Back to Journals » Journal of Inflammation Research » Volume 17
Serum Albumin Level Can Predict Immunotherapy Response of Neuromyelitis Optica Spectrum Disorders in the Acute Phase
Authors Xiang W, Wu Y, Li H, Zhu D , Yao X, Ding J, Wang Z, Guan Y
Received 29 September 2023
Accepted for publication 16 January 2024
Published 12 February 2024 Volume 2024:17 Pages 909—917
DOI https://doi.org/10.2147/JIR.S442532
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Monika Sharma
Weiwei Xiang,1,* Yifan Wu,2,* Hongyan Li,1,* Desheng Zhu,1 Xiaoying Yao,1 Jie Ding,1 Ze Wang,1 Yangtai Guan1
1Department of Neurology, Ren Ji Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, People’s Republic of China; 2Department of Neurology, Xuanwu Hospital, Capital Medical University, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yangtai Guan, Department of Neurology, Ren Ji Hospital, Shanghai Jiao Tong University School of Medicine, No. 160, Pujian Road, Shanghai, People’s Republic of China, Email [email protected]
Background: Neuromyelitis optica spectrum disorder (NMOSD) is an autoimmune demyelinating disease of the central nervous system. However, few biomarkers have been found to predict the outcome of immunotherapy. We investigated the relationship between the serum albumin (S-Alb) and response to immunotherapy in acute NMOSD patients.
Methods: A total of 107 consecutive Chinese patients with acute NMOSD diagnosed between January 2013 and January 2022 were included in our prospective observational study. S-Alb was measured by the use of bromocresol green and immunoturbidimetric methods on admission. The immunotherapy response was assessed by the percentage change in the expanded disability status scale (EDSS) score from admission to discharge after treatment. We evaluated the association between S-Alb and immunotherapy response through multivariate logistic regression analysis.
Results: S-Alb levels were significantly lower in patients who were resistant to immunotherapy than in those who were responsive to treatment (p< 0.001). S-Alb levels were positively related to a favorable response to immunotherapy (r=0.386, p< 0.001). The odds ratio (95% CI) for the association between S-Alb level and response to immunotherapy was 1.27 (95% CI=1.08, 1.50; p=0.004) after adjusting for potential factors. ROC analysis showed that patients with S-Alb levels lower than 40.85 g/L were likely to be resistant to immunotherapy.
Conclusion: Our study indicated that a higher S-Alb was an independent indicator of response to immunotherapy in acute NMOSD patients.
Keywords: immune diseases, NMOSD, immunotherapy, albumin, multivariate analysis
Introduction
Neuromyelitis optica spectrum disorder (NMOSD) is an autoimmune demyelinating disease of the central nervous system, which is characterized by recurrent longitudinal extensive myelitis (LETM) and optical neuritis (ON).1,2 NMOSD was once thought to be a subtype of multiple sclerosis (MS). Since the discovery of immunoglobulin against aquaporin-4 (also known as AQP4-lgG), a water channel mainly located on astrocytes in the brain, NMOSD has been confirmed to be a distinct entity.3 Acute attacks can cause severe morbidity or can even be fatal. Therefore, reducing the severity of acute attacks in NMOSD patients is essential.
The most common treatment for acute NMOSD is a high-dose corticosteroid, typically intravenous methylprednisolone (IVMP).4 Intravenous immunoglobulin (IVIG) and plasma exchange (PLEX) are also treatment choices for patients who respond poorly to the steroid therapy.2,4 Only few studies have evaluated clinical factors that may be related to a favorable outcome of treatment with PLEX, such as a short-delay to initiation and a less severe attack.5–7 However, few biomarkers have been found to predict the outcome of immunotherapy.
Serum albumin (S-Alb) is an inherent drug delivery platform with a long circulatory half-life.8 Recent studies have revealed its neuroprotective effects, such as acting as a target for oxidation and nitration reactions and reducing the production of reactive oxygen and reactive nitrogen species in the CNS.9 Clinically, S-Alb is a prognostic factor in various autoimmune diseases in the CNS, such as autoimmune encephalitis10 and Guillain–Barré syndrome (GBS).11 We hypothesized that S-Alb levels may be a predictor of the response to immunotherapy in acute NMOSD patients. In this study, we aimed to determine the serum level of albumin in patients with NMOSD and its association with the outcome of immunotherapy in the acute phase.
Methods
Design
This was a prospective observational study designed to explore the relationship between the S-Alb and immunotherapy response in acute NMOSD patients. Consecutive patients were enrolled in our study from Renji Hospital in China from January 2013 to January 2022, and patient data were recorded in the NMOSD Registry Database of the hospital. The cerebrospinal fluid (CSF) and blood samples were collected before the administration of any corticosteroids or other immunosuppressive therapy. The study conforms to the precepts of the World Medical Association Declaration of Helsinki. Our study was approved by the Ethics Committee of Renji Hospital. Written informed consent was obtained from all the included patients. Our data are available upon request due to privacy/ethical restrictions.
Study Population
Patients were diagnosed with NMOSD according to the criteria defined by Wingerchuk et al.12 The inclusion criteria were as follows: (1) Patients who were diagnosed with NMOSD (2) receiving IVMP treatment in the acute phase. The exclusion criteria were as follows: (1) incomplete information (gender, symptoms, EDSS score, S-Alb and details of treatment); (2) receiving the initial dose of IVMP less than 0.5g /d during the hospitalization; (3) receiving other treatments for the acute phase, such as cyclophosphamide; (4) having severe hepatic or renal insufficiency before treatment; (5) having malignant tumors.
Clinical and Laboratory Data Collection
The clinical information, including age, gender, clinical features (age at first attack, disease duration, total number of attacks), lesion distribution [optic neuritis (ON), myelitis, longitudinally extensive transverse myelitis (LETM), length of spinal lesion, characteristic brain lesions on MR images] and treatment data (initial dose of IVMP, total dose of IVMP, IVIG, and adverse effects), was collected during hospitalization. The adverse effects identified in our study focused on immunotherapy-related adverse effects, such as infection, allergy, gastrointestinal bleeding and abnormal laboratory results (such as elevated transaminase levels and hypokalemia).
Fasting venous blood samples were collected upon admission, and S-Alb was measured via the use of bromocresol green and immunoturbidimetric methods upon admission. Blood samples were also collected to measure routine blood indices and blood biochemical indicators, including serum data [serum status of AQP4-IgG, blood white blood cell (WBC), alanine transaminase (ALT), and blood urine nitrogen (BUN)].
The expanded disability status scale (EDSS) score before treatment was checked at the time of sampling, and the EDSS score after treatment was recorded at the time of discharge. We defined resistance to treatment as no change in EDSS score or no improvement in neurological examination results or function and response to treatment as improvement in EDSS score or resolution of primary symptoms (such as area postrema syndrome) during hospitalization.13 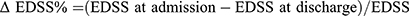 at hospitalization.
at hospitalization.
All patients were administered immunotherapy during their hospitalization at an initial IVMP dose of 1 g/d or 0.5 g/d, followed by a reduction to a half dose every 3 days until oral administration of 60 mg of prednisone per day, guided by the Guidelines for diagnosis and treatment of neuromyelitis in China.
Disease duration refers to the period from the time of the current attack to the time of sampling. Characteristic brain lesions in NMOSD refer to increased signals on T2-weighted MRI sequences according to the diagnostic criteria.12 We defined NMOSD relapse as the occurrence of new or worsening symptoms accompanied by neurological signs and the presence of new or enhanced correlated MRI lesions. The interval should be at least 30 days since the previous relapse. AQP4-IgG antibodies were tested in all patients using a cell-based assay.
Statistical Analysis
The data analysis was performed using SPSS 25.0 software (SPSS, Inc., Chicago, IL, USA). The Kolmogorov‒Smirnov Z-test was used to verify the normality of the data. The patients in our study were grouped according to immunotherapy response assessed by the percentage difference in EDSS score between admission and discharge after treatment. Categorical variables were presented as counts (percentage) and continuous variables were presented as the means (standard error, SE) for normal data or medians (interquartile ranges, IQRs) for nonnormal data. Student’s t-test and Mann‒Whitney U-test were applied for normally and nonnormally distributed data, respectively. Pearson’s correlation and Spearman’s rank correlation coefficients were used to evaluate correlations between different clinical parameters for normal and nonnormal data, respectively. Multivariate logistic regression analysis was used to detect the independent factors predictive of the response to immunotherapy after adjusting for known confounders. A receiver operating characteristic (ROC) curve was used to identify the optimal cutoff value of S-Alb level for predicting the response to immunotherapy. A two-tailed probability value < 0.05 was considered significant.
Results
Basic Characteristics
A total of 124 consecutive candidates were recruited for the study at the time of the final survey in January 2022. Among these candidates, those who had missing data related to S-Alb, EDSS, gender and age were excluded from the eligible candidates for this study (n=12). Those who underused IVMP (initial dose < 0.5 g/d) (n=5) were also excluded from the pool of eligible candidates for this study. As a result, a total of 107 subjects were included in the final analyses. A flowchart of the study is shown in Figure 1.
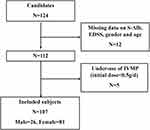 |
Figure 1 Flowchart of the study. |
The baseline characteristics of all subjects are presented in Table 1. Among the 107 patients with NMOSD, 75.7% were female (n=81). The ages ranged from 15 to 81 years, with a mean age of 48.51±14.91 years. The mean EDSS score was 4.64±2.38 at sampling. The S-Alb levels varied from 29.9 to 49.9 g/L, with a mean S-Alb of 41.04±4.30 g/L. S-Alb levels were significantly lower in patients who were resistant to immunotherapy than in those who were responsive to treatment (p<0.001). This difference was also observed in patients treated with IVMP (39.11±4.26 g/L versus 42.65±3.71 g/L, p<0.001) (Figure 2). However, in patients treated with IVMP+IVIG, S-Alb levels were greater in those who responded to treatment (38.17±4.63 g/L versus 40.97±2.97 g/L, p=0.140) but the difference was not statistically significant (Figure 2).
 |
Table 1 Baseline Characteristics of Subjects |
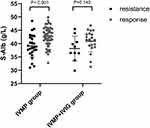 |
Figure 2 Levels of S-Alb according to responses to immunotherapy in IVMP and IVMP+IVIG groups. |
The serum AQP4-IgG was positive in 90 patients (84.1%). Twenty-nine patients were experiencing their first attack (27.1%), while 78 had relapsed (72.9%). Forty-two patients (39.3%) had ON, 72 patients (67.3%) had myelitis, among whom 58 patients (54.20%) had LETM, and 37 patients (34.6%) had characteristic brain lesions on cranial MR images. All NMOSD patients were treated with intravenous methylprednisolone (IVMP), and 30 were also treated with intravenous immunoglobulin (IVIG), among whom 68 patients responded to treatment. The median total disease duration was 15 days (IQR, 7–30 days).
Comparison of the Response to Treatment According to the S-Alb Level
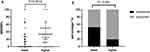
The ΔEDSS% was significantly greater in the higher S-Alb subgroup (0% versus 33.33%, p=0.0012) (Figure 3A). Among all the patients, 52.73% and 19.23% were resistant to immunotherapy in the lower and higher S-Alb groups, respectively (p<0.001) (Figure 3B).
Relationship Between S-Alb Level and Response to Treatment
The Spearman correlation coefficient (95%) for the correlation between S-Alb level and response to immunotherapy was 0.386 (p<0.001) (Figure 4). The odds ratios (95% CI) for the association between S-Alb level and response to immunotherapy were 1.29 (1.14–1.45, p<0.001) for all patients and 1.21 (1.07, 1.37, p=0.002) for the AQP4-IgG positive patients. After multivariate analysis was performed for age, gender, length of spinal lesions, EDSS score, WBC count and CRP concentration, the odds ratios (95% CI) were 1.27 (1.08–1.50, p=0.004) and 1.24 (1.04–47, p=0.015) for the total patients and AQP4-IgG-positive patients, respectively, which showed that S-Alb was an independent factor of immunotherapy outcomes. The OR (95%) showed a grade increase according to the S-Alb tertiles by trend analysis (p=0.005) (Table 2).
 |
Table 2 Association Between S-Alb and Response to Immunotherapy |
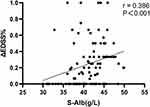 |
Figure 4 Relationship between S-Alb and immunotherapy response. |
In this model, the odds ratios (95% CI) of the other covariates were as follows. Female, 2.51 (0.72–8.77), p=0.15; age (per year), 0.96 (0.92–0.99), p=0.03; length of spinal lesion, 0.89 (0.78–1.01), p=0.078; WBC count, 0.86 (0.75–0.98), p=0.03; EDSS score, 0.925 (0.72–1.19), p=0.55; and CRP levels, 1.03 (0.939–1.12), p=0.57.
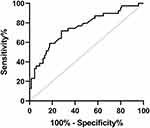
ROC Analysis to Identify the Optimal Cutoff Value of the S-Alb Level
The ROC curve for the ability of S-Alb level to predict the response to immunotherapy in patients with NMOSD is shown in Figure 5. The cutoff value of the S-Alb level was 40.85 g/L with a sensitivity of 72.1%, a specificity of 71.8%, and an AUC of 0.751 (0.653–0.850) (p <0.001).
Discussion
Our study revealed that patients who responded to immunotherapy had higher S-Alb levels and that S-Alb levels are associated with the response to immunotherapy in acute NMOSD attacks. In addition, patients with higher S-Alb levels had greater improvements in EDSS score. Furthermore, the association between S-Alb levels and response to immunotherapy was independent after adjustment for confounding risk factors. Therefore, these results demonstrated that a higher S-Alb level is a prognostic factor for a favorable outcome in acute NMOSD patients. Furthermore. ROC analysis revealed that immunotherapy was more likely to fail in patients with an S-Alb level less than 40.85 g/L. In practice, clinicians usually use their own experience to judge whether or when to use a second-line therapy. This study provides evidence for clinicians to identify potential nonresponders earlier. A short delay to some second-line therapies, such as PLEX, has proven to be more beneficial5 than other therapies; therefore, detecting nonresponders to IVMP or IVIG may help to initiate a more aggressive treatment in the early stage.
S-Alb, which is most abundant in human blood, is a single polypeptide chain of 585 amino acids with various ligand-binding sites.14 The affinity of this protein for the neonatal Fc receptor (FcRn), which also interacts with immunoglobulin, enables its long circulation half-life of 19 days.8,15 Therefore, albumin is an inherent drug transporter and a significant factor for the pharmacokinetics of drugs due to its abundance and stability. Corticosteroids have been confirmed to bind to albumin16 and this reversible combination could buffer fluctuations in the concentrations of steroids and their free fractions.17 Currently, albumin binding or fusion is the dominant strategy for extending half-life of a material.18 Studies have revealed that S-Alb nanoparticles increased the effectiveness of MPs mostly by improving their absorption.19,20 Our study is the first to demonstrate the association between S-Alb and the response to immunotherapy, including IVMP and IVIG. We assumed that a higher albumin concentration provides more binding sites for methylprednisolone to prolong its half-life and facilitates its distribution, leading to a more favorable prognosis in the short term.
In other pathological conditions, low albumin levels are associated with poor prognosis in GBS patients and patients with autoimmune encephalitis treated with IVIG. These studies also revealed that some patients developed a decrease in the serum albumin concentration after the use of IVIG and had a poor prognosis.10,11 The level of Albumin is thought to be able to reflect the recycling capacity of the protein, for example, the expression of FcRn and immunoglobulin pharmacokinetics. Additionally, the exhaustion of FcRn by IVIG may explain the reduction of albumin. However, our study did not provide supporting evidence for these studies. On the one hand, IVMP is now regarded as a first-line therapy, while IVIG is now an add-on treatment with for which data are scarce. Patients who choose to use IVIG are limited. On the other hand, our study focused on the short-term efficacy. A long-term follow-up is needed in the future.
In addition to its drug delivery ability, S-Alb also has multiple clinical value, such as maintaining colloid osmotic pressure, acting as an antioxidant and removing free radicals.21 Recent studies revealed the neuroprotective role of albumin. Albumin scavenges ROS and RON as a target for oxidation and nitration reactions.22 The upregulation of oxidative stress has been studied in NMO pathogenesis.23 S-Alb can extravasate via the broken blood-brain barrier during the pathogenesis of NMOSD and decrease local oxidation and nitration, thereby alleviating tissue damage.9 Therefore, a high S-Alb could be a protective factor against severe disease. The S-Alb is also a well-known predictor of the prognosis in many acute or severe conditions.24,25 Hypoalbuminemia is correlated with poor clinical outcomes. A meta-analysis revealed that when S-Alb exceeds 30 g/L complication rates may be reduced in acutely ill patients.26 In a recent study by Shi et al, NMOSD patients were divided into mild/moderate disability and severe disability groups according to their EDSS score, and some of the patients were in remission.27 The results demonstrated that total bilirubin was an independent factor that influenced the severity of disability. Besides, albumin was closely related to NMOSD severity. We grouped NMOSD patients according to their response to immunotherapy, and the patients were in the acute phase. Our study indicated that a higher level of S-Alb was an independent indicator of the response to immunotherapy in acute NMOSD patients. The results of our study, combined with the findings of Shi et al, could better reflect the role of albumin in the pathophysiology of NMOSD.
Our current study has a few limitations. First, the prospective study included data from one single center, a large number of patients need to be included in the future, especially patients treated with other therapies. Second, our study concluded that S-Alb is a predictor of the short-term outcome of immunotherapy, so a long-term follow-up is needed to determine the role of S-Alb in immunotherapy. Finally, the EDSS score, which is used for the evaluation of disease severity, is primarily used in MS. Therefore, some specific symptoms of NMOSD, such as area postrema syndrome, were not included in the scoring.
Conclusion
This study concluded that higher S-Alb levels could be considered a predictor of a favorable short-term outcome in NMOSD patients during the acute phase. However, further studies with long-term follow-up and larger sample sizes are needed.
Acknowledgments
Weiwei Xiang, Yifan Wu and Hongyan Li are co-first authors for this study.
Funding
This work was supported by the Key projects of basic research of Shanghai Municipal Science and Technology Commission, 20JC1412000(YG). Innovative research team of high-level local universities in Shanghai, SHSMU-ZDCX20211901(YG). The National Natural Science Foundation of China, 82071341(YG).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Kim HH, Jeong IH, Hyun JS, Kong BS, Kim HJ, Park SJ. Metabolomic profiling of CSF in multiple sclerosis and neuromyelitis optica spectrum disorder by nuclear magnetic resonance. PLoS One. 2017;12(7):1.
2. Marignier R, Cobo Calvo A, Vukusic S. Neuromyelitis optica and neuromyelitis optica spectrum disorders. Curr Opin Neurol. 2017;30(3):208–215. doi:10.1097/WCO.0000000000000455
3. Lennon VA, Wingerchuk DM, Kryzer TJ, et al. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet. 2004;364(9451):2106–2112. doi:10.1016/S0140-6736(04)17551-X
4. Bruscolini A, Sacchetti M, La Cava M, et al. Diagnosis and management of neuromyelitis optica spectrum disorders - an update. Autoimmunity Rev. 2018;17(3):195–200. doi:10.1016/j.autrev.2018.01.001
5. Bonnan M, Valentino R, Debeugny S, et al. Short delay to initiate plasma exchange is the strongest predictor of outcome in severe attacks of NMO spectrum disorders. J Neurol Neurosurg. 2018;89(4):346–351. doi:10.1136/jnnp-2017-316286
6. Banerjee A, Ng J, Coleman J, Ospina JP, Mealy M, Levy M. Outcomes from acute attacks of neuromyelitis optica spectrum disorder correlate with severity of attack, age and delay to treatment. Mult Scler Relat Disord. 2019;28:60–63. doi:10.1016/j.msard.2018.12.010
7. Jiao Y, Cui L, Zhang W, et al. Plasma exchange for neuromyelitis optica spectrum disorders in Chinese patients and factors predictive of short-term outcome. Clin Ther. 2018;40(4):603–612. doi:10.1016/j.clinthera.2018.03.007
8. Sleep D. Albumin and its application in drug delivery. Expert Opin Drug Deliv. 2015;12(5):793–812. doi:10.1517/17425247.2015.993313
9. LeVine SM. Albumin and multiple sclerosis. BMC Neurol. 2016;16(1):47. doi:10.1186/s12883-016-0564-9
10. Jang Y, Lee S-T, Kim T-J, et al. High albumin level is a predictor of favorable response to immunotherapy in autoimmune encephalitis. Sci Rep. 2018;8(1):1012. doi:10.1038/s41598-018-19490-z
11. Fokkink W-JR, Walgaard C, Kuitwaard K, Tio-Gillen AP, van Doorn PA, Jacobs BC. Association of albumin levels with outcome in intravenous immunoglobulin-treated guillain-barré syndrome. JAMA Neurol. 2017;74(2):189–196. doi:10.1001/jamaneurol.2016.4480
12. Wingerchuk DM, Banwell B, Bennett JL, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2015;85(2):177–189. doi:10.1212/WNL.0000000000001729
13. Keegan M, Pineda AA, McClelland RL, Darby CH, Rodriguez M, Weinshenker BG. Plasma exchange for severe attacks of CNS demyelination: predictors of response. Neurology. 2002;58(1):143–146. doi:10.1212/WNL.58.1.143
14. Matos MJ. Learning from nature: the role of albumin in drug delivery. Future Med Chem. 2018;10(9):983–985. doi:10.4155/fmc-2018-0053
15. Pyzik M, Rath T, Lencer WI, Baker K, Blumberg RS. FcRn: the architect behind the immune and nonimmune functions of IgG and albumin. J Immunol. 2015;194(10):4595–4603. doi:10.4049/jimmunol.1403014
16. Abboud R, Akil M, Charcosset C, Greige-Gerges H. Interaction of glucocorticoids and progesterone derivatives with human serum albumin. Chem Phys Lipids. 2017;207(Pt B):271–278. doi:10.1016/j.chemphyslip.2017.04.007
17. Hammond GL. Plasma steroid-binding proteins: primary gatekeepers of steroid hormone action. J Endocrinol. 2016;230(1):R13–R25. doi:10.1530/JOE-16-0070
18. Kontermann RE. Half-life extended biotherapeutics. Expert Opin Biol Ther. 2016;16(7):903–915. doi:10.1517/14712598.2016.1165661
19. Lin Y, Li C, Li J, et al. NEP(1-40)-modified human serum albumin nanoparticles enhance the therapeutic effect of methylprednisolone against spinal cord injury. J Nanobiotechnol. 2019;17(1):12. doi:10.1186/s12951-019-0449-3
20. Wu L, Chen M, Mao H, et al. Albumin-based nanoparticles as methylprednisolone carriers for targeted delivery towards the neonatal Fc receptor in glomerular podocytes. Int J Mol Med. 2017;39(4):851–860. doi:10.3892/ijmm.2017.2902
21. Rozga J, Piątek T, Małkowski P. Human albumin: old, new, and emerging applications. Ann Transplant. 2013;18:205–217. doi:10.12659/AOT.889188
22. Roche M, Rondeau P, Singh NR, Tarnus E, Bourdon E. The antioxidant properties of serum albumin. FEBS Lett. 2008;582(13):1783–1787. doi:10.1016/j.febslet.2008.04.057
23. Pentón-Rol G, Cervantes-Llanos M, Martínez-Sánchez G, et al. TNF-alpha and IL-10 downregulation and marked oxidative stress in neuromyelitis optica. J Inflamm. 2009;6:18. doi:10.1186/1476-9255-6-18
24. Kendall H, Abreu E, Cheng A-L. Serum albumin trend is a predictor of mortality in ICU patients with sepsis. Biol Res Nurs. 2019;21(3):237–244. doi:10.1177/1099800419827600
25. Leite HP, Rodrigues da Silva AV, de Oliveira Iglesias SB, Koch Nogueira PC. Serum albumin is an independent predictor of clinical outcomes in critically ill children. Pediatr Crit Care Med. 2016;17(2):e50–e57. doi:10.1097/PCC.0000000000000596
26. Vincent J-L, Dubois M-J, Navickis RJ, Wilkes MM. Hypoalbuminemia in acute illness: is there a rationale for intervention? A meta-analysis of cohort studies and controlled trials. Ann Surg. 2003;237(3):319–334. doi:10.1097/01.SLA.0000055547.93484.87
27. Shi L, Li D, Zhang Y, et al. Factors influencing the degree of disability in patients with neuromyelitis optica spectrum disorders. Eur J Med Res. 2023;28(1):426. doi:10.1186/s40001-023-01404-z
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.