Back to Journals » Open Access Rheumatology: Research and Reviews » Volume 14
Salivary Gland Ultrasound in Primary Sjögren’s Syndrome: Current and Future Perspectives
Authors Lorenzon M, Spina E, Tulipano Di Franco F, Giovannini I , De Vita S, Zabotti A
Received 17 May 2022
Accepted for publication 24 August 2022
Published 1 September 2022 Volume 2022:14 Pages 147—160
DOI https://doi.org/10.2147/OARRR.S284763
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Chuan-Ju Liu
Michele Lorenzon,1 Erica Spina,1 Francesco Tulipano Di Franco,1 Ivan Giovannini,2 Salvatore De Vita,2 Alen Zabotti2
1Institute of Radiology, Department of Medicine, University of Udine, Udine, Italy; 2Rheumatology Clinic, Department of Medical and Biological Sciences, Azienda Sanitaria Universitaria Friuli Centrale c/o University of Udine, Udine, Italy
Correspondence: Michele Lorenzon, Institute of Radiology, Department of Medicine, University of Udine, Udine, Italy, Tel +39 0432 559232, Email [email protected]
Abstract: Salivary gland ultrasound (SGUS) is the imaging modality of choice for the assessment of parotid and submandibular gland parenchyma. Being highly effective, non-invasive and easy to perform, SGUS has become increasingly popular among specialists in assessing salivary gland (SG) abnormalities, including those commonly found in primary Sjögren’s syndrome (pSS). SGUS may be useful in the assessment of pSS and its complications, the most serious being the development of non-Hodgkin’s lymphoma (NHL). SGUS may also be useful in the characterization and differential diagnosis of diffuse and focal abnormalities commonly associated with pSS, and may act as a guide for core-needle biopsy (CNB), an established, safe, and feasible technique, which provides enough viable tissue for the diagnosis and assessment of lymphoproliferative diseases of the SG. The combination of SGUS with other tools, such as sonoelastography and artificial intelligence (AI), could further improve the usefulness of SGUS in the management of pSS. In this perspective, we summarize current and future applications of SGUS in pSS.
Keywords: primary Sjögren’s syndrome, major salivary glands, ultrasound, lymphoma, core needle biopsy
Key Points
- Ultrasound (US) is the imaging modality of choice to assess salivary gland (SG) parenchyma in patients with primary Sjögren’s syndrome (pSS). The Outcome Measures in Rheumatology Clinical Trials (OMERACT) working group has standardized SGUS in pSS with a 0−3 score.
- SGs can be involved in several diseases, with US features similar to those seen in pSS.
- In patients with pSS, newly diagnosed focal lesions should raise the suspicion of non-Hodgkin’s lymphoma (NHL).
- Lymphoproliferative disease is the most serious complication of pSS. SG lymphoma can be revealed by SGUS with either a diffuse or focal appearance.
- US-guided CNB of the major SGs is an established, safe and feasible technique that provides sufficient viable tissue for the diagnosis and assessment of lymphoproliferative diseases.
Introduction
Primary Sjögren’s syndrome (pSS) is a chronic, systemic, autoimmune disease, characterized by lacrimal and salivary gland (SG) involvement, with consequent keratoconjunctivitis sicca and xerostomia and heterogeneous extra-glandular involvement1,2 The pathogenesis of pSS is not completely understood. Immune-mediated destruction of exocrine glands, hyperactivation of lymphocytes, and activation of inflammatory cells expose pSS patients to an increased risk of lymphoproliferative diseases, reported as the highest among autoimmune diseases.3
Today, the diagnosis of pSS relies on a combination of clinical, serological, histological, functional, and instrumental parameters aimed at detecting systemic, SG, and lacrimal gland changes.4 Although the most recent classification criteria for pSS do not mention imaging modalities,5 since the publication of a study by De Vita in 1992, salivary gland ultrasound (SGUS) has been increasingly applied to evaluate parotid and submandibular gland structural abnormalities and parenchymal lesions.6–8 Other imaging modalities, such as sialography and scintigraphy, were used in the past to assess SG involvement,9,10 but these are nowadays considered obsolete.11
SGUS is a non-invasive, easy to perform and inexpensive method to assess in detail superficial anatomical structures, including the parotid and submandibular glands. Thus, it is the imaging modality of choice today when evaluating major SGs in patients with pSS.12 Several studies have reported that the inclusion of SGUS in pSS classification criteria may improve their diagnostic accuracy, feasibility, and sensitivity.13–15 In recent years, in addition to its diagnostic role, new applications for SGUS have emerged, such as disease activity monitoring and pSS-related lymphoproliferative diseases assessment.16,17 Furthermore, SGUS may be useful as a guide in biopsies to ensure adequate histological sampling in pSS patients with suspected lymphoma, opening new frontiers in clinical research.18–20 Aim of our review is to outline the state of the art and future perspectives of SGUS in pSS.
pSS Diagnosis
Key Points
- Ultrasound (US) is the imaging modality of choice to assess SG parenchyma in patients with pSS.
- The Outcome Measures in Rheumatology Clinical Trials (OMERACT) working group has standardized SGUS in pSS to validate the use of SGUS as a possible outcome measurement instrument. According to its system, a score of 0 denotes normal salivary glands, and a score of 3 denotes extensive inhomogeneity with hypo/anechoic areas occupying the entire glandular surface. A higher score increases the likelihood of a diagnosis of pSS.
The diagnosis of pSS is based on a set of clinical, laboratory, imaging, and pathological features.21 In recent decades, key features of SGUS have made it the imaging modality of choice for SG assessment over sialography, magnetic resonance imaging and salivary scintigraphy.12 These include good reliability and high spatial and contrast resolution due to the superficial position of the SGs.12 Furthermore, SGUS is non-invasive, nonionizing, inexpensive, and widely available.22,23
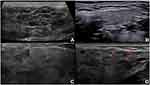
SGUS can detect parenchymal abnormalities of the major SGs.24–27 Distinctive sonographic features of the parotid and submandibular glands in pSS were highlighted almost 30 years ago and consisted mainly of glandular heterogeneity with hypo/anechoic areas and hyperechoic bands (Figure 1A).28 Since then, other SGUS abnormalities, such as glandular calcifications, fatty infiltration, and a visible posterior border, have been reported and scored.24,29 More recently, SGUS has been shown to be a valuable tool in the assessment of pSS, and some authors have suggested that it could be a minor classification criterion in patients with ocular or oral dryness30 or with a suspicion of pSS due to systemic features derived from the EULAR Sjögren’s syndrome disease activity index (ESSDAI), with remarkable sensitivity and specificity (97.3% and 90.2%, respectively).13 The scoring system of the American College of Rheumatology – European League Against Rheumatism (ACR-EULAR), together with SGUS, showed improved accuracy and the potential to replace the ocular staining score, Schirmer’s test, or unstimulated whole saliva flow in pSS classification.13 In addition, SGUS seems to improve the sensitivity, even in patients with systemic disease and mild or no sicca symptoms.14
The increasing application of SGUS in the assessment of pSS requires standardization of imaging modalities. The OMERACT working group recently established a novel, semiquantitative scoring system (0–3) for glandular alterations in pSS.29 The OMERACT scoring system is based on the presence and distribution of hypoechoic areas within SGs, which are the main sonographic features characterizing pSS.24 The hypoechoic areas must be small (some millimeters), not compressible by the probe, generate few or no echoes, without blood flow at color-Doppler imaging.28 These hypoechoic areas can be located anywhere within the SGs.29 Based on the OMERACT scoring system, “normal parenchyma” is classed as score 0, whereas diffuse changes and extensive inhomogeneity, with hypo/anechoic areas occupying the entire glandular surface is classed as score 3 (Figures 1A–D). Scores 1 and 2 represent mild and moderate SG involvement, respectively.29 A higher score increases the likelihood of a diagnosis of pSS.31 The OMERACT score shows substantial inter-reader agreement and is helpful in identifying pSS patients with an increased risk of non-Hodgkin’s lymphoma (NHL) of the major SGs (see in the “Lymphoma” section).32,33
In clinical practice, a diagnosis of pSS can be made in the absence of fulfilling established classification criteria. In such cases, positive SGUS can add information that can help guide the clinician in the diagnostic process and potentially influence patient management.7,34,35 To sum up, SGUS has potential in the diagnostic work-up in cases of suspected pSS, it is emerging as a valid complementary tool to histopathology and as an alternative tool in cases where a biopsy cannot be performed.36,37
Differential Diagnosis
Diseases with Diffuse Pattern at SGUS
Key Points
- SGs can be involved in several diseases, with US features similar to those seen in pSS.
- When SGUS shows diffuse SG involvement, with inhomogeneity and hypoechogenicity, the differential diagnosis relies mainly on clinical, laboratory, and, if necessary, histological findings.
From a clinical viewpoint, pSS is not the only disease that can lead to xerostomia, xerophthalmia, and SG swelling.38 Thus, the exclusion of other disorders is crucial. Conditions that may cause misdiagnosis of pSS are listed as exclusion criteria in the ACR-EULAR classification criteria. In detail, these are 1) a history of head and neck radiation treatment, 2) active hepatitis C infection, 3) AIDS, 4) sarcoidosis, 5) amyloidosis, 6) graft-versus-host disease, and 7) IgG4-related disease.5 These conditions may exclude a diagnosis of pSS because of overlapping clinical features or interference with criteria tests.39
On US imaging, major SGs with a diffuse, heterogeneous, hypoechoic appearance are typical of pSS.40,41 However, a similar presentation may be seen in rheumatoid arthritis, systemic lupus erythematosus, or systemic sclerosis. In these conditions, autoimmune sialadenitis with sicca syndrome is related to underlying connective tissue disease, and it is defined as secondary SS.28,42 Therefore, in patients with sicca syndrome and diffuse, heterogeneous, hypoechoic SGs on US imaging, the differential diagnosis of primary and secondary SS relies mainly on clinical features.43
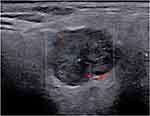
Infiltrative conditions, such as sarcoidosis and amyloidosis, can cause SG enlargement, with a pattern on US similar to that seen in pSS (Figure 2).44 In such cases, SGUS is not particularly helpful in the differential diagnosis, and the diagnosis depends on clinical, laboratory, and possibly histological findings.43
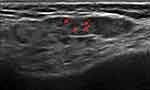 |
Figure 2 Left, nonenlarged submandibular gland with multiple small hypoechoic foci (arrows) in a patient with sicca syndrome. Following a CNB, the diagnosis was sarcoidosis. |
IgG4-related disease is characterized by SG infiltration of IgG4+ lymphocytes that cause bilateral and painless SG swelling. IgG4-related disease shows specific features on SGUS that can aid the differential diagnosis of pSS.45,46 Unlike pSS, IgG4 disease presents with a nodal and/or reticular US pattern and more commonly affect the submandibular gland (Figure 3A and B).43,47 However, clinical and ultrasonographic overlap between IgG4-related disease and pSS makes a histopathological assessment of the SGs mandatory to establish the diagnosis.48,49
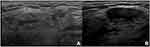 |
Figure 3 Submandibular gland with several confluent hypoechoic areas (arrows) (A) and a large hypoechoic lesion (arrow). (B) Following a CNB, the diagnosis was IgG4 disease. |
ANCA-associated vasculitis has SGUS appearance similar to that observed in IgG4-related disease, but SG swelling is often unilateral and/or painful.50,51 In clinical practice, a biopsy is suggested but not always performed, and the diagnosis is often made based on clinical features, in combination with positive ANCA serology.52 pSS-like manifestations may also be found in patients following allogeneic hematopoietic stem cell transplantation due to a major immune attack against patients’ own tissues, including SGs, leading to graft versus host disease.53 In addition, they may be seen in in oncological patients exposed to radiation therapy of the head and neck.54 In these cases, the diagnosis is based on the patient’s clinical history.55
Factors other than an immune response may account for parenchymal abnormalities in SGs. For example, lithiasis and infections can induce acute or chronic inflammation, with inhomogeneous hypoechoic changes.56 Glandular volume and blood flow on color Doppler imaging distinguish acute versus chronic inflammation on SGUS, both of which are increased in the presence of acute inflammation and normal/reduced in cases of chronic inflammation.57 In terms of lithiasis, associated SGUS findings consist of the stone and the dilated duct. In cases of viral or bacterial infections, multiple, small, oval, hypoechoic lesions may be distributed throughout the glandular parenchyma of the SGs.58 HCV and HIV infections may be additional causes of chronic salivary inflammation.59 In these cases, serological tests may aid the differential diagnosis.60 Finally, chronic nontender bilateral SG swelling may be associated with alcohol consumption, diabetes mellitus, and malnutrition.61–64
Diseases with Focal Appearance at SGUS
Key Points
- In patients with pSS, particularly those with OMERACT scores of 2 or 3 for major SGs, newly diagnosed focal lesions should raise a suspicion of NHL.
- Other benign and malignant focal lesions typically found in the general population should be considered in the differential diagnosis.
Lymphoproliferative diseases are a serious complication of pSS,65 with the most common histotype being NHL of mucosa-associated lymphoid tissue.66 In pSS patients with inhomogeneous glandular patterns, especially those with higher OMERACT scores (2 and 3), the presence of a newly diagnosed focal lesion is suggestive of lymphoma.33 However, in this clinical scenario, other focal benign and malignant lesions typically found in the general population must first be ruled out (Figure 4).67,68 In the general population, focal lesions in SGs are rare. When present, they are often (70−80%) benign and usually (80−90%) located in the parotid glands.58 Focal lesions are less common (10−12%) in submandibular glands;69 however, when present, 50% of these lesions are malignant.69 Among benign focal lesions, the most common are pleomorphic adenomas and Warthin tumors.70 Pleomorphic adenomas are the most frequent (45−75%) of all SG tumors.71 A pleomorphic adenoma is a benign lesion but presents a high risk of local recurrence. On SGUS, the typical appearance of a pleomorphic adenoma is a hypoechoic, often inhomogeneous, lobulated lesion, well defined and with posterior acoustic enhancement, without evidence of internal vascularization on color Doppler imaging (Figure 5A and B).72 Calcifications may be present.73 Warthin tumors are less frequent, accounting for 5−10% of all benign SG lesions.70 On SGUS, the appearance is typically a hypoechoic, oval, well-defined lesion, with anechoic areas inside, hypervascular on color Doppler imaging.74 The tumor may present as a completely cystic lesion. Despite the differences in the typical appearances of these lesions, there is significant overlap among their presentations and with malignant lesions.75
Among malignant SG lesions, mucoepidermoid carcinomas (Figure 6A and B) and adenoid cystic carcinomas are the most frequent.76 Squamous cell carcinomas, acinic cell carcinomas, and adenocarcinomas are less common.77 The imaging features of SG cancer can be variable.78 Well-differentiated neoplasms may be homogeneous and well defined, like benign lesions,79,80 whereas poorly differentiated or advanced malignant neoplasms can appear as hypoechoic, inhomogeneous, irregular lesions, with blurred margins, with or without cystic components.55,78,81
Lymphoma
Key Points
- Lymphoproliferative diseases are the most serious complication in patients with pSS.
- SG swelling is a clinical sign of lymphoma.
- SGUS is a useful tool in the assessment of swollen parotid and submandibular glands in patients with pSS.
- Lymphomas of the SGs can have either a diffuse or focal appearance.
- An OMERACT score of 3 is associated with a higher risk of lymphoma.
- Lymphomas of the SGs with a focal appearance show distinctive features on SGUS.
Lymphomas are the most serious complication of pSS, with a consistent impact on the mortality rate of pSS patients.82,83 Non-Hodgkin’s lymphoma (NHL) of mucosa-associated lymphoid tissue (MALT) is the usual histotype involving the major SGs in these patients.66 The risk of NHL in patients with pSS is estimated to be increased 4 to 40-fold, resulting in the occurrence of NHL in about 5% of patients.84 The latter has been attributed to chronic SG inflammation in pSS.66 SG swelling in patients with pSS is a clinical sign of NHL. SGUS is useful to evaluate echo-structural abnormalities of the parotid and submandibular glands.85,86 In this clinical scenario, SGUS has demonstrated that NHL of the major SGs can have either a diffuse (Figure 7) or focal appearance (Figure 8A and B).
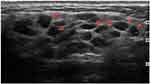 |
Figure 7 Enlarged right parotid gland, with multiple, oval, well-defined very hypoechoic lesions (arrows) in a patient with pSS. A CNB revealed the presence of NHL with diffuse glandular involvement. |
Recent studies have attempted to shed light on which sonographic features are most associated with NHL of the major SGs in pSS.33,87,88 In case of a diffuse appearance, an OMERACT score of 3 is described as the main feature associated with NHL, which is consistent with the hypothesis that NHL is more likely to develop in a more inflamed SG.29,33 However, recent studies highlighted that NHL of major SGs can also present as well-defined focal abnormalities in SGs with no diffuse background lymphomatous infiltration.19,33 It has been hypothesized that such areas may represent foci of actively expanding B-cell clones,33 in keeping with the currently accepted model of lymphomagenesis in pSS.66 Research has also demonstrated that focal NHL exhibits recurrent, distinctive sonographic features and tends to present as a very hypoechoic, oval, well-defined, homogenous lesion, with posterior acoustic enhancement, which frequently contains thin, hyperechoic septa and signs of hypervascularity on SGUS and color Doppler imaging.33 For these reasons, when NHL of major SGs is suspected in pSS, it is highly advisable to prioritize assessing the presence of focal lesions, as this could potentially aid the selection of the best target and guide the resulting biopsy.33
Core Needle Biopsy (CNB)
Key Points
- Generally, in percutaneous assessments of SGs, US-guided fine needle aspiration cytology (FNAC) is the method of choice, as it is easy to perform, safe, and accurate.
- In patients with pSS, when a SG lymphoma is clinically suspected, FNAC is inadequate for diagnosis. In this scenario, US-guided CNB of major SGs is an established, safe, and feasible technique, which provides enough viable tissue for the diagnosis and assessment of lymphoproliferative diseases.
- When properly executed, US-guided CNB carries only a moderate risk of transient adverse effects and extremely little risk of permanent adverse effects (the most serious being facial nerve damage) and is safer than an open biopsy.
Generally, in non-pSS patients, in the percutaneous diagnosis of SG lesions, FNAC is the method of choice, as it is a well-established, easy to perform, safe, and minimally invasive technique to acquire material for cytological analysis.89 However, in patients with pSS, due to the specific pathological features of the disease and possible complications (such as NHL), FNAC has been reported to be inadequate by itself and inferior to CNB in terms of its diagnostic potential.12,90–92 In this clinical scenario, US-guided CNB biopsy of major SGs, most commonly the parotid glands (Figure 9), has the potential to overcome FNAC limitations and can be used when NHL is suspected, for example, in cases of persistent SG swelling. Compared to FNAC, a CNB has the advantage of providing viable tissue samples, which allows immunohistochemical staining and flow cytometry:19,93 the sampling is of paramount importance in assessing lymphomatous lesions.18,19
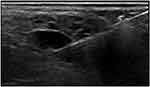 |
Figure 9 US-guided CNB of a focal area suspicious of NHL in a patient with pSS. |
Current evidence suggests that a CNB of submandibular glands is very safe. For parotid glands, US-guided CNB can be safely performed in the postero-caudal part of the gland, from the so-called “safety-zone” (ie, the posterior, caudal, superficial part of the parotid gland). A posterior to anterior approach should be preferred, and the needle should be kept as superficial as possible, within 10−15 mm from the glandular surface, to minimize the risk of facial nerve injury (Figure 10A−C).94 Despite the theoretical risk of facial nerve injury and other complications, when properly performed, a CNB of major SGs may determine transient adverse reactions, with no permanent complications.19,94,95 Therefore, US-guided CNB of the SGs can be considered a safe, feasible, and accurate technique in patients with pSS.
A number of recent studies have demonstrated that US-guided CNB is an effective and a safer alternative to an open biopsy for the characterization of major SG lesions, particularly NHL, in pSS patients.18,19,33 Compared to a surgical biopsy, the possibility of causing serious complications, such as facial nerve damage, sialoceles, and salivary fistulae,96 in a CNB is reduced, while maintaining similar diagnostic value.19 Furthermore, with US-guided CNB, it is possible to target specific focal areas within major SGs, which is of significant added value in cases of NHL with a focal appearance. In this regard, a previous study reported that a sample obtained from a parotid focal lesion in a patient with pSS was characterized as NHL at pathology, whereas a sample obtained from the adjacent parenchyma of the same gland showed only diffuse chronic sialadenitis.19 This report raises the suspicion that focal presenting NHL of major SGs could be underdiagnosed when relying on the classic surgical biopsy approach.
Monitoring Disease Activity with SGUS
Key Points
- SGUS may play a role in monitoring pSS activity and treatment effectiveness.
- Nowadays, further studies are needed to establish SGUS value in this clinical setting.
The role of SGUS as a tool to assess pSS disease activity and monitor treatment effectiveness is still under investigation. Studies reported an association between SGUS scores and the presence of extra-glandular pSS manifestations and systemic, disease activity evaluated by ESSDAI and EULAR Sjogren’s Syndrome Patient Reported Index (ESSPRI).34,97–99
SGUS may represent a disease activity marker in the ESSDAI glandular domain, as well as a marker of damage progression.25 Studies reported that SGUS may detect morphological changes in some salivary gland features as a consequence of pharmacologic intervention with rituximab or ianalumab, likely related to disease activity.100–102
Recently, the use of Doppler assessment has been thoroughly investigated, and the OMERACT ultrasound working group proposed a semiquantitative scoring systems to evaluate vascularization by color Doppler in pSS patients.103,104
In the future, the combination of Doppler and grey scale scoring system may lead to a global and comprehensive SGUS scoring, allowing a better assessment of disease activity and damage in the major salivary glands.
Sonoelastography
Key Points
- Sonoelastography is an adjunct to US.
- Sonoelastography of SGs could be useful in diagnosing and monitoring pSS; however, lack of data prevents to make a final statement about the usefulness of elastography in diagnosing and monitoring pSS.
Sonoelastography is a US imaging method to evaluate tissue stiffness.105 There are three techniques available: strain-based elastography, shear-wave elastography, and virtual touch tissue imaging and quantification.106 In strain-based elastography, the operator applies direct mechanical compression to the tissue surface to obtain a qualitative measure of elasticity.107 No quantitative analysis is possible using this technique. In contrast, shear-wave elastography provides qualitative and quantitative information, expressed in m/s or kPa. This technique is based on measurements of wave propagation after tissue excitation by acoustic radiation force impulse imaging.108 Finally, virtual touch imaging and quantification produce a qualitative gray-scale map of variations in tissue stiffness and shear wave velocity.22
Some studies have shown that elastography combined with SGUS may be useful for the diagnosis of pSS.109–111 pSS is associated with inflammation and fibrosis. Thus, patients with pSS can present with increased SG stiffness, mainly because of structural changes of glandular parenchima.112 Stiffness values vary, depending on the level of inflammation and fibrosis. These values can serve as a marker of histological damage in pSS.113–115 Thus far, elastography studies of SGs of pSS patients have been conducted in small cohorts of patients.111–115 Moreover, there are substantial differences among the three aforementioned modalities to obtain elastography.108 Therefore, it is not possible to make a final statement about the usefulness of elastography in diagnosing and monitoring pSS.
Artificial Intelligence (AI)
Key Points
- Artificial intelligence is being developed as a diagnostic tool to support SGUS assessments.
- The application of AI tools, such as machine learning and deep learning, can be expected to aid clinical decision making in pSS.
In pSS, SGUS is used to score glandular parenchyma using various scoring systems, such as the De Vita score or OMERACT score.28,29 The scoring of glandular patterns during SGUS evaluations is operator dependent, and it has shown low inter- and intraobserver reliability in some cases.116 The use of computer algorithms for texture analysis could eventually lead to the development of AI tools to assist human experts in the evaluation of SGs.117,118 Such tools might become available in the near future, as AI has been shown to be able to individuate complex patterns in a large variety of images, reaching excellent technical expertise that could support clinical decisions.119,120 In SGUS in patients with pSS, some authors have analyzed a series of radiomics-based AI algorithms using the scoring system proposed by De Vita et al.28 Among these algorithms, a multilayer perceptron classifier proved to be the most accurate and least error prone in SG segmentation.121
Developments in AI research and computerized software tools may herald a progress in SG screening by SGUS, reducing screening times and reliance on human experts.120 However, automatized tools for segmentation and reconstruction of SGs from SGUS are not yet in routine use in the clinic in the pSS management.22 The current lack of data may be justified by the small cohorts of patients in previous studies. Multicenter studies of a large number of pSS patients may aid the development of robust automatized SGUS assessment and reduce both screening times and operator dependency.122 With the aim of improving automated image segmentation of SGs, as part of the HarmonicSS initiative, a group of pSS experts have begun to recruit large European cohorts of patients with different grades of pSS.32 Thus far, they have reported reaching close-to-human performances in terms of SGUS assessment. It seems reasonable to assume that AI methods will lead to the use of novel tools for the diagnosis of pSS in the future.121
Conclusions
In pSS patients, the usefulness of SGUS in the assessment of major SG parenchyma has been clearly demonstrated, and it is currently applied in daily clinical practice as a complementary tool for the diagnosis of pSS. SGUS may also have uses in monitoring disease activity, in assessing glandular damage and in early detection of focal or diffuse patterns indicative of lymphoproliferative diseases, although its potential usefulness in this area needs further investigation. The importance of SGUS may increase in the future. SGUS could play a pivotal role in pSS as a guide for CNB, a feasible and accurate technique that recent studies showed to be safer than open surgical biopsy. In addition, advances in AI, with the development of more accurate AI algorithms, could expand and support the use of SGUS in clinical practice in the future.
Abbreviations
AI, artificial intelligence; CNB, core-needle biopsy; FNAC, fine needle aspiration cytology; NHL non-Hodgkin’s lymphoma; OMERACT, Outcome Measures in Rheumatology Clinical Trials; pSS, primary Sjögren’s syndrome; SG, salivary gland; SGUS, salivary gland ultrasound; US, ultrasound; ACR-EULAR, American College of Rheumatology – European League Against Rheumatism; ESSDAI, EULAR Sjogren syndrome disease activity index; ESSPRI, EULAR Sjogren’s Syndrome Patient Reported Index.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Maciel G, Crowson CS, Matteson EL, Cornec D. Prevalence of primary Sjögren’s syndrome in a US population-based cohort: primary SS prevalence. Arthritis Care Res. 2017;69(10):1612–1616. doi:10.1002/acr.23173
2. Mavragani CP, Moutsopoulos HM. Sjögren’s syndrome. Annu Rev Pathol Mech Dis. 2014;9(1):273–285. doi:10.1146/annurev-pathol-012513-104728
3. Zintzaras E. The risk of lymphoma development in autoimmune diseases: a meta-analysis. Arch Intern Med. 2005;165(20):2337. doi:10.1001/archinte.165.20.2337
4. Baldini C, Luciano N, Tarantini G, et al. Salivary gland ultrasonography: a highly specific tool for the early diagnosis of primary Sjögren’s syndrome. Arthritis Res Ther. 2015;17(1):146. doi:10.1186/s13075-015-0657-7
5. Shiboski CH, Shiboski SC, Seror R, et al. 2016 American College of Rheumatology/European League against rheumatism classification criteria for primary Sjögren’s syndrome: a consensus and data‐driven methodology involving three international patient cohorts. Arthritis Rheumatol. 2017;69(1):35–45. doi:10.1002/art.39859
6. Jousse-Joulin S, Milic V, Jonsson MV, et al. Is salivary gland ultrasonography a useful tool in Sjögren’s syndrome? A systematic review. Rheumatology. 2016;55(5):789–800. doi:10.1093/rheumatology/kev385
7. Cornec D, Jousse-Joulin S, Pers JO, et al. Contribution of salivary gland ultrasonography to the diagnosis of Sjögren’s syndrome: toward new diagnostic criteria. Arthritis Rheum. 2013;65(1):216–225. doi:10.1002/art.37698
8. Salaffi F, Carotti M, Iagnocco A, et al. Ultrasonography of salivary glands in primary Sjogren’s syndrome: a comparison with contrast sialography and scintigraphy. Rheumatology. 2008;47(8):1244–1249. doi:10.1093/rheumatology/ken222
9. Baldini C, Talarico R, Tzioufas AG, Bombardieri S. Classification criteria for Sjogren’s syndrome: a critical review. J Autoimmun. 2012;39(1–2):9–14. doi:10.1016/j.jaut.2011.12.006
10. Mavragani CP, Moutsopoulos HM. The geoepidemiology of Sjögren’s syndrome. Autoimmun Rev. 2010;9(5):A305–A310. doi:10.1016/j.autrev.2009.11.004
11. Cornec D, Jousse-Joulin S, Marhadour T, et al. Salivary gland ultrasonography improves the diagnostic performance of the 2012 American College of Rheumatology classification criteria for Sjogren’s syndrome. Rheumatology. 2014;53(9):1604–1607. doi:10.1093/rheumatology/keu037
12. Baldini C, Zabotti A, Filipovic N, et al. Imaging in primary Sjögren’s syndrome: the “obsolete and the new”. Clin Exp Rheumatol. 2018;36 Suppl 112(3):215–221.
13. Nimwegen JF, Mossel E, Delli K, et al. Incorporation of salivary gland ultrasonography into the American College of Rheumatology/European League against rheumatism criteria for primary sjögren’s syndrome. Arthritis Care Res. 2020;72(4):583–590. doi:10.1002/acr.24017
14. Le Goff M, Cornec D, Jousse-Joulin S, et al. Comparison of 2002 AECG and 2016 ACR/EULAR classification criteria and added value of salivary gland ultrasonography in a patient cohort with suspected primary Sjögren’s syndrome. Arthritis Res Ther. 2017;19(1):269. doi:10.1186/s13075-017-1475-x
15. Takagi Y, Nakamura H, Sumi M, et al. Combined classification system based on ACR/EULAR and ultrasonographic scores for improving the diagnosis of Sjögren’s syndrome. PLoS One. 2018;13(4):e0195113. doi:10.1371/journal.pone.0195113
16. Jousse-Joulin S, Devauchelle-Pensec V, Morvan J, et al. Ultrasound assessment of salivary glands in patients with primary Sjögren’s syndrome treated with rituximab: quantitative and Doppler waveform analysis. Biol Targets Ther. 2007;1(3):311–319.
17. Milic V, Colic J, Cirkovic A, Stanojlovic S, Damjanov N, Abu-Shakra M. Disease activity and damage in patients with primary Sjogren’s syndrome: prognostic value of salivary gland ultrasonography. PLoS One. 2019;14(12):e0226498. doi:10.1371/journal.pone.0226498
18. Baer AN, Grader‐Beck T, Antiochos B, Birnbaum J, Fradin JM. Ultrasound‐guided biopsy of suspected salivary gland lymphoma in Sjögren’s syndrome. Arthritis Care Res. 2021;73(6):849–855. doi:10.1002/acr.24203
19. Zabotti A, Zandonella Callegher S, Lorenzon M, et al. Ultrasound-guided core needle biopsy compared with open biopsy: a new diagnostic approach to salivary gland enlargement in Sjögren’s syndrome? Rheumatology. 2021;60(3):1282–1290. doi:10.1093/rheumatology/keaa441
20. Manfrè V, Giovannini I, Zandonella Callegher S, et al. Ultrasound and bioptic investigation of patients with primary Sjögren’s syndrome. J Clin Med. 2021;10(6):1171. doi:10.3390/jcm10061171
21. Goules AV, Tzioufas AG. Primary Sjögren’s syndrome: clinical phenotypes, outcome and the development of biomarkers. Immunol Res. 2017;65(1):331–344. doi:10.1007/s12026-016-8844-4
22. Devauchelle-Pensec V, Zabotti A, Carvajal-Alegria G, Filipovic N, Jousse-Joulin S, De Vita S. Salivary gland ultrasonography in primary Sjögren’s syndrome: opportunities and challenges. Rheumatology. 2021;60(8):3522–3527. doi:10.1093/rheumatology/kez079
23. Obinata K, Sato T, Ohmori K, Shindo M, Nakamura M. A comparison of diagnostic tools for Sjögren syndrome, with emphasis on sialography, histopathology, and ultrasonography. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontol. 2010;109(1):129–134. doi:10.1016/j.tripleo.2009.08.033
24. Hočevar A, Ambrožič A, Rozman B, Kveder T, Tomšič M. Ultrasonographic changes of major salivary glands in primary Sjögren’s syndrome. Diagnostic value of a novel scoring system. Rheumatology. 2005;44(6):768–772. doi:10.1093/rheumatology/keh588
25. Vitali C, Palombi G, Baldini C, et al. Sjögren’s syndrome disease damage index and disease activity index: scoring systems for the assessment of disease damage and disease activity in Sjögren’s syndrome, derived from an analysis of a cohort of Italian patients. Arthritis Rheum. 2007;56(7):2223–2231. doi:10.1002/art.22658
26. de Vita S, Boiocchi M, Sorrentino D, et al. Characterization of prelymphomatous stages of B cell lymphoproliferation in Sjögren’s syndrome. Arthritis Rheum. 1997;40(2):318–331. doi:10.1002/art.1780400217
27. Delli K, Arends S, van Nimwegen J, et al. Ultrasound of the major salivary glands is a reliable imaging technique in patients with clinically suspected primary Sjögren’s syndrome. Ultraschall Med. 2018;39(03):328–333. doi:10.1055/s-0043-104631
28. De Vita S, Lorenzon G, Rossi G, Sabella M, Fossaluzza V. Salivary gland echography in primary and secondary Sjögren’s syndrome. Clin Exp Rheumatol. 1992;10(4):351–356.
29. Jousse-Joulin S, D’Agostino MA, Nicolas C, et al. Video clip assessment of a salivary gland ultrasound scoring system in Sjögren’s syndrome using consensual definitions: an OMERACT ultrasound working group reliability exercise. Ann Rheum Dis. 2019;78(7):967–973. doi:10.1136/annrheumdis-2019-215024
30. Vitali C, Carotti M, Salaffi F. Is it the time to adopt salivary gland ultrasonography as an alternative diagnostic tool for the classification of patients with Sjögren’s syndrome? Comment on the article by cornec et al: letters. Arthritis Rheum. 2013;65(7):1950. doi:10.1002/art.37945
31. Fana V, Dohn UM, Krabbe S, Terslev L. Application of the OMERACT grey-scale ultrasound scoring system for salivary glands in a single-centre cohort of patients with suspected Sjögren’s syndrome. RMD Open. 2021;7(2):e001516. doi:10.1136/rmdopen-2020-001516
32. Zabotti A, Zandonella Callegher S, Tullio A, et al. Salivary gland ultrasonography in Sjögren’s syndrome: a European multicenter reliability exercise for the HarmonicSS project. Front Med. 2020;7:581248. doi:10.3389/fmed.2020.581248
33. Lorenzon M, Tulipano Di Franco F, Zabotti A, et al. Sonographic features of lymphoma of the major salivary glands diagnosed with ultrasound-guided core needle biopsy in Sjögren’s syndrome. Clin Exp Rheumatol. 2021;39(6):175–183. doi:10.55563/clinexprheumatol/4c36nr
34. Zandonella Callegher S, Zabotti A, Giovannini I, Treppo E, Quartuccio L, De Vita S. Normal-appearing salivary gland ultrasonography identifies a milder phenotype of primary Sjögren’s syndrome. Front Med. 2020;7:602354. doi:10.3389/fmed.2020.602354
35. Zabotti A, Zandonella Callegher S, Gandolfo S, et al. Hyperechoic bands detected by salivary gland ultrasonography are related to salivary impairment in established Sjögren’s syndrome. Clin Exp Rheumatol. 2019;37 Suppl 118(3):146–152.
36. van Ginkel MS, Glaudemans AWJM, van der Vegt B, et al. Imaging in primary Sjögren’s syndrome. J Clin Med. 2020;9(8):2492. doi:10.3390/jcm9082492
37. Jousse-Joulin S, Coiffier G. Current status of imaging of Sjogren’s syndrome. Best Pract Res Clin Rheumatol. 2020;34(6):101592. doi:10.1016/j.berh.2020.101592
38. Cornec D, Saraux A, Jousse-Joulin S, et al. The differential diagnosis of dry eyes, dry mouth, and parotidomegaly: a comprehensive review. Clin Rev Allergy Immunol. 2015;49(3):278–287. doi:10.1007/s12016-014-8431-1
39. Shiboski CH, Shiboski SC, Seror R, et al. 2016 American College of Rheumatology/European League Against Rheumatism classification criteria for primary Sjögren’s syndrome: a consensus and data-driven methodology involving three international patient cohorts. Ann Rheum Dis. 2017;76(1):9–16. doi:10.1136/annrheumdis-2016-210571
40. Gilboe IM. Sicca symptoms and secondary Sjogren’s syndrome in systemic lupus erythematosus: comparison with rheumatoid arthritis and correlation with disease variables. Ann Rheum Dis. 2001;60(12):1103–1109. doi:10.1136/ard.60.12.1103
41. La Paglia GMC, Sanchez-Pernaute O, Alunno A, et al. Ultrasound salivary gland involvement in Sjogren’s syndrome vs. other connective tissue diseases: is it autoantibody and gland dependent? Clin Rheumatol. 2020;39(4):1207–1215. doi:10.1007/s10067-019-04780-2
42. Avouac J, Sordet C, Depinay C, et al. Systemic sclerosis–associated Sjögren’s syndrome and relationship to the limited cutaneous subtype: results of a prospective study of sicca syndrome in 133 consecutive patients. Arthritis Rheum. 2006;54(7):2243–2249. doi:10.1002/art.21922
43. Law ST, Jafarzadeh SR, Govender P, Sun X, Sanchorawala V, Kissin EY. Comparison of ultrasound features of major salivary glands in sarcoidosis, amyloidosis, and Sjögren’s syndrome. Arthritis Care Res. 2020;72(10):1466–1473. doi:10.1002/acr.24029
44. Ramos-Casals M, Brito-Zerón P, García-Carrasco M, Font J. Sarcoidosis or Sjögren syndrome? Clues to defining mimicry or coexistence in 59 cases. Medicine. 2004;83(2):85–95. doi:10.1097/01.md.0000121237.98962.1e
45. Palazzo E, Palazzo C, Palazzo M. IgG4-related disease. Joint Bone Spine. 2014;81(1):27–31. doi:10.1016/j.jbspin.2013.06.001
46. Yamamoto M, Takahashi H, Shinomura Y. Mechanisms and assessment of IgG4-related disease: lessons for the rheumatologist. Nat Rev Rheumatol. 2014;10(3):148–159. doi:10.1038/nrrheum.2013.183
47. Zhang X, Zhang S, He J, et al. Ultrasonographic evaluation of major salivary glands in primary Sjögren’s syndrome: comparison of two scoring systems. Rheumatology. 2015;54(9):1680–1687. doi:10.1093/rheumatology/kev103
48. Shimizu M, Okamura K, Kise Y, et al. Effectiveness of imaging modalities for screening IgG4-related dacryoadenitis and sialadenitis (Mikulicz’s disease) and for differentiating it from Sjögren’s syndrome (SS), with an emphasis on sonography. Arthritis Res Ther. 2015;17(1):223. doi:10.1186/s13075-015-0751-x
49. Abraham M, Khosroshahi A. Diagnostic and treatment workup for IgG4-related disease. Expert Rev Clin Immunol. 2017;13(9):867–875. doi:10.1080/1744666X.2017.1354698
50. Akiyama M, Takanashi S, Takeuchi T, Kaneko Y. Salivary gland involvement in ANCA-associated vasculitis. Autoimmun Rev. 2021;20(11):102940. doi:10.1016/j.autrev.2021.102940
51. Barrett AW. Wegener’s granulomatosis of the major salivary glands: wegener’s granulomatosis. J Oral Pathol Med. 2012;41(10):721–727. doi:10.1111/j.1600-0714.2012.01141.x
52. Gaffo AL. Diagnostic Approach to ANCA-associated Vasculitides. Rheum Dis Clin N Am. 2010;36(3):491–506. doi:10.1016/j.rdc.2010.05.009
53. Imanguli MM, Atkinson JC, Mitchell SA, et al. Salivary gland involvement in chronic graft-versus-host disease: prevalence, clinical significance, and recommendations for evaluation. Biol Blood Marrow Transplant. 2010;16(10):1362–1369. doi:10.1016/j.bbmt.2010.03.023
54. Roesink JM, Moerland MA, Hoekstra A, Rijk PPV, Terhaard CHJ. Scintigraphic assessment of early and late parotid gland function after radiotherapy for head-and-neck cancer: a prospective study of dose–volume response relationships. Int J Radiat Oncol. 2004;58(5):1451–1460. doi:10.1016/j.ijrobp.2003.09.021
55. Ahuja AT, Evans RM, eds. Practical Head and Neck Ultrasound.
56. Gritzmann N, Rettenbacher T, Hollerweger A, Macheiner P, Hübner E. Sonography of the salivary glands. Eur Radiol. 2003;13(5):964–975. doi:10.1007/s00330-002-1586-9
57. Tschammler A, Ott G, Schang T, Seelbach-Goebel B, Schwager K, Hahn D. Lymphadenopathy: differentiation of benign from malignant disease–color Doppler US assessment of intranodal angioarchitecture. Radiology. 1998;208(1):117–123. doi:10.1148/radiology.208.1.9646801
58. Bialek EJ, Jakubowski W, Zajkowski P, Szopinski KT, Osmolski A. US of the major salivary glands: anatomy and spatial relationships, pathologic conditions, and pitfalls. RadioGraphics. 2006;26(3):745–763. doi:10.1148/rg.263055024
59. Martinoli C, Pretolesi F, Del Bono V, Derchi LE, Mecca D, Chiaramondia M. Benign lymphoepithelial parotid lesions in HIV-positive patients: spectrum of findings at gray-scale and Doppler sonography. Am J Roentgenol. 1995;165(4):975–979. doi:10.2214/ajr.165.4.7677004
60. Brook I. Acute bacterial suppurative parotitis: microbiology and management. J Craniofac Surg. 2003;14(1):37–40. doi:10.1097/00001665-200301000-00006
61. Kastin B, Mandel L. Alcoholic sialosis. N Y State Dent J. 2000;66(6):22–24.
62. Moerman RV, Bootsma H, Kroese FGM, Vissink A. Sjögren’s syndrome in older patients: aetiology, diagnosis and management. Drugs Aging. 2013;30(3):137–153. doi:10.1007/s40266-013-0050-7
63. Romanos GE, Javed F, Romanos EB, Williams RC. Oro-facial manifestations in patients with eating disorders. Appetite. 2012;59(2):499–504. doi:10.1016/j.appet.2012.06.016
64. Kim D, Uy C, Mandel L. Sialosis of unknown origin. N Y State Dent J. 1998;64(7):38–40.
65. Alunno A, Leone MC, Giacomelli R, Gerli R, Carubbi F. Lymphoma and lymphomagenesis in primary Sjögren’s syndrome. Front Med. 2018;5:102. doi:10.3389/fmed.2018.00102
66. Stergiou IE, Poulaki A, Voulgarelis M. Pathogenetic mechanisms implicated in Sjögren’s syndrome lymphomagenesis: a review of the literature. J Clin Med. 2020;9(12):3794. doi:10.3390/jcm9123794
67. Heřman J, Sedláčková Z, Vachutka J, et al. Differential diagnosis of parotid gland tumors: role of shear wave elastography. BioMed Res Int. 2017;2017:1–6. doi:10.1155/2017/9234672
68. Shimizu M, Ussmüller J, Hartwein J, Donath K, Kinukawa N. Statistical study for sonographic differential diagnosis of tumorous lesions in the parotid gland. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontol. 1999;88(2):226–233. doi:10.1016/S1079-2104(99)70120-4
69. Spiro RH. Management of malignant tumors of the salivary glands. Oncol Williston Park N. 1998;12(5):
70. Luers JC, Guntinas-Lichius O, Klussmann JP, Küsgen C, Beutner D, Grosheva M. The incidence of Warthin tumours and pleomorphic adenomas in the parotid gland over a 25-year period. Clin Otolaryngol. 2016;41(6):793–797. doi:10.1111/coa.12694
71. Bokhari MR, Greene J. Pleomorphic adenoma. In: StatPearls. StatPearls Publishing; 2022. Available from. http://www.ncbi.nlm.nih.gov/books/NBK430829/.
72. Dumitriu D, Dudea SM, Botar-Jid C, Băciuţ G. Ultrasonographic and sonoelastographic features of pleomorphic adenomas of the salivary glands. Med Ultrason. 2010;12(3):175–183.
73. Khalife A, Bakhshaee M, Davachi B, Mashhadi L, Khazaeni K. The diagnostic value of B-mode sonography in differentiation of malignant and benign tumors of the parotid gland. Iran J Otorhinolaryngol. 2016;28(88):305–312.
74. Kim J, Kim EK, Park CS, Choi YS, Kim YH, Choi EC. Characteristic sonographic findings of Warthin’s tumor in the parotid gland. J Clin Ultrasound. 2004;32(2):78–81. doi:10.1002/jcu.10230
75. Bialek EJ, Jakubowski W, Karpinska G. Role of ultrasonography in diagnosis and differentiation of pleomorphic adenomas: work in progress. Arch Otolaryngol Neck Surg. 2003;129(9):929. doi:10.1001/archotol.129.9.929
76. Paris J, Coulet O, Facon F, Chrestian MA, Giovanni A, Zanaret M. Cancers primitifs de la parotide: approche anatomo-clinique. Rev Stomatol Chir Maxillofac. 2004;105(6):309–315. doi:10.1016/S0035-1768(04)72333-5
77. Lewis AG, Tong T, Maghami E. Diagnosis and management of malignant salivary gland tumors of the parotid gland. Otolaryngol Clin North Am. 2016;49(2):343–380. doi:10.1016/j.otc.2015.11.001
78. Howlett DC, Kesse KW, Hughes DV, Sallomi DF. The role of imaging in the evaluation of parotid disease. Clin Radiol. 2002;57(8):692–701. doi:10.1053/crad.2001.0865
79. Hardee PSGF, Carter JLB, Piper KM, Ng SY. Metachronous bilateral primary adenocarcinoma of the submandibular glands. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontol. 2001;91(4):455–461. doi:10.1067/moe.2001.113547
80. Yoshihara T, Suzuki S, Nagao K. Mucoepidermoid carcinoma arising in the accessory parotid gland. Int J Pediatr Otorhinolaryngol. 1999;48(1):47–52. doi:10.1016/S0165-5876(98)00187-6
81. Goto TK, Yoshiura K, Nakayama E, et al. The combined use of US and MR imaging for the diagnosis of masses in the parotid region. Acta Radiol Stockh Swed. 2001;42(1):88–95.
82. Nocturne G, Pontarini E, Bombardieri M, Mariette X. Lymphomas complicating primary Sjögren’s syndrome: from autoimmunity to lymphoma. Rheumatology. 2021;60(8):3513–3521. doi:10.1093/rheumatology/kez052
83. Skarlis C, Raftopoulou S, Mavragani CP. Sjogren’s syndrome: recent updates. J Clin Med. 2022;11(2):399. doi:10.3390/jcm11020399
84. Skarlis C, Argyriou E, Mavragani CP. Lymphoma in Sjögren’s syndrome: predictors and therapeutic options. Curr Treat Options Rheumatol. 2020;6(1):1–17. doi:10.1007/s40674-020-00138-x
85. Vitali C. Classification criteria for Sjogren’s syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002;61(6):554–558. doi:10.1136/ard.61.6.554
86. Fragkioudaki S, Mavragani CP, Moutsopoulos HM. Predicting the risk for lymphoma development in Sjogren syndrome: an easy tool for clinical use. Medicine. 2016;95(25):e3766. doi:10.1097/MD.0000000000003766
87. Bacon CM, Du MQ, Dogan A. Mucosa-associated lymphoid tissue (MALT) lymphoma: a practical guide for pathologists. J Clin Pathol. 2006;60(4):361–372. doi:10.1136/jcp.2005.031146
88. Raderer M, Kiesewetter B, Ferreri AJM. Clinicopathologic characteristics and treatment of marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma): characteristics and management of MALT Lymphoma. CA Cancer J Clin. 2016;66(2):152–171. doi:10.3322/caac.21330
89. Chrabańska M, Kiczmer P, Drozdzowska B. Salivary gland lesions: diagnostic reliability and challenges of fine needle aspiration cytology. Int J Clin Exp Pathol. 2021;14(1):54–62.
90. Pfeiffer J, Ridder GJ. Diagnostic value of ultrasound-guided core needle biopsy in patients with salivary gland masses. Int J Oral Maxillofac Surg. 2012;41(4):437–443. doi:10.1016/j.ijom.2011.12.005
91. Huang YC, Wu CT, Lin G, Chuang WY, Yeow KM, Wan YL. Comparison of ultrasonographically guided fine-needle aspiration and core needle biopsy in the diagnosis of parotid masses. J Clin Ultrasound. 2012;40(4):189–194. doi:10.1002/jcu.20873
92. Eom HJ, Lee JH, Ko MS, et al. Comparison of fine-needle aspiration and core needle biopsy under ultrasonographic guidance for detecting malignancy and for the tissue-specific diagnosis of salivary gland tumors. Am J Neuroradiol. 2015;36(6):1188–1193. doi:10.3174/ajnr.A4247
93. Silva JL, Azevedo S, Faria DS, Costa JT, Teixeira F. Ultrasound-guided core biopsy of the parotid gland: the procedure from the Rheumatology point of view. Acta Reumatol Port. 2020;45(1):69–70.
94. Tulipano Di Franco F. Feasibility and safety issues of Ultrasound-guided core biopsy of focal lesions of major salivary glands: our experience; 2021:1643. DOI:10.26044/ECR2021/C-12973
95. Giovannini I, Lorenzon M, Manfrè V, et al. Safety, patient acceptance and diagnostic accuracy of ultrasound core needle biopsy of parotid or submandibular glands in primary Sjögren’s syndrome with suspected salivary gland lymphoma. RMD Open. 2022;8(1):e001901. doi:10.1136/rmdopen-2021-001901
96. Pijpe J, Kalk WWI, van der Wal JE, et al. Parotid gland biopsy compared with labial biopsy in the diagnosis of patients with primary Sjogren’s syndrome. Rheumatology. 2006;46(2):335–341. doi:10.1093/rheumatology/kel266
97. Theander E, Mandl T. Primary Sjögren’s syndrome: diagnostic and prognostic value of salivary gland ultrasonography using a simplified scoring system. Arthritis Care Res. 2014;66(7):1102–1107. doi:10.1002/acr.22264
98. Inanc N, Şahinkaya Y, Mumcu G, et al. Evaluation of salivary gland ultrasonography in primary Sjögren’s syndrome: does it reflect clinical activity and outcome of the disease. Clin Exp Rheumatol. 2019;37 Suppl 118(3):140–145.
99. Fidelix T, Czapkowski A, Azjen S, Andriolo A, Trevisani VFM, Antoniou AN. Salivary gland ultrasonography as a predictor of clinical activity in Sjögren’s syndrome. PLoS One. 2017;12(8):e0182287. doi:10.1371/journal.pone.0182287
100. Fisher BA, Everett CC, Rout J, et al. Effect of rituximab on a salivary gland ultrasound score in primary Sjögren’s syndrome: results of the TRACTISS randomised double-blind multicentre substudy. Ann Rheum Dis. 2018;77(3):412–416. doi:10.1136/annrheumdis-2017-212268
101. Cornec D, Jousse-Joulin S, Costa S, et al. High-grade salivary-gland involvement, assessed by histology or ultrasonography, is associated with a poor response to a single rituximab course in primary Sjögren’s syndrome: data from the TEARS randomized trial. PLoS One. 2016;11(9):e0162787. doi:10.1371/journal.pone.0162787
102. Diekhoff T, Fischer T, Schefer Q, et al. Ianalumab (VAY736) in primary Sjögren’s syndrome: assessing disease activity using multi-modal ultrasound. Clin Exp Rheumatol. 2020;38 Suppl 126(4):228–236.
103. Hočevar A, Bruyn GA, Terslev L, et al. Development of a new ultrasound scoring system to evaluate glandular inflammation in Sjögren’s syndrome: an OMERACT reliability exercise. Rheumatol Oxf Engl. 2021:keab876. doi:10.1093/rheumatology/keab876
104. Damjanov N, Milic V, Nieto-González JC, et al. Multiobserver reliability of ultrasound assessment of salivary glands in patients with established primary Sjögren syndrome. J Rheumatol. 2016;43(10):1858–1863. doi:10.3899/jrheum.151220
105. Al-Qahtani M. Shear-wave and strain elastography: a comparative review on principles, basic techniques and applications. Curr Med Imaging Rev. 2016;12(4):269–278. doi:10.2174/1573405612666160402002105
106. DeWall RJ. Ultrasound elastography: principles, techniques, and clinical applications. Crit Rev Biomed Eng. 2013;41(1):1–19. doi:10.1615/CritRevBiomedEng.2013006991
107. Cosgrove D, Piscaglia F, Bamber J, et al. EFSUMB guidelines and recommendations on the clinical use of ultrasound elastography.Part 2: clinical applications. Ultraschall Med. 2013;34(03):238–253. doi:10.1055/s-0033-1335375
108. Martire MV, Santiago ML, Cazenave T, Gutierrez M. Latest advances in ultrasound assessment of salivary glands in Sjögren syndrome. J Clin Rheumatol. 2018;24(4):218–223. doi:10.1097/RHU.0000000000000625
109. Turnaoglu H, Kural Rahatli F, Pamukcu M, Haberal KM, Uslu N. Diagnostic value of acustic radiation force impulse imaging in the assessment of salivary gland involvement in primary Sjögren’s sydrome. Med Ultrason. 2018;20(3):313. doi:10.11152/mu-1397
110. Knopf A, Hofauer B, Thürmel K, et al. Diagnostic utility of Acoustic Radiation Force Impulse (ARFI) imaging in primary Sjoegren`s syndrome. Eur Radiol. 2015;25(10):3027–3034. doi:10.1007/s00330-015-3705-4
111. Oruk YE, Çildağ MB, Karaman CZ, Çildağ S. Effectiveness of ultrasonography and shear wave sonoelastography in Sjögren syndrome with salivary gland involvement. Ultrasonography. 2021;40(4):584–593. doi:10.14366/usg.21014
112. Zhang S, Zhu J, Zhang X, He J, Li J. Assessment of the stiffness of major salivary glands in primary Sjögren’s syndrome through quantitative acoustic radiation force impulse imaging. Ultrasound Med Biol. 2016;42(3):645–653. doi:10.1016/j.ultrasmedbio.2015.11.009
113. Hofauer B, Mansour N, Heiser C, et al. Sonoelastographic modalities in the evaluation of salivary gland characteristics in Sjögren’s syndrome. Ultrasound Med Biol. 2016;42(9):2130–2139. doi:10.1016/j.ultrasmedbio.2016.04.011
114. Chen S, Wang Y, Chen S, Wu Q, Chen S. Virtual touch quantification of the salivary glands for diagnosis of primary Sjögren syndrome. J Ultrasound Med. 2016;35(12):2607–2613. doi:10.7863/ultra.16.01085
115. Bădărînză M, Serban O, Maghear L, et al. Shear wave elastography as a new method to identify parotid lymphoma in primary Sjögren syndrome patients: an observational study. Rheumatol Int. 2020;40(8):1275–1281. doi:10.1007/s00296-020-04548-x
116. Fox RI. Is salivary gland ultrasonography a useful tool in Sjögren’s syndrome? Rheumatology. 2016;55(5):773–774. doi:10.1093/rheumatology/kev409
117. LeCun Y, Bengio Y, Hinton G. Deep learning. Nature. 2015;521(7553):436–444. doi:10.1038/nature14539
118. Castellano G, Bonilha L, Li LM, Cendes F. Texture analysis of medical images. Clin Radiol. 2004;59(12):1061–1069. doi:10.1016/j.crad.2004.07.008
119. Litjens G, Kooi T, Bejnordi BE, et al. A survey on deep learning in medical image analysis. Med Image Anal. 2017;42:60–88. doi:10.1016/j.media.2017.07.005
120. Kise Y, Shimizu M, Ikeda H, et al. Usefulness of a deep learning system for diagnosing Sjögren’s syndrome using ultrasonography images. Dentomaxillofacial Radiol. 2020;49(3):20190348. doi:10.1259/dmfr.20190348
121. Vukicevic A, Zabotti A, de Vita S, Filipovic N. Assessment of machine learning algorithms for the purpose of primary Sjögren’s syndrome grade classification from segmented ultrasonography images. In: Fratu O, Militaru N, Halunga S editors, Future Access Enablers for Ubiquitous and Intelligent Infrastructures. Vol. 241. Lecture Notes of the Institute for Computer Sciences, Social Informatics and Telecommunications Engineering. Springer International Publishing; 2018:239–245. doi:10.1007/978-3-319-92213-3_35
122. Mahmood H, Shaban M, Rajpoot N, Khurram SA. Artificial Intelligence-based methods in head and neck cancer diagnosis: an overview. Br J Cancer. 2021;124(12):1934–1940. doi:10.1038/s41416-021-01386-x
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.