing interests.
References
1. Artasensi A, Pedretti A, Vistoli G., et al. Type 2 diabetes mellitus: a review of multi-target drugs. Molecules. 2020;25(8):1987. doi:10.3390/molecules25081987
2. Martínez-Castelao A, Navarro-González JF, Górriz JL, et al. The concept and the epidemiology of diabetic nephropathy have changed in recent years. J Clin Med. 2015;4(6):1207–1216. doi:10.3390/jcm4061207
3. Fu H, Liu S, Bastacky SI, et al. Diabetic kidney diseases revisited: a new perspective for a new era. Mol Metab. 2019;30:250–263. doi:10.1016/j.molmet.2019.10.005
4. Wight TN. Versican: a versatile extracellular matrix proteoglycan in cell biology. Curr Opin Cell Biol. 2002;14(5):617–623. doi:10.1016/S0955-0674(02)00375-7
5. Xu Q, Li B, Wang Y, et al. Identification of VCAN as hub gene for diabetic kidney disease immune injury using integrated bioinformatics analysis. Front Physiol. 2021;12:651690. doi:10.3389/fphys.2021.651690
6. Han R, Hu S, Qin W, et al. C3a and suPAR drive versican V1 expression in tubular cells of focal segmental glomerulosclerosis. JCI Insight. 2019;4(7):e122912. doi:10.1172/jci.insight.122912
7. Li S, Zhan JK, Wang YJ, et al. Exosomes from hyperglycemia-stimulated vascular endothelial cells contain versican that regulate calcification/senescence in vascular smooth muscle cells. Cell Biosci. 2019;9(1):1. doi:10.1186/s13578-018-0263-x
8. Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15(7):539–553.
9. American Diabetes Association. Standards of medical Care in diabetes—2022 abridged for primary care providers. Clin Diabetes. 2022;40(1):10–38. doi:10.2337/cd22-as01
10. The Japanese Society of Nephrology. Clinical practice guidebook for diagnosis and treatment of chronic kidney disease. Jpn J Nephrol. 2012;54:1031–1189.
11. Christofides EA, Desai N. Optimal early diagnosis and monitoring of diabetic kidney disease in type 2 diabetes mellitus: addressing the barriers to albuminuria testing. J Prim Care Commun Health. 2021;12:21501327211003683. doi:10.1177/21501327211003683
12. Jackson CE, Solomon SD, Gerstein HC, et al. Albuminuria in chronic heart failure: prevalence and prognostic importance. Lancet. 2009;374(9689):543–550. doi:10.1016/S0140-6736(09)61378-7
13. Matsushita K, Coresh J, Sang Y, et al. Estimated glomerular filtration rate and albuminuria for prediction of cardiovascular outcomes: a collaborative meta-analysis of individual participant data. Lancet Diabetes Endocrinol. 2015;3(7):514–525. doi:10.1016/S2213-8587(15)00040-6
14. Al-Trad B, Al-Batayneh K, El-Metwally S, et al. Nigella sativa oil and thymoquinone ameliorate albuminuria and renal extracellular matrix accumulation in the experimental diabetic rats. Eur Rev Med Pharmacol Sci. 2016;20(12):2680–2688.
15. Rudnicki M, Perco P, Neuwirt H, et al. Increased renal versican expression is associated with progression of chronic kidney disease. PLoS One. 2012;7(9):e44891. doi:10.1371/journal.pone.0044891
16. Wight TN, Kang I, Evanko SP, et al. Versican-A critical extracellular matrix regulator of immunity and inflammation. Front Immunol. 2020;11:512. doi:10.3389/fimmu.2020.00512
17. Han CY, Kang I, Harten IA, et al. Adipocyte-derived versican and macrophage-derived biglycan control adipose tissue inflammation in obesity. Cell Rep. 2020;31(13):107818. doi:10.1016/j.celrep.2020.107818
18. Wight TN, Kang I, Merrilees MJ. Versican and the control of inflammation. Matrix Biol. 2014;35:152–161. doi:10.1016/j.matbio.2014.01.015
19. Liu L, Bai F, Song H, et al. Upregulation of TIPE1 in tubular epithelial cell aggravates diabetic nephropathy by disrupting PHB2 mediated mitophagy. Redox Biol. 2022;50:102260. doi:10.1016/j.redox.2022.102260
20. Jiang N, Zhao H, Han Y, et al. HIF-1α ameliorates tubular injury in diabetic nephropathy via HO-1-mediated control of mitochondrial dynamics. Cell Prolif. 2020;53(11):e12909. doi:10.1111/cpr.12909
21. Hung PH, Hsu YC, Chen TH, et al. Recent advances in diabetic kidney diseases: from kidney injury to kidney fibrosis. Int J Mol Sci. 2021;22(21):11857. doi:10.3390/ijms222111857
22. Li S, Li N, Li L, et al. Association of serum bilirubin levels with macro- and microvascular complications in Chinese people with type 2 diabetes mellitus: new insight on gender differences. Diabetes Metab Syndr Obes. 2023;16:597–606. doi:10.2147/DMSO.S403483
23. Mu PW, Jiang P, Wang MM, et al. Oestrogen exerts anti-inflammation via p38 MAPK/NF-κB cascade in adipocytes. Obes Res Clin Pract. 2016;10(6):633–641. doi:10.1016/j.orcp.2016.02.007
24. Dellê H, Rocha JR, Cavaglieri RC, et al. Antifibrotic effect of tamoxifen in a model of progressive renal disease. J Am Soc Nephrol. 2012;23(1):37–48. doi:10.1681/ASN.2011010046
25. Nahavandi S, Ahmadi S, Sobhani SA, et al. A high dose of estrogen can improve renal ischemia-reperfusion-induced pulmonary injury in ovariectomized female rats. Can J Physiol Pharmacol. 2021;99(12):1241–1252. doi:10.1139/cjpp-2021-0130
26. Holmes S, Singh M, Su C, et al. Effects of oxidative stress and testosterone on pro-inflammatory signaling in a female rat dopaminergic neuronal cell line. Endocrinology. 2016;157(7):2824–2835. doi:10.1210/en.2015-1738
27. Retnakaran R, Cull CA, Thorne KI, et al. Risk factors for renal dysfunction in type 2 diabetes: U.K. Prospective diabetes study 74. Diabetes. 2006;55(6):1832–1839. doi:10.2337/db05-1620
28. Salgado RM, Covarrubias AC, Favaro RR, et al. Estradiol induces transcriptional and posttranscriptional modifications in versican expression in the mouse uterus. J Mol Histol. 2013;44(2):221–229. doi:10.1007/s10735-012-9476-1
29. Theofilatos K, Stojkovic S, Hasman M, et al. Proteomic atlas of atherosclerosis: the contribution of proteoglycans to sex differences, plaque phenotypes, and outcomes. Circ Res. 2023;133(7):542–558. doi:10.1161/CIRCRESAHA.123.322590
30. Coresh J, Heerspink HJL, Sang Y, et al. Change in albuminuria and subsequent risk of end-stage kidney disease: an individual participant-level consortium meta-analysis of observational studies. Lancet Diabetes Endocrinol. 2019;7(2):115–127. doi:10.1016/S2213-8587(18)30313-9
31. Persson F, Bain SC, Mosenzon O, et al. Changes in albuminuria predict cardiovascular and renal outcomes in type 2 diabetes: a post hoc analysis of the LEADER Trial. Diabetes Care. 2021;44(4):1020–1026. doi:10.2337/dc20-1622
32. Buyadaa O, Magliano DJ, Salim A, et al. Risk of rapid kidney function decline, all-cause mortality, and major cardiovascular events in nonalbuminuric chronic kidney disease in type 2 diabetes. Diabetes Care. 2020;43(1):122–129. doi:10.2337/dc19-1438
33. Looker HC, Mauer M, Saulnier PJ, et al. Changes in albuminuria but not GFR are associated with early changes in kidney structure in type 2 diabetes. J Am Soc Nephrol. 2019;30(6):1049–1059. doi:10.1681/ASN.2018111166
34. Guo H, Li Y, Qiu L, et al. Gua Lou Er Chen decoction attenuates atherosclerosis by reducing proteoglycans accumulation and inflammation. Phytomedicine. 2023;115:154811. doi:10.1016/j.phymed.2023.154811
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.