Back to Journals » OncoTargets and Therapy » Volume 15
Sequential Afatinib and Osimertinib in Asian Patients with EGFR Mutation-Positive Non-Small Cell Lung Cancer and Acquired T790M: Combined Analysis of Two Global Non-Interventional Studies
Authors Miura S, Jung HA, Lee SY, Lee SH , Lee MK, Lee YC, Hochmair MJ, Yang CT , Märten A, Yang JCH , Popat S
Received 18 March 2022
Accepted for publication 12 July 2022
Published 22 August 2022 Volume 2022:15 Pages 873—882
DOI https://doi.org/10.2147/OTT.S362535
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr William C. Cho
Satoru Miura,1 Hyun Ae Jung,2 Shin Yup Lee,3 Seung Hyeun Lee,4 Min Ki Lee,5 Yong Chul Lee,6 Maximilian J Hochmair,7 Cheng-Ta Yang,8 Angela Märten,9 James Chih-Hsin Yang,10 Sanjay Popat11,12
1Department of Internal Medicine, Niigata Cancer Center Hospital, Niigata, Japan; 2Division of Hematology-Oncology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, South Korea; 3Department of Internal Medicine, School of Medicine, Kyungpook National University, Daegu, South Korea; 4Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Kyung Hee University Medical Center, Kyung Hee University School of Medicine, Seoul, South Korea; 5Department of Internal Medicine, Pusan National University School of Medicine, Busan, South Korea; 6Department of Internal Medicine, Research Center for Pulmonary Disorders, Jeonbuk National University Medical School, Jeonju, South Korea; 7Department of Respiratory & Critical Care Medicine, Karl Landsteiner Institute of Lung Research & Pulmonary Oncology, Vienna, Austria; 8Department of Thoracic Medicine, Chang Gung Memorial Hospital, Taoyuan, Taiwan; 9Boehringer Ingelheim International GmbH, Ingelheim am Rhein, Germany; 10Department of Medical Oncology, National Taiwan University Cancer Center and Department of Oncology, National Taiwan University Hospital, Taipei, Taiwan; 11Lung Unit, Royal Marsden National Health Service Foundation Trust, London, United Kingdom; 12The Institute of Cancer Research, London, United Kingdom
Correspondence: Sanjay Popat, Lung Unit, Royal Marsden National Health Service Foundation Trust, 203 Fulham Road, Chelsea, London, SW3 6JJ, United Kingdom, Tel +44-20-7808-2132, Email [email protected]
Objective: Two recent non-interventional trials, GioTag and UpSwinG, demonstrated encouraging time-to-treatment failure (TTF) and overall survival (OS) in patients with epidermal growth factor receptor (EGFR) mutation-positive non-small cell lung cancer (NSCLC) (Del19 or L858R) who received sequential afatinib/osimertinib, especially in Asians. Here, we have undertaken a combined analysis of Asian patients from both studies.
Materials and Methods: Existing medical/electronic records were identified for consecutive EGFR-tyrosine kinase inhibitor (TKI)-naïve patients who received first-line afatinib/second-line osimertinib in “real-world” practice (all T790M-positive). Patients with active brain metastases were excluded. The primary objective was TTF. OS was a key secondary objective.
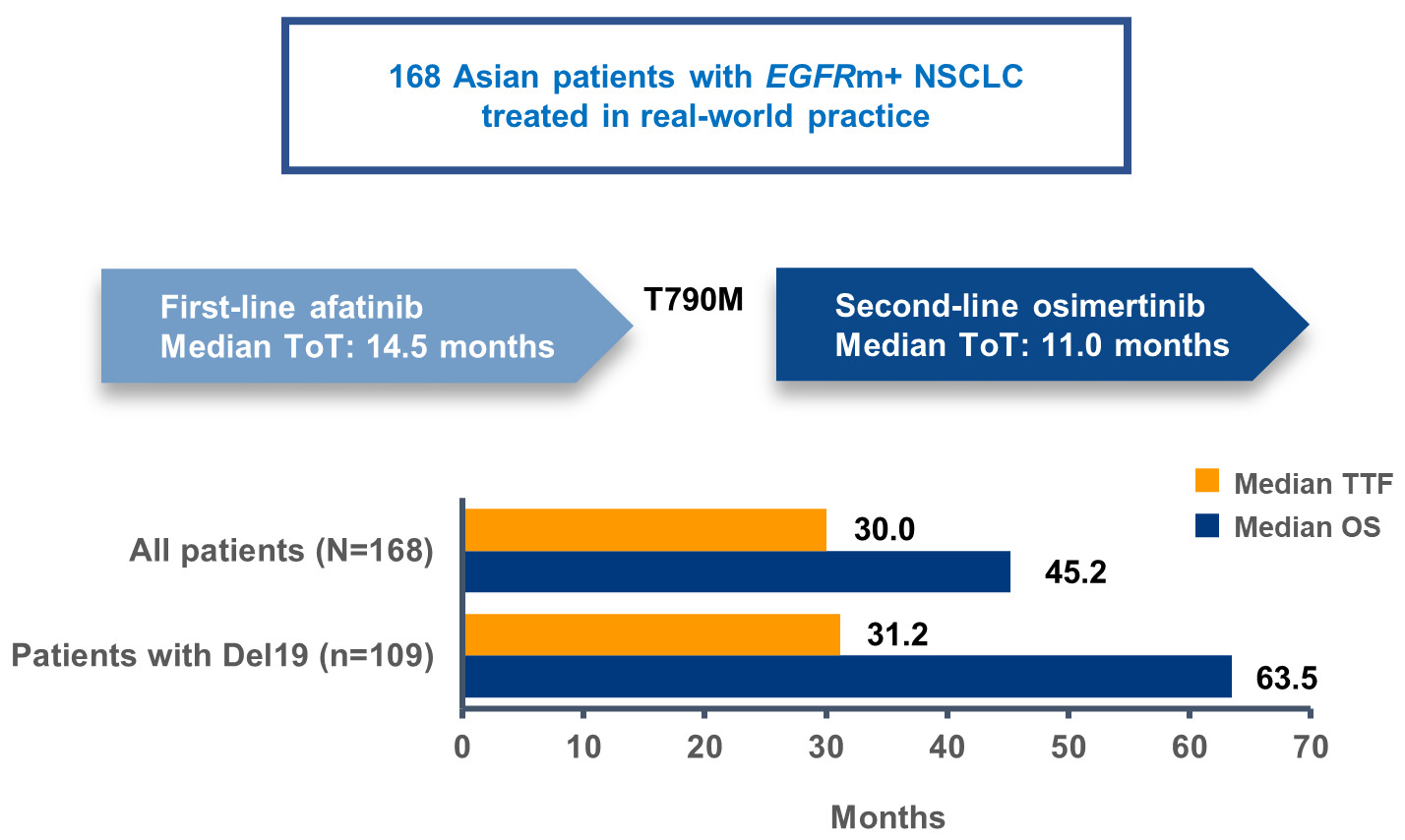
Results: One hundred and sixty-eight patients were analyzed. Most patients were recruited from South Korea or Japan (52/21%). At the start of afatinib, median age (range) was 61.5 years (35– 88), 58% were female, Eastern Cooperative Oncology Group Performance Status (ECOG PS) (0/1/≥ 2) was 29/62/9%, 17% had brain metastases, and EGFR mutation status (Del19/L858R) was 65/35%. At the start of osimertinib, ECOG PS (0/1/≥ 2) was 22/61/17% and 14% had brain metastases. Median TTF and OS were 30.0 months (95% CI: 24.5– 32.5) and 45.2 months (95% CI: 41.7– 71.1), respectively. Median OS was 63.5 months in patients with a Del19 mutation. Median OS in patients with brain metastases or ECOG PS ≥ 2 was 26.4 and 33.1 months, respectively.
Conclusion: Sequential afatinib/osimertinib showed encouraging activity in Asian patients with EGFR mutation-positive NSCLC and T790M-mediated acquired resistance, especially those with Del19-positive disease. Activity was observed across “real-world” patients including those with poor ECOG PS and/or brain metastases. ECOG PS and incidence of brain metastases remained stable prior to, and after, afatinib.
Keywords: EGFR, afatinib, osimertinib, sequential, T790M
Graphical Abstract:
Introduction
Three generations of epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI) are available for the first-line treatment of patients with EGFR mutation-positive non-small cell lung cancer (NSCLC): the first-generation reversible EGFR TKIs (erlotinib and gefitinib), the second-generation ErbB family blockers (afatinib and dacomitinib) and the third-generation irreversible EGFR TKI, osimertinib.1 The head-to-head randomized trials, LUX-Lung 7,2 ARCHER-1050,3 and FLAURA4 have shown that afatinib, dacomitinib and osimertinib confer significant clinical benefit versus first-generation EGFR TKIs. However, second- and third-generation EGFR TKIs have never been directly compared in prospective trials. Accordingly, optimal treatment in individual patients is still open to debate. This is particularly the case in Asian patients, as no second- or third-generation agent has shown a survival advantage over first-generation agents in this setting, probably reflecting the wide implementation of subsequent treatment options beyond acquired resistance.5
As the emergence of the gatekeeper EGFR mutation, T790M, is the predominant mechanism of resistance to afatinib, occurring in up to 50‒70% of cases6,7 (~75% in patients with Del19-positive disease),8 many patients could potentially go on to receive subsequent osimertinib (which is active against T790M)9 after afatinib. Several recent non-interventional trials have assessed clinical outcomes in EGFR-TKI-naïve NSCLC patients treated with sequential afatinib and osimertinib in real-world clinical practice. The global GioTag study (NCT03370770) analyzed 203 such patients and reported median time-to-treatment failure (TTF) and overall survival (OS) of 27.7 months and 37.6 months, respectively. For Asian patients, median TTF was 37.1 months and median OS was 44.8 months.10 Likewise, the global UpSwinG study (NCT04179890) analyzed 193 patients and reported median TTF and OS of 27.7 months and 36.5 months, respectively (28.8 and 42.3 months in Asians).11 In a real-world study of 126 patients treated in South Korea, median TTF was 35.4 months. Median OS was not reached; 2- and 3-year survival rates were 86% and 69%, respectively.12 In another recent real-world study in Japan, sequential afatinib and osimertinib conferred median OS of over 5 years, albeit on a small dataset.13 Together, these findings suggest that sequential afatinib and osimertinib could be a particularly promising regimen in Asian patients with EGFR mutation-positive NSCLC.
Here, in order to increase cohort size and facilitate subgroup analysis, we have undertaken a combined analysis of Asian patients from GioTag and UpSwinG.
Materials and Methods
Study Design
Both GioTag and UpSwinG were non-interventional, global, multicenter studies. GioTag was conducted across ten countries (Austria, Canada, Israel, Italy, Japan, Singapore, Slovenia, Spain, Taiwan, United States of America). UpSwinG was conducted across nine countries (Austria, France, Germany, Italy, Japan, South Korea, Spain, Taiwan, United Kingdom). Medical and electronic health records of consecutive patients treated in a real-world practice were retrospectively reviewed between November 2019 and July 2020 (UpSwinG) and December 2017 to December 2019 (GioTag). The studies were mutually exclusive.
Patients were aged ≥18 years with EGFR mutation-positive (Del19 or L858R), TKI-naïve, advanced NSCLC treated with first-line afatinib and, following detection of T790M, second-line osimertinib. Patients who received any drugs other than afatinib as a first-line treatment and patients with active brain metastases or treated as part of a clinical trial were excluded (treatment with osimertinib within an expanded access/compassionate use program was permitted). EGFR mutation detection (activating mutations and T790M) was undertaken locally using different methodologies as per standard care. In both studies, patients must have started osimertinib treatment at least 10 months prior to data entry but did not need to still be on treatment. A maximum of 15 consecutive patients were enrolled per site.
Both studies were undertaken in compliance with the principles laid down in the Declaration of Helsinki, in accordance with the International Conference on Harmonisation (ICH) Harmonized Tripartite Guideline for Good Clinical Practice, Good Epidemiological Practice and Good Pharmacoepidemiology Practice, and relevant local regulations. Informed and privacy consent signatures were obtained depending on local regulations.
Outcomes and Assessments
In both studies, the primary outcome was TTF, defined as the time from the first dose of afatinib to the last dose of osimertinib, or death by any cause. Secondary objectives included OS and time on treatment with first- and second-line treatment.
Statistical Analyses
TTF and OS were estimated using the Kaplan–Meier method. Medians and two-sided 95% confidence intervals (CIs) were calculated using Greenwood’s variance estimate. In both studies, the following subgroup analyses were prespecified: EGFR mutation type (Del19 or L858R); Eastern Cooperative Oncology Group performance status (ECOG PS); presence or absence of brain metastasis, and starting dose of afatinib. Subgroup analyses were limited to descriptive statistics.
Results
Patients
Fifty of 203 patients (24.6%) from GioTag and 118 of 191 patients (61.8%) from UpSwinG were Asian. All of these patients (n = 168) were analyzed in this study. Most patients were treated in South Korea (n = 88; 52.3%) or Japan (n = 35; 20.8%). The remainder of patients were treated in the USA (n = 19; 11.3%), Taiwan (n = 17; 10.1%), UK (n = 4; 2.4%), Italy (n = 2; 1.2%), Canada, Singapore, and Austria (all n = 1). Baseline characteristics were generally similar across the two studies (Table 1). Overall, the median age at the start of afatinib treatment was 61.5 years (range 35–88), 58% were female and most had a Del19 EGFR mutation (65%). The patient cohort reflected real-world clinical practice with 16.7% having brain metastases and 9.0% of those with known ECOG PS having a score of ≥2. Notably, the incidence of brain metastases, and ECOG PS were largely stable prior to, and following afatinib treatment (Table 1).
 |
Table 1 Baseline Characteristics |
Clinical Outcomes
Overall, median TTF was 30.0 months (95% CI: 24.5–32.5; Figure 1A, Table 2). Median time on afatinib was 14.5 months (95% CI: 13.3–16.7) and median time on osimertinib was 11.1 months (95% CI: 9.3–14.6). TTF was largely consistent across patient subgroups (Table 2). Median TTF was 31.2 months (95% CI: 25.8–38.2; Figure 2A) in patients with a Del19 EGFR mutation (n = 109) and 25.1 months (95% CI: 20.5–30.4; Figure 2A) in patients with the L858R EGFR mutation (n = 59).
 |
Table 2 TTF and OS in Patient Subgroups |
 |
Figure 1 TTF (A) and OS (B) in Asian patients receiving sequential Afatinib and osimertinib (n = 168). Abbreviations: CI, confidence interval; OS, overall survival; TTF, time-to-treatment failure. |
Median OS was 45.2 months (95% CI: 41.7–71.1; Figure 1B, Table 2). OS rates at 24, 30, and 36 months were 81.4%, 70.4%, and 62.8%, respectively. OS was also generally consistent across subgroups. Median OS was 63.5 months (95% CI: 42.3–71.1) and 39.1 months (95% CI: 29.3–48.5), respectively, in patients with a Del19 or the L858R EGFR mutation (Figure 2B, Table 2). In patients with brain metastases (n = 28) or ECOG PS ≥2 (n = 15) median OS was 26.4 months (95% CI: 19.1–29.6) and 33.1 months (95% CI: 17.1–NR), respectively (Table 2). In patients with a Del19 EGFR mutation treated in Asian countries (n = 93) median OS was 63.5 months (95% CI: 41.7–71.1).
Outcomes were also analyzed in Asian patients who received the approved starting dose of afatinib of 40 mg/day. TTF and OS in the overall Asian dataset (n = 147) and in patient subgroups are shown in Supplementary Table 1 and Supplementary Figures 1 and 2.
Discussion
Previous observational studies in a real-world clinical setting have demonstrated promising outcomes in patients with EGFR mutation-positive NSCLC treated with sequential afatinib and osimertinib.7,10,12,14–16 Outcomes with this sequential approach appeared to be particularly encouraging in Asian patients.10,11 This combined study of UpSwinG and GioTag facilitated a detailed analysis of 168 Asian patients treated with sequential afatinib and osimertinib. In these patients, median TTF was 2.5 years and median OS was nearly four years; the three-year survival rate was 63%. Almost two thirds of patients had a Del19 EGFR mutation. In these patients, median OS was over 5 years. Moreover, OS was encouraging even in patients with poor prognostic features, such as poor ECOG PS and presence of brain metastases, although patient numbers were small. Another interesting observation was that ECOG PS and the incidence of brain metastases were largely stable prior to, and after, treatment with afatinib, although patients with active brain metastases were excluded from the study. These observations imply that overall patient fitness might not be negatively impacted by treatment with afatinib prior to osimertinib and there was no evidence of significant central nervous system (CNS) progression, consistent with afatinib’s CNS activity.17 Notwithstanding the inherent limitations of retrospective observational studies, and the potential for selection bias, these are interesting findings and suggest that sequential afatinib and osimertinib could be considered in Asian patients, especially in those with a Del19 EGFR mutation. These patients demonstrated particularly favorable outcomes and are known to have high rates of T790M-mediated acquired resistance to first- and second-generation EGFR TKIs.8,18 Therefore, a high proportion of patients, perhaps up to 75%,8 with a Del19 mutation could be eligible to receive subsequent osimertinib, which has demonstrated strong activity in a second-line setting in Asian patients.9
As well as being a second-line option in patients with T790M-positive disease, osimertinib is a first-line treatment option in patients with EGFR mutation-positive NSCLC. In the global Phase III FLAURA trial, first-line osimertinib significantly improved both progression-free survival (PFS) and OS versus gefitinib/erlotinib in this setting,4,19 with a favorable tolerability profile reflecting its wild-type EGFR sparing mechanism of action. Notably, however, in an Asian subanalysis of FLAURA, there was no evidence of OS benefit with osimertinib over gefitinib/erlotinib (median OS 37.1 months vs 35.8 months; hazard ratio (HR) 1.0; 95% CI: 0.75–1.32).5 Indeed, in Japanese patients, there was a trend towards lower OS with osimertinib than with gefitinib/erlotinib (median 39.3 months vs not reached; HR 1.39; 95% CI: 0.83–2.34).5 These observations could reflect high rates of subsequent therapy in Asian countries, especially those, like Korea and Japan, with advanced healthcare systems.5 Such countries are associated with high rates of tumor rebiopsy and/or liquid biopsies at the point of acquired resistance, thus facilitating routine testing for T790M.20,21 While treatment with sequential afatinib and osimertinib is dependent on the availability of T790M testing, there may be other factors that underlie apparent favorable outcomes in Asian patients treated with sequential afatinib and osimertinib. In both GioTag (44.8 versus 36.7 months)10 and UpSwing (42.3 and 31.3 months)11 median OS was higher in Asian patients than non-Asian patients, indicating that ethnic factors could contribute to efficacy. In addition to socioeconomic factors and differences in healthcare systems, background biological/genetic factors in Asians might explain this phenomenon.5
In the absence of prospective data, it is unclear whether sequential afatinib and osimertinib could elicit an OS advantage compared with first-line osimertinib. Currently, despite intensive clinical development of treatment possibilities including fourth-generation EGFR TKIs and MET, HER2, JAK1, AXL and BRAF V600E inhibitors,22,23 no targeted treatment options are approved for use post-osimertinib yet. This reflects the observation that resistance mechanisms to osimertinib are highly heterogeneous and encompass both EGFR-dependent and EGFR-independent mechanisms.22,23 Accordingly, at the moment, most patients who receive subsequent therapy after osimertinib are given platinum-doublet chemotherapy.24 Therefore, sequential afatinib and osimertinib in patients with T790M-positive disease could potentially help maximize net time on targeted treatment and delay chemotherapy compared with first-line osimertinib. On the other hand, some patients treated with first-line afatinib may never have the opportunity to benefit from osimertinib if they develop T790M-negative acquired resistance and other treatment options would have to be considered in this scenario.25 For this reason, it would be extremely useful to be able to predict patients who would be most likely to develop T790M-mediated resistance to first-line therapy. In addition to the presence of an EGFR Del19 mutation, other emerging patient- and disease-related factors seem to have the potential as T790M predictive biomarkers including age, white blood cell count, liver metastases, lactate dehydrogenase and cytokeratin 19 fragment levels.26 Further research and validation of these biomarkers are required. Ultimately, head-to-head trials are required to compare sequential EGFR TKI regimens, with OS as the key endpoint. A randomized Phase II trial that compares initial treatment with afatinib versus osimertinib in patients with EGFR mutation-positive NSCLC is currently ongoing in Japan.27
This study has several limitations. As a retrospective study, it was prone to selection bias. For example, the study was restricted to sites that had access to osimertinib at the time, which probably excluded some patients, especially those treated in smaller regional centers. Furthermore, many patients treated with first-line afatinib could not be considered for inclusion in the study, such as those who developed T790M-independent acquired resistance, those who died during afatinib treatment, those who were unfit or unwilling to receive second-line treatment and those with active brain metastases. Conversely, patients with long-term benefits from afatinib had a lower likelihood of being included in the study. Another group of patients who could not be included in the study were those who had progression in tissues that were not amenable to testing for T790M, eg, the brain. Ultimately, therefore, the study is not generalizable to all patients treated with first-line afatinib, with the cohort possibly representing a fitter group of patients than generally encountered in real-world practice. It was notable, for example, that only three patients had baseline ECOG PS ≥2. Despite these inherent weaknesses of the study, we tried to limit selection bias by restricting enrollment from individual centers (maximum of 15 patients). Furthermore, in order to avoid early censoring, patients must have initiated osimertinib treatment at least 10 months prior to data entry. Finally, there was no comparator arm in this study. Accordingly, these real-world observations do not substitute for prospective data but do provide important hypothesis-generating observations.
Conclusions
In conclusion, this observational study suggests that withholding osimertinib for second-line use after afatinib could be a possible strategy in patients with EGFR mutation-positive NSCLC with T790M-positive disease. This seems to be particularly the case in Asian patients, given that sequential lines of therapy, and monitoring of the molecular evolution of tumors are commonplace in everyday clinical practice in several Asian countries. Sequential regimens of EGFR TKIs require prospective evaluation in Asian patients to help identify optimal treatment in terms of maximizing survival, tolerability, and quality of life.
Abbreviations
CI, confidence interval; CNS, central nervous system; HR, hazard ratio; ECOG PS, Eastern Cooperative Oncology Group performance status; EGFR, epidermal growth factor receptor; OS, overall survival; NR, not reached; NSCLC, non-small cell lung cancer; PFS, progression-free survival; TKI, tyrosine kinase inhibitor; TTF, time-to-treatment failure.
Data Sharing Statement
The datasets generated and analyzed during the study are available from AM on reasonable request.
Ethics Approval and Informed Consent
Both constituent studies were undertaken in compliance with the principles laid down in the Declaration of Helsinki, in accordance with the International Conference on Harmonisation (ICH) Harmonized Tripartite Guideline for Good Clinical Practice, Good Epidemiological Practice and Good Pharmacoepidemiology Practice, relevant local regulations and relevant sponsor Standard Operating Procedures. The studies were initiated only after all required legal documentation was reviewed and approved by the respective Institutional Review Board/Independent Ethics Committee and competent authority according to national and international regulations. Ethical approval of the UpSwinG and GioTag study protocols (for principal investigators) was provided by the UK National Health Service Health Research Authority (submission ID, 1-4V2NYEY; approval on 11 December 2019) and Ethics Committee of the City of Vienna, respectively. All other ethics committees that approved the constituent studies are listed in Supplementary Table 2. Informed and privacy consent signatures were obtained depending on local regulations.
Acknowledgments
Medical writing assistance, supported financially by Boehringer Ingelheim, was provided by Lynn Pritchard of Ashfield MedComms, an Inizio Company, during the preparation of this article. SP reports that this study represents independent research supported by the National Institute for Health Research (NIHR) Biomedical Research Centre at The Royal Marsden NHS Foundation Trust and the Institute of Cancer Research, London and that the views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was funded by Boehringer Ingelheim. The sponsor played a role in the collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication and, as such, are included in the author list. BI was given the opportunity to review the manuscript for medical and scientific accuracy as well as intellectual property considerations. The authors received no direct compensation related to the development of the manuscript and met criteria for authorship as recommended by the International Committee of Medical Journal Editors (ICMJE).
Disclosure
SM reports receiving honoraria from Chugai Pharma, AstraZeneca, Eli Lilly, Merck Sharp & Dohme, Boehringer Ingelheim, Bristol Myers Squibb, Taiho Pharma, and Pfizer. HAJ, SYL, SHL, MKL, and YCL declare no potential conflict of interest. MJH reports receiving consulting fees from Boehringer Ingelheim, Merck Sharp & Dohme, Pfizer, Novartis, Roche, and honoraria from AstraZeneca, Bristol Myers Squibb, Boehringer Ingelheim, Merck Sharp & Dohme, Pfizer, Roche. C-TY declares no potential conflict of interest. AM declares employment with Boehringer Ingelheim. JC-HY reports receiving personal and/or institutional fees from Amgen; personal and institutional fees from AstraZeneca, Boehringer Ingelheim, Novartis, Roche/Genentech, Takeda Oncology, Yuhan Pharmaceuticals; institutional fees from Bayer, Daiichi Sankyo, Eli Lilly, Merck KGaA (Darmstadt, Germany), Merck Sharp & Dohme, Johnson & Johnson; personal fees from Bristol Myers Squibb, Ono Pharmaceuticals, Pfizer; grants from AstraZeneca. SP reports receiving grant support, honoraria, consulting fees, and travel support from Boehringer Ingelheim; consulting fees and travel support from Bristol Myers Squibb; honoraria and consulting fees from Roche, Takeda, AstraZeneca; honoraria from Chugai Pharma; consulting fees from Novartis, Guardant Health, AbbVie, Pfizer; consulting fees and travel support from Merck Sharp & Dohme.
References
1. Shah R, Lester JF. Tyrosine kinase inhibitors for the treatment of EGFR mutation-positive non-small-cell lung cancer: a clash of the generations. Clin Lung Cancer. 2020;21(3):e216–e228. doi:10.1016/S1470-2045(16).30033-X
2. Park K, Tan EH, O’Byrne K, et al. Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): a phase 2B, open-label, randomised controlled trial. Lancet Oncol. 2016;17(5):577–589. doi:10.1016/S1470-2045(16).30033-X
3. Wu YL, Cheng Y, Zhou X, et al. Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): a randomised, open-label, Phase 3 trial. Lancet Oncol. 2017;18(11):1454–1466. doi:10.1016/S1470-2045(17).30608.3
4. Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. 2018;378(2):113–125. doi:10.1056/NEJMoa1713137
5. Kim ES, Melosky B, Park K, Yamamoto N, Yang JC. EGFR tyrosine kinase inhibitors for EGFR mutation-positive non-small-cell lung cancer: outcomes in Asian populations. Future Oncol. 2021;17(18):2395–2408. doi:10.2217/fon-2021-0195
6. Yang JC, Ahn MJ, Kim DW, et al. Osimertinib in pretreated T790M-positive advanced non-small-cell lung cancer: AURA study phase II extension component. J Clin Oncol. 2017;35(12):1288–1296. doi:10.1200/jco.2016.70.3223
7. Hochmair MJ, Buder A, Schwab S, et al. Liquid-biopsy-based identification of EGFR T790M mutation-mediated resistance to Afatinib treatment in patients with advanced EGFR mutation-positive NSCLC, and subsequent response to osimertinib. Target Oncol. 2019;14(1):75–83. doi:10.1007/s11523-018-0612-z
8. Jenkins S, Yang JC, Jänne PA, et al. EGFR mutation analysis for prospective patient selection in two phase II registration studies of osimertinib. J Thorac Oncol. 2017;12(8):1247–1256. doi:10.1016/j.jtho.2017.05.002
9. Mok TS, Wu YL, Ahn MJ, et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med. 2017;376(7):629–640. doi:10.1056/NEJMoa1612674
10. Hochmair MJ, Morabito A, Hao D, et al. Sequential afatinib and osimertinib in patients with EGFR mutation-positive non-small-cell lung cancer: final analysis of the GioTag study. Future Oncol. 2020;16(34):2799–2808. doi:10.2217/fon-2020-0740
11. Popat S, Jung HA, Lee SY, et al. Sequential Afatinib and osimertinib in patients with EGFR mutation-positive NSCLC and acquired T790M: a global non-interventional study (UpSwinG). Lung Cancer. 2021;162:9–15. doi:10.1016/j.lungcan.2021.09.009
12. Kim T, Jang TW, Choi CM, et al. Sequential treatment of Afatinib and osimertinib or other regimens in patients with advanced non-small-cell lung cancer harboring EGFR mutations: results from a real-world study in South Korea. Cancer Med. 2021;10(17):5809–5822. doi:10.1002/cam4.4127
13. Mitsuya S, Tsuruoka K, Kanaoka K, et al. Comparison between second- and third-generation epidermal growth factor receptor tyrosine kinase inhibitors as first-line treatment in patients with non-small-cell lung cancer: a retrospective analysis. Anticancer Res. 2021;41(10):5137–5145. doi:10.21873/anticanres.15331
14. Popat S, Jung HA, Lee SY, et al. 1223P - Sequential afatinib (afa) and osimertinib (osi) in patients (pts) with advanced EGFR mutation-positive (EGFRm+) NSCLC who acquire the T790M resistance mutation: a non-interventional cohort study (UpSwinG). Ann Oncol. 2021;32(Suppl5):S949–S1039. doi:10.1016/j.annonc.2021.08.1828
15. Park K, Bennouna J, Boyer M, et al. Sequencing of therapy following first-line Afatinib in patients with EGFR mutation-positive non-small cell lung cancer. Lung Cancer. 2019;132:126–131. doi:10.1016/j.lungcan.2019.04.014
16. Tamiya M, Tamiya A, Suzuki H, et al. Which is better EGFR-TKI followed by osimertinib: afatinib or gefitinib/erlotinib? Anticancer Res. 2019;39(7):3923–3929. doi:10.21873/anticanres.13544
17. Hochmair M. Medical treatment options for patients with epidermal growth factor receptor mutation-positive non-small cell lung cancer suffering from brain metastases and/or leptomeningeal disease. Target Oncol. 2018;13(3):269–285. doi:10.1007/s11523-018-0566-1
18. Ke EE, Zhou Q, Zhang QY, et al. A higher proportion of the EGFR T790M mutation may contribute to the better survival of patients with exon 19 deletions compared with those with L858R. J Thorac Oncol. 2017;12(9):1368–1375. doi:10.1016/j.jtho.2017.05.018
19. Ramalingam SS, Vansteenkiste J, Planchard D, et al. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med. 2020;382(1):41–50. doi:10.1056/NEJMoa1913662
20. Lee K, Kim Y, Jung HA, et al. Repeat biopsy procedures and T790M rates after Afatinib, gefitinib, or erlotinib therapy in patients with lung cancer. Lung Cancer. 2019;130:87–92. doi:10.1016/j.lungcan.2019.01.012
21. Seto T, Nogami N, Yamamoto N, et al. Real-world EGFR T790M testing in advanced non-small-cell lung cancer: a prospective observational study in Japan. Oncol Ther. 2018;6(2):203–215. doi:10.1007/s40487-018-0064-8
22. Schmid S, Li JJN, Leighl NB. Mechanisms of osimertinib resistance and emerging treatment options. Lung Cancer. 2020;147:123–129. doi:10.1016/j.lungcan.2020.07.014
23. Wu L, Ke L, Zhang Z, Yu J, Meng X. Development of EGFR TKIs and options to manage resistance of third-generation EGFR TKI osimertinib: conventional ways and immune checkpoint inhibitors. Front Oncol. 2020;10:602762. doi:10.3389/fonc.2020.602762
24. Planchard D, Boyer MJ, Lee JS, et al. Postprogression outcomes for osimertinib versus standard-of-care EGFR-TKI in patients with previously untreated EGFR-mutated advanced non-small cell lung cancer. Clin Cancer Res. 2019;25(7):2058–2063. doi:10.1158/1078-0432.Ccr-18-3325
25. Liao BC, Griesing S, Yang JC. Second-line treatment of EGFR T790M-negative non-small cell lung cancer patients. Ther Adv Med Oncol. 2019;11:1758835919890286. doi:10.1177/1758835919890286
26. Tamiya M, Fujikawa K, Suzuki H, et al. Classification and regression tree for estimating predictive markers to detect T790M mutations after acquired resistance to first line EGFR-TKI: HOPE-002. Invest New Drugs. 2022;40(2):361–369. doi:10.1007/s10637-021-01203-5
27. Morikawa K, Tanaka H, Itani H, et al. Hypothesis generative head-to-head study comparing efficacy of Afatinib and osimertinib based on immunological biomarkers in Japanese NSCLC patients with EGFR mutations (Heat on Beat study). Ther Adv Med Oncol. 2020;12:1758835920967254. doi:10.1177/1758835920967254
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

