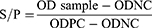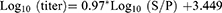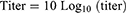Back to Journals » Veterinary Medicine: Research and Reports » Volume 13
Seroprevalence and Associated Risk Factors of Mycoplasma gallisepticum Infection in Poultry Farms of Hawasa and Bishoftu, Central Ethiopia
Authors Shiferaw J , Shifara F, Tefera M , Feyisa A, Tamiru Y
Received 18 February 2022
Accepted for publication 6 May 2022
Published 18 May 2022 Volume 2022:13 Pages 101—107
DOI https://doi.org/10.2147/VMRR.S360669
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Young Lyoo
Jirata Shiferaw,1 Firaol Shifara,2 Misgana Tefera,1 Abdi Feyisa,3 Yobsan Tamiru4
1Department of Pathology and Parasitology, Addis Ababa University College of Veterinary Medicine and Agriculture, Bishoftu, Ethiopia; 2College of Natural and Computational Science, Faculty of Veterinary Medicine, Hawassa University, Hawassa, Ethiopia; 3Department of Clinical Studies, College of Veterinary Medicine and Agriculture Addis Ababa University, Bishoftu, Ethiopia; 4School of Veterinary Medicine, Wollega University, Nekemte, Ethiopia
Correspondence: Jirata Shiferaw, Department of Pathology and Parasitology, Addis Ababa University College of Veterinary Medicine and Agriculture, Bishoftu, Ethiopia, Email [email protected]
Background: Mycoplasma gallisepticum (MG) infection is an economically important respiratory disease in the poultry production system worldwide. It is characterized by rapid transmission and causing many obstacles to poultry industries in different parts of Ethiopia.
Methods: A cross-sectional study was employed from January to September 2021 to estimate the seroprevalence and associated risk factors of MG in domestic layer chickens in large and small commercial poultry farms in Hawassa and Bishoftu area, Ethiopia. A total of 368 blood samples were collected. Data were analyzed using SPSS version-20, reported as percent prevalence, and Pearson’s chi-square was used to assess the association between factors considered to have association with MG infection. The samples were processed by using an indirect ELISA (ProFLOKIBV, USA) test coated with antibody against MG.
Results: The total seroprevalence of MG infection was found to be 70.65%. Significant variation in environmental risk factors with seroprevalence was assessed and the higher prevalence of MG was significantly (χ2 = 14.42; p < 0.05) higher in layer chicken farms found in Bishoftu. Likewise, it was significantly observed in the adult chicken and commercial production system. There were significant difference between breeds and ages of birds with the occurrences of MG (χ2 = 19.60 and χ2 = 17.46, respectively). Management related risk factors found around the types of farms were significantly different with the occurrences of MG (OR = 52.5; p < 0.05).
Conclusion: The evidence from seroprevalence of the MG infection in the current finding provides an indication of the eminence of infection in the study areas. Moreover, it provides an insight on the prevalence of MG infection and further molecular characterization of the organism needs to be conducted in the areas. Biosecurity measures combined with vaccination and sero-monitoring should also be implemented in the farms.
Keywords: Bishoftu, Hawassa, indirect ELISA, M. gallisepticum, Ethiopia
Introduction
Chickens are important livestock resources, easily breaking the vicious cycle of poverty and malnutrition in developing countries. Poultry production plays a major role in the raising up of the economy, in addition to alleviating poverty and malnutrition. The greater percentage of smallholder poultry in the national flock population of developing countries makes them worth paying attention to the management and improves breeding.1 Commercial production hires local people; also it contracts day-old chickens to local people to raise them on a payment basis.2
In Ethiopia, like any other countries found under development, poultry production is raised rapidly and taken as a food insecurity solving sector by providing a major source of protein supplementation. Taking this into account, the Ethiopian Ministry of Agriculture (MoA) has identified poultry production as a key sector to deal with food security issues to raise the amount of meat and eggs produced per year through increasing the number of poultry farms.3 However, many infectious diseases affecting these production types are found as constraint factors affecting the poultry industry. Infectious diseases are among the several factors responsible for considerable losses. The infectious diseases that are of growing concern in poultry production are chicken mycoplasmosis.4
The bacterial infection of avian mycoplasmas was first discovered in 1935. It belongs to the class Mollicutes, family Mycoplasmataceae, and is the most important economically significant mycoplasma pathogen of poultry. Among different species of Mycoplasma, Mycoplasma gallisepticum (MG) is one of the bacterial species highly affecting chickens. It is economically the most important pathogen of poultry. The MG infections are known as chronic respiratory disease (CRD) of chickens, infectious sinusitis of turkeys, and house finch conjunctivitis.5–7
Even though all age groups of birds can be affected, young chickens are more susceptible than the adult individuals.8 It affects different production types.9 MG can be directly transmitted vertically through contamination of oviduct and egg and laterally through direct contact with clinically affected chickens and indirectly in contact with secretion. It may persist also in the environment and can be the source of infection.5,10 Contact between birds also aids in the rapid spread of the infection within flocks.11 MG strains vary in infectivity and virulence, and infections may sometimes be inapparent.12 Chickens may have not obvious symptoms or may exhibit coughing, sticky nasal discharge, difficulty breathing, swelling of the face, sneezing, foamy secretion in the eyes, and a drop in body weight as well. It also causes chronic respiratory diseases in chickens.10,13
MG infection can be identified based on the characteristics associated to their morphology, cultural characteristics, metabolic activities, and serological and molecular characteristics.14 Among serological tests the serum plate agglutination (SPA) test could be used as a tool for quick detection of MG infection. The control of avian mycoplasmosis by vaccination is limited since only few vaccines are available. Total eradication through test and slaughter is the most effective control method.15
Mycoplasma gallisepticum (MG) is an economically expensive and globally prevalent pathogen of poultry.16 Currently the MG infection is circulating in different parts of the country and causing a huge loss. Despite its significant importance in commercial and smallholder poultry farms, a few works were undertaken as to the status and distribution of MG infection in Ethiopia. The conducted studies were reported by Chaka et al17 and Jibril et al4 in East Shewa. Therefore, the objectives of the study were: serological identification of MG in layer chickens and assessing the risk factors related to occurrences of MG in the selected study area.
Materials and Methods
Study Area and Period
The study was conducted from June 2021 to September 2021 in large commercial and small-scale poultry farms found in Hawassa and Bishoftu, Southern and Central Ethiopia, respectively. Hawassa is the capital city of Sidama regional state which is located at 38°29ʹE and 7°05ʹN, 275 km south of Addis Ababa. Its elevation is about 1790 m above sea level and the respective minimum and maximum average temperatures are 12 and 28 °C. The mean annual rainfall is 960 mm. The short rainy season/“spring” starts from February to May and the long rainy season/“winter” starts from mid-June to October. The total chicken population of the zone is approximately 2,123,579 poultry.18
Bishoftu town is located 45 km southeast of Addis Ababa, with an altitude of 1850 m above sea level. Farmers in the vicinity of Bishoftu use a mixed crop and livestock farming system.19
Ethics Approval
The approval on animal handling ethics was accepted before starting sample collection. All the animal handling and sample collection methods were performed in accordance with the Addis Ababa University College of Veterinary Medicine research ethics (AAU-CVMA-REC) and animal welfare guide for the care and use of animals (Ref. No. 01223/2021).
Study Population
A study was conducted on randomly selected chickens kept under an intensive management system in three purposively selected farms of Elfora and Genesis in the Bishoftu town and one farm from Hawassa town. Small-scale farms were selected from both districts where 40 samples were from Hawassa and 20 samples were from Bishoftu. Different age and breed groups were considered in the study. Accordingly, Sasso and Bovans brown were the two layer breeds of chickens sampled in the present study. There was no vaccination history for the flock included in the current study.
Study Design
A cross-sectional study was employed on the study of seroprevalence and its associated risk factors of MG infections in the study areas. Blood samples were collected from apparently healthy chickens. Chickens with age groups greater than four months were randomly selected from different farms in the area. Breed, age, location, and types of production of the farms were recorded as risk factors.
Sample Size Determination
The sample size for the current study was estimated based on the formula set by Thrusfield20 with a 5% desired precision and confidence interval (CI) of 95% by considering an expected prevalence of 67.7%16 in East Shewa. Thus the required sample size (N = 1.962) multiplied by expected prevalence rate (p-exp) and divided by desired absolute precision (d2 = 0.05). Hence, using this formula, the calculated sample size for the current study was 336. However, to increase precision, 10% was added and a total of 368 samples were collected and processed.
Sample Collection and Sampling Technique
For serological study, 3 ml of blood samples were collected by sterile disposable randomly from the brachial vein of each chicken under sterile circumstances and then it was immediately conveyed to clean labelled tubes. Then it was allowed to clot at room temperature in the slant position for about two hours. Then clear serum was collected into labelled cryovial tubes and maintained under cold chain and then transported to Addis Ababa University, College of Veterinary Medicine (CVMA) serological analysis.
Laboratory Examination
Indirect ELISA test with MG antibody specific kit was conducted to determine antibody against MG infection. All the serological procedures were conducted according to the manufacturer's (flock screen cat. no. v050/v054, GUILDHAY Company, Surrey, England) guidelines. The MG ELISA test kit used in this study was selected based on its higher specificity of company guidelines. Finally, the result was interpreted based on the OD value. Likewise, a validity test was done two times depending on the controls and result. A serum sample having SP value greater than 0.3 was taken as positive; while SP values less than or equal to 0.3 were considered as negative. The test serum and ELISA test reagents were brought to room temperature. Prior to being assayed, a 1:50 dilution of the sample was prepared with manufacturer's diluents in a 2-step process. The 100 µL of each diluted sample was pipette into the appropriate well on the antigen-coated plate.21
For each sample, the calculation of the SP ratio and antibody titer was as follows:
Statistical Methods and Data Analysis
Data were entered into a Microsoft Excel spread sheet and analyzed using STATA (version 20) software analysis. The total prevalence of MG infection was calculated by dividing the total number of ELISA positive samples by the total number of animals tested. Then association between the potential risk factors and prevalence of the disease was made using the Pearson chi-square (χ2) test. Significant association at 95% CI and P-value of 5% was considered.
Data Quality Management
Quality of data was secured through appropriate collection and record of the samples. Consequently, history of vaccination in the farm was assessed by asking the owners and the farm managers during sample collection.
Results
Seroprevalence of Mycoplasma gallisepticum Infection
From the total of 368 sera samples collected in the study area, 70.65% were found to be positive to MG infection. A higher prevalence (79.80%) of the disease was observed in the Bishoftu farm than Hawassa (Table 1). The sensitivity and specificity of the MG ELISA test was resulted 89.7% and 93.4% respectively. It was obtained from the sample positive ratios and antibody titers according to manufacturer's guidelines.
 |
Table 1 Sero-Prevalence of MG per the Study Area |
Seroprevalence of MG Infection Across Different Risk Factors
Seroprevalence of MG infection was analyzed across different associated risk factors. Accordingly, the breed-wise analysis of the disease was significantly (p < 0.05) found to be higher in Bovans brown than Sasso breeds of chicken. Likewise, comparing the prevalence of the infection in different stages of production, it was relatively higher in commercial farms than in the small-scale farms and are significantly associated (p < 0.05) (Table 2). There were significant differences between ages of birds with the occurrences of MG (χ2 = 17.46) although there is collinearity found in total sample size. The management factors particularly biosecurity and the farm types (informed from stakeholders during sample collection) were found to be significantly different (OR = 52.5 and p < 0.05) with the occurrences of MG (Table 3).
 |
Table 2 Sero-Prevalence of MG Infection in Poultry Farms Across Different Associated Risk Factors |
 |
Table 3 Logistic Regression Analysis on the Occurrences of MG with Associated Risk Factors |
Discussion
The present study was carried out on seroprevalence of MG infection and its associated risks in both commercial and small-scale poultry farms of Bishoftu and Hawassa kept under intensive type. The incidence of infectious diseases of poultry poses a significant threat to production and productivity.22 The overall seroprevalence of the current study is relatively consistent with the earlier studies conducted by Chaka et al17 and Mera and Mudasir,7 who reported 67.7% and 65.5%% seropositive prevalence of MG in Ethiopia and Kenya, respectively. Whereas the current result was much higher than the earlier findings of Sarkar et al23 in Bangladesh, Jibril et al4 in Ethiopia and Baksi et al24 in Bangladesh, who reported 58.9%, 49.4%, and 32% of Avian MG seroprevalence respectively. The disparity among the indicated above reports with the current study is possibly associated with variation in sample size, age and breed of chicken, management system, hygiene status of the farms, and difference in agro-ecological location.25
The present study also revealed considerable variations in seroprevalence of MG among the study areas. The higher prevalence of MG was significantly (χ2 = 14.42; p < 0.05) found in layer chicken farms found in Bishoftu area. This variation could be due to the difference in the number of samples collected from the two sites, the difference in types of geo-location and hygienic practices in the area.
The current finding indicated that there was significant variation (χ2 = 19.60; p < 0.05) in seroprevalence of MG between breeds of chickens. Accordingly, a higher prevalence was observed in Bovans brown breeds of chicken than Sasso breeds in the study area. The differences in prevalence between the breeds might be attributed to the difference in genetic resistance against the disease.
The result of the current study indicated that age was significantly associated (χ2 = 17.46; p = 0.02) with seroprevalence of MG in which an increasing prevalence with increasing age was observed. This result was in agreement with the previous reports of Talha26 and Jibril et al,4 who reported that higher seroprevalence of MG was found in adult chickens. This suggests that the possibility of getting MG increase with age which might be dependent on environmental exposure. In addition to this fact, as the chicken get older, the risk of disease also likely increases particularly in commercial production systems when they enter into different production cycles.
The present finding showed that there was a significant difference in seroprevalence of MG between the types of production (farm types), in which a higher prevalence of the cases was found in commercial production type (χ2 = 15.93; p = 0.03). Similar findings were reported by Jibril et al.4 As compared to commercial chickens of large scale, small scales are easily managed and the problems in management systems that might favor the existence of MG are very rare. Moreover, as the intensification gets higher, the likely hood of stress also gets worse. These all add up together and results in a relatively higher prevalence of mycoplasmosis in large-scale commercial farms than in small-scale ones.27
Conclusion
The evidence from seroprevalence of the MG infection using indirect ELISA in the current finding provides an indication of the status of infection in the study areas. Accordingly, the study finding relatively indicates a higher seroprevalence of MG in relation to similar studies in the country. The present study generally provides an insight on the presence of MG in the study areas, although there is unidentified species of mycoplasma occurrences through further molecular characterization of the agent. Therefore, further molecular detection and characterization of MG should be conducted; vaccination and sero-monitoring should be implemented in commercial farms of Ethiopia.
Data Sharing Statement
Data sets endorsing the current result are accessible from the principal author whenever required.
Ethical Clearance and Consent
Before starting sample collection, oral informed consent was obtained from all poultry farmers. Then the owners were agreed to allow researchers to collect samples without stress and injury to animals. All sampling procedures were conducted as per the guidelines approved by animal welfare and research ethics review committee at the Addis Ababa University College of Veterinary Medicine and Agriculture Ethical Review Committee.
Acknowledgments
The authors thank the Addis Ababa University, Associate research and Technology Transfer (RTT) support under the them entitled “Improving poultry production” for this project, and the anonymous commercials and small-scale farms for providing and allowing us to collect blood samples from their chicks. We also acknowledge the support of Zoetis ALPHA Company for supporting ELISA Kits for serological survey used in this research. Further thanks go to National Veterinary Institute (NVI), Ethiopia for providing the serology laboratory and College of Veterinary Medicine and Agriculture for providing materials used during data collection.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Farrell D. The Role of Poultry in Human Nutrition. Poultry Development Review. Rome: Food and Agriculture Organization; 2013:2–9.
2. Habte T, Amare A, Bettridge J, Collins M, Christley R, Wigley P. Guide to chicken health and management in Ethiopia: for farmers and development agents; 2017.
3. Jenberie S, Lynch S, Kebede F, et al. Genetic characterization of infectious bursal disease virus isolates in Ethiopia. Acta Trop. 2014;130:39–43. doi:10.1016/j.actatropica.2013.09.025
4. Jibril Y, Asfaw Y, Gebregziabher B, Issa A. Seroprevalence of Mycoplasma gallisepticum in domestic chickens, East Shewa, Ethiopia. Ethiop Vet J. 2018;22(1):74–86. doi:10.4314/evj.v22i1.6
5. Ley DH. Mycoplasma gallisepticum infection. In: Fadly AM, Gilson JR, McDougald LR, Nolan LK, Swanye DE, editors. Disease of Poultry. Ames, Iowa, USA: Iowa State University Press; 2008:807–834.
6. Kleven SH. Mycoplasmas in the etiology of multifactorial respiratory disease. Poult Sci. 1998;77(8):1146–1149. doi:10.1093/ps/77.8.1146
7. Mera U, Mudasir H. Prevalence of Mycoplasma gallisepticum (MG) antibodies in chicken in Sokoto, Nigeria. EAS J Vet Med Sci. 2019;1(5):58–59.
8. Seifi S, Shirzad MR. Seroprevalence and risk factors of Mycoplasma gallisepticum infection in Iranian broiler breeder farms. Int J Animal Vet Adv. 2012;4:45–48.
9. Manimaran K, Mishra A, Roy P, Kumanan K. Development of a promising agglutination-based diagnostic kit for detection of Mycoplasma gallisepticum (MG) infection in chickens. Indian J Anim Res. 2021;55(7):849–852.
10. Ley DH. Mycoplasma gallisepticum infection. In: Calnek BW, Barnes HJ, Beard CW, McDougald LR, Saif YM, editors. Disease of Poultry. Ames, Iowa, USA: Iowa State University Press; 2003:722–744.
11. Ayim M, Asafu-Adjaye A, Beckley C, et al. Serological survey of Mycoplasma gallisepticum infection in layer chickens in the Ga-East District of the Greater Accra region. JGSA. 2012;14(1):22–29.
12. Sayed R, Ahmed H, Shasha F, Real-Time AA. PCR quantification and differentiation of both challenge and vaccinal Mycoplasma gallisepticum strains used in vaccine quality control. J World Poult Res. 2018;8(3):50–58.
13. Hennigan SL, Driskell JD, Ferguson-Noel N, et al. Detection and differentiation of avian mycoplasmas by surface-enhanced Raman spectroscopy based on a silver nanorod array. Appl Environ Microbiol. 2012;78(6):1930–1935. doi:10.1128/AEM.07419-11
14. Ley DH, Yoder HW. Mycoplasma gallisepticum infection. In: Calnek BW, Barnes HJ, Beard CW, Dougald LR M, Saif YM, editors. Disease of Poultry.
15. Yoder HW. Mycoplasma gallisepticum infections. In: Calnek BW, Barnes HJ, Beard CW, Reid WM, Yoder HW
16. Levisohn S, Kleven SH. Avian mycoplasmosis (Mycoplasma gallisepticum). Revue Scientifique Et Technique. 2000;19(2):425–442.
17. Chaka H, Goutard F, Bisschop SP, Thompson PN. Seroprevalence of Newcastle disease and other infectious diseases in backyard chickens at markets in Eastern Shewa zone, Ethiopia. Poult Sci. 2012;91(4):862–869. doi:10.3382/ps.2011-01906
18. Central statistical agency (CSA). Agricultural sample survey 2016/2017 (2009 E.C). volume I report on area and production of major crops (private peasant holdings, Meher season). Addis Ababa; 2017:23.
19. ADARDO (Ada’a District Agricultural and Rural Development Office). Annual report. Bishoftu, Ethiopia; 2007.
20. Thrustfield M. Veterinary Epidemiology. A Comprehensive Introduction to the Role of Epidemiology in Veterinary Medicine. John Wiley & Sons; 2018.
21. Alcorn M. How to Carry Out Filed Investigation: In Poultry Disease.
22. Adebiyi O, Ferdinand C, Kufre J, Okop A. A modified delphi study towards developing a guideline to inform policy on fetal alcohol spectrum disorders in South Africa: a study protocol. BMJ. 2018;8:1–12.
23. Sarkar S, Rahman M, Rahman M, Amin K, Khan M, Rahman M. Sero-prevalence of Mycoplasma gallisepticum infection of chickens in model breeder poultry farms of Bangladesh. Int J Poult Sci. 2005;4(1):32–35.
24. Baksi S, Savaliya BF, Trivedi B, Rao N. Seroprevalence of Mycoplasma gallisepticumin different parts of India. Indian J Comp Microbiol Immunol Infect Dis. 2016;37(2):63–66. doi:10.5958/0974-0147.2016.00012.X
25. Pradhan M, Amin M, Taimur MJ. A sero-prevalence study of avian Mycoplasma in Bangladesh.
26. Talha A. Investigation on the prevalence and significance of M. gallisepticum in village chickens and the possibility of establishing M. gallisepticum free flocks and significance of M. gallisepticum on different production parameters in layer chickens in Bangladesh [Doctoral dissertation, MSc Thesis]. Mymensingh: The Royal Veterinary, and Agricultural University, Denmark and Bangladesh Agricultural University; 2003.
27. Heleili N, Mamache B, Chelihi A. Incidence of avian Mycoplasmosis in the region of Batna, Eastern Algeria. Vet World. 2011;4(3):101–105. doi:10.5455/vetworld.2011.101-105
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.