Back to Journals » Drug Design, Development and Therapy » Volume 16
Ruscogenin Attenuates Lipopolysaccharide-Induced Septic Vascular Endothelial Dysfunction by Modulating the miR-146a-5p/NRP2/SSH1 Axis
Authors Pan D, Zhu J, Cao L, Zhu B, Lin L
Received 14 January 2022
Accepted for publication 21 March 2022
Published 12 April 2022 Volume 2022:16 Pages 1099—1106
DOI https://doi.org/10.2147/DDDT.S356451
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Tin Wui Wong
Danhong Pan,1 Jinqiang Zhu,2 Liexiang Cao,2 Beilei Zhu,3 Lili Lin1
1Emergency Care Unit, The First People’s Hospital of Wenling, Wenling, Zhejiang, 317500, People’s Republic of China; 2Emergency Department, The First People’s Hospital of Wenling, Wenling, Zhejiang, 317500, People’s Republic of China; 3Department of Neurology, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, Zhejiang, 325000, People’s Republic of China
Correspondence: Lili Lin, Emergency Care Unit, The First People’s Hospital of Wenling, No. 333, Chuan’an South Road, Chengxi Street, Wenling, Zhejiang, 317500, People’s Republic of China, Tel +86– 13616760809, Email [email protected]
Introduction: Endothelial dysfunction (ED) is associated with the progression of sepsis. Ruscogenin (RUS) has shown considerable efficacy in treating ED and sepsis. In the current study, the effects of RUS on sepsis-induced ED were assessed, and the mechanism was explored by focusing on the interactions of RUS with miRs.
Methods: Sepsis was induced in mice and in human umbilical vein endothelial cells (HUVECs) using LPS method. Expression profile of miRs responding to sepsis was determined. Symptoms associated with sepsis and ED were examined after treatment with RUS. Changes in mouse survival, arterial structure, systemic inflammation, cell viability, apoptosis, and the miR-146a-5p/NRP2/SSH1 axis were analyzed.
Results: Based on the microarray results, miR-146a-5p was selected as the therapeutic target. RUS improved survival rates and arterial structure, suppressed proinflammatory cytokines, down-regulated miR-146a-5p, and up-regulated NPR2 and SSH1 in septic mice. In HUVECs, RUS increased cell viability, suppressed apoptosis, inhibited inflammation, downregulated miR-146a-5p, and increased NRP2 and SSH1 levels. The re-induction of miR-146a-5p-5p impaired the protective effects of RUS on HUVECs.
Discussion: Effects of RUS on sepsis-induced impairments in endothelium relied on the suppression of miR-146a-5p.
Keywords: endothelial dysfunction, ruscogenin, miR-146a-5p, NRP2, sepsis
Introduction
Sepsis is a complex syndrome1 that has been characterized as a major factor contributing to morbidity and mortality worldwide.2 One of the most devastating complications associated with sepsis is systemic endothelial dysfunction (ED), which leads to poor tissue perfusion, persistent hypotension, and multiple organ failure during the progression of sepsis and has been recognized as an independent indicator of mortality.3–5 As a major risk factor for cardiovascular disorders (CVDs),6–8 ED can be induced by multiple mechanisms,9,10 and failure to control ED development always promotes the development of atherosclerosis (AS).11,12 Thus, the effective control of ED has become a promising strategy for handling AS as well as CVDs.
Recent progression in understanding pathogenesis of sepsis reveals the diagnostic and treatment potentials of multiple biological factors in the initiation of the disorder. Among which, microRNAs (miRs) elicit increasing interests for their involvement in sepsis. miR-25 contributed to the treatment of sepsis by ginkgolide A,13 and miR-22-3p served as an early diagnostic biomarker for sepsis.14 Moreover, miRs also play critical roles in the development of ED. For example, miR-221 and miR-21 were up-regulated in human umbilical vein ECs (HUVECs).15 Moreover, inhibited expression of Dicer impaired the expression endothelial cell‑specific receptor kinase, VEGFR2, and IL-8, and suppressed the proliferation of ECs.16 Thus, it is reasonable to infer that there must be some miRs that play important roles in sepsis-induced ED and serve as potential therapeutic targets for the treatment of sepsis-induced ED. In our preliminary microarray experiment (Supplementary Materials Tables S1 and S2), the expression of miR-146a-5p was induced by sepsis. MiR-146a-5p has been reported to contribute to the progression of the cardiac innate immune response and cardiomyocyte dysfunction.17 One of the typical downstream effectors of miR-145a-5p (TargetScan Human 7.1 prediction), NRP2, plays a protective role against ED by inhibiting the inflammatory response during ED progression.18 Thus, we hypothesized that the targeted regulation of miR-146a-5p could attenuate sepsis-induced ED by restoring the function of NRP2 and other downstream pathways.
Traditional Chinese medicine (TCM) has shown its potential in the treatment of different chronic disorders. Ruscogenin (RUS) is an active component isolated from Ruscus aculeatus that has shown significant anti-inflammatory activity during long-term applications in TCM.19–22 Wang showed that RUS could attenuate sepsis-induced acute lung injury and pulmonary endothelial barrier dysfunction.23 A study also demonstrated the protective effects of RUS against ED, which were further verified by studies by Cao et al and Bi et al.24,25 Collectively, this compound shows considerable efficacy against sepsis-induced ED. However, the mechanism of the protective effects of RUS on the vascular system remains incompletely understood. MiRs play key roles in the progression of both sepsis and ED. The current study aimed to explore the interaction between RUS and miRs during the treatment of sepsis-induced ED. Our preliminary experiments verified the potential regulatory effects of RUS on miR-146a-5p (Supplementary Materials Figure S1); thus, miR-146a-5p and the downstream factor NPR2 were selected as potential targets of the compound.
To verify this hypothesis, mice were subjected to lipopolysaccharide (LPS) to induce sepsis and ED. The mice were treated with RUS, and changes in mouse survival, aortic histology, and the miR-146a-5p/NRP2/SSH1 axis were analyzed. HUVECs were incubated with LPS to induce a septic ED model that was handled with RUS. Then, the level of miR-146a-5p was modulated to explore the potential mechanism driving the function of RUS.
Materials and Methods
Microarray Analysis of miR Expression Profile
To select miRs response to sepsis induction, HUVECs with or without LPS treatment were collected, and total extracted RNA was subjected to microarray analyses using Illumina NextSeq 500 based on miRDeep2 database by Sango Biotech (Shanghai, China). The data were summarized by normalizing the expression levels of all dys-expressed miRs (Supplementary Materials Tables S1 and S2) and the differentially expressed miRs were filtered to exclude those with changes less than 2.0-fold compared with control HUVECs.
Septic Model Induction Using LPS Method and RUS Administration
Eight-week-old male C57BL/6 mice (18–22 g) were purchased from Liaoning Changsheng Biotechnology Co., Ltd (Shenyang, China) and housed individually under a 12-h light/dark cycle with ad libitum access to water. Animal experiments were approved by the Ethics Committee of Liaoning Changsheng Biotechnology Co., Ltd (Shenyang, China) and followed the US National Institutes of Health (NIH) animal care and use protocol. Sepsis was induced using the LPS method: briefly, the mice received an intraperitoneal injection of LPS (dissolved in normal saline, 5 mg/kg body weight) (from Escherichia coli O111:B4, Sigma, USA) 1 h after RUS or Dex treatment.26 For grouping, 75 mice were randomly divided into five groups (15 in each group): Control group, mice received an intraperitoneal injection of saline of the same volume; LPS group, mice an intraperitoneal injection of LPS (5 mg/kg body weight); LPS + Low group, mice were orally administered with 0.01 mg/kg RUS (dissolved in DMSO, J&K Scientific Ltd., China) 1 h before LPS injection; LPS + High group, mice were orally administered with 0.1 mg/kg RUS 1 h before LPS; LPS + Dex group, mice were orally administered with 5 mg/kg dexamethasone (Dex) (Sigma-Aldrich, USA) 1 h before LPS injection. To calculate the effect of RUS on the survival rate of mice, 10 mice in each group were housed for 24 hours after LPS injection, and the survival rate was calculated. The other five mice were executed using pentobarbital sodium (150 mg/kg BW) 8 hours after LPS injection. Mesenteric artery tissues and blood samples were collected and preserved.
Hematoxylin-Eosin (H&E) Staining and Enzyme Linked Sorbent Immune Assay (ELISA)
Injuries in the mesenteric artery were detected with H&E staining: tissues were immersed and fixed in 8% formalin for embedding, sectioned, and incubated with hematoxylin and eosin. The injury degree of the tissues was evaluated by determining the intima-to-medial thickness.27 The levels of cytokines, including IL-6, IL-1β, and TNF-α were detected using corresponding ELISA kits.
Reverse Transcription Quantitative PCR (RT-qPCR)
Total RNA was extracted using the TRIzol method, and reversely transcribed into cDNA templates using M-MLV. The relative expression levels of different targets (miR‐146a-5p, forward: 5′‐CGGATCCTTGGTCTCCTCCAGATGTTTAT‐3′, reverse: 5′‐CCTCGAGTCATTAAAGTGATTTCTCCCAAG‐3′; NRP2, forward, 5’- CGCATTGCATCAGCCATGAT-3’, reverse, 5’-GGGAGATGTGTTCTGCTTCA-3’; SSH1, forward, 5’-GATGGAGATGGTGGGTTCAG-3’, reverse, 5’-GGGGAAGTAGTTGTGCCTC-3’; GAPDH, forward, 5’-GAAGGTGAAGGTCGGAGTC-3’, reverse, 5’-GAAGATGGTGATGGGATTTC-3’; U6, 5′‐AGAGAAGATTAGCATGGCCCCTG‐3′, 5′‐ATCCAGTGCAGGGTCCGAGG‐3′) were calculated following method of 2−ΔΔct in reference to control group (data represented as 1).
Cell Treatment
To determine the effects of RUS on sepsis-induced injuries in vitro, HUVECs (purchased from Chi Scientific, no. 1-0025, China) were divided into four groups: Control group, healthy cells (2 × 105 cells/mL); LPS group, cells (2 × 105 cells/mL) incubated with LPS (10 μg/mL, dissolved in normal saline) (from Escherichia coli O111:B4, Sigma, USA) for 12 h; LPS + RUS group, cells (2 × 105 cells/mL) incubated with LPS (10 μg/mL) and 10 μM RUS for 12 h; LPS + RUS + Mimic group, cells (2 × 105 cells/mL) were pre-transfected with a miR-146a-5p mimic and then incubated with LPS (10 μg/mL) and 10 μM RUS for 12 h. Then, the cells were collected, and the cell viability, apoptosis, and plasma levels of cytokines, including IL-6, IL-1β, and TNF-α were detected.
CCK-8 Assays
CCK-8 assays were performed to determine cell proliferation in response to different treatment conditions. Exponentially growing HUVECs were incubated in 96-well plates (3×103/well) for 24 h. Then, 10 μL of CCK-8 solution was added to the wells, and the mixture was incubated at 37°C for an additional hour. The OD values at 450 nm were detected using a microplate reader (ELX-800, BIOTEK, USA) and employed as representative of cell viability.
Flow Cytometry
HUVEC apoptosis was analyzed using an apoptosis detection kit (KGA106, KeyGEN BioTECH, China) according to the manufacturer's instruction. The total apoptotic rate was the sum of the late apoptotic rate and the early apoptotic rate.
Statistical Analysis
The data are expressed as the mean ± standard deviation (SD). Survival was compared using the Log rank test. One-way analysis of variance (ANOVA) with Tukey’s post hoc comparisons was performed using GraphPad Prism version 6.0. A p value smaller than 0.05 was determined as significant level (two-tailed).
Results
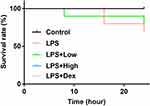
Survival of Septic Mice Was Improved by RUS
The survival rate of LPS group was lower compared mice in Control group, and for septic mice treated with RUS of high dose and Dex, the survival rates clearly increased (Figure 1), indicating that RUS and Dex could decrease death rate of septic mice. However, based on the Log rank test analysis, the difference regarding survival rate between different groups was statistically insignificant (p = 0.1795), which might be attributed to the fact that the septic symptoms induced by LPS method were relatively mild compared with other methods, such as cecal ligation and puncture method.
RUS Attenuated Artery Injury and Suppressed the Production of Cytokines in Septic Mice
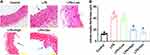
Mice in LPS group showed gross evidence of injuries in the intestinal tissue (Figure 2A), which was attenuated by RUS in both doses. The results of H&E staining provided preliminary evidence for supporting the anti-ED function of the compound. The intima-media thickness was recorded and the results showed that the thickness of LPS group was higher than that of Control group, and the difference was statistically significant (Figure 2B) (p < 0.05). After the administration of RUS at both doses, the thickness was reduced. Moreover, the effect of RUS at high dose was comparable to that of Dex.
The production of serum levels of IL-6, IL-1β, and TNF-α was also induced in LPS group (Figure 3A–C), indicating that the initiation of the inflammatory response in septic mice. RUS suppressed the levels of the cytokines, and the function was also exerted in a dose-dependent manner.
RUS Inhibited miR-146a-5p Level and Induced the Expression Levels of NRP2 and SSH1 in Septic Mice
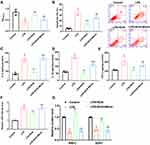
Based on the microarray assay and RT-qPCR validation (Supplementary Materials Tables S1 and S2; and Figure S1), miR-146a-5p and miR-12-5p showed the highest sensitivity to RUS treatment (p = 0.000). The current study selected miR-146a-5p due to its previously proved pro-ED function.17 LPS injection increased miR-146a-5p level (Figure 4A), while decreased the expressions of NRP2 and SSH1 (Figure 4B). In mice co-administrated with RUS, miR-146a-5p expression was inhibited, contributing to the induced levels of NRP2 and SSH1 (Figure 4). Changes in miR-146a-5p mediated-NRP2/SSH1 axis might infer that RUS exerted anti-ED effects by modulating the function of miR-146a-5p/NRP2/SSH1 axis.
RUS Exerted Its Protective Effects in HUVECs Against Sepsis-Induced ED by Inhibiting miR-146a-5p Level
LPS incubation reduced cell viability (Figure 5A), increased apoptosis (Figure 5B), and induced production of cytokines (Figure 5C–E). The effects of LPS on cell phenotypes were associated with the upregulation of miR-146a-5p (Figure 5F), and downregulation of NRP2 and SSH1 (Figure 5G). MiR-146a-5p, and NRP2 and SSH1 levels were reversed by RUS (Figure 5F and G), which contributed to increased cell viability, suppressed apoptosis and cytokine levels.
Afterwards, the level of the miR was induced prior to LPS and RUS treatment (Figure 5F). The increased level of miR-146a-5p suppressed levels of NRP2 and SSH1 (Figure 5G). Moreover, the changes in miR-146a-5p/NRP2/SSH1 after miR-146a-5p induction suppressed cell viability, induced apoptosis, and increased levels of cytokines (Figure 5) even with RUS treatment. Collectively, effects of RUS on LPS-induced septic ED were exerted by inhibiting miR-146a-5p level.
Discussion
ED is closely related to the development of AS and CVD, and causes leading mortality and morbidity worldwide.28 Accumulating studies demonstrate that TCM holds great potential for treating ED.24,29,30 RUS is one of the major functional components of R. aculeatus. In the current study, we used RUS to protect the endothelium against sepsis-induced ED. Our data showed that RUS improved the survival rate and arterial histology in septic mice, as well as increasing viability, inhibiting apoptosis and cytokine levels in LPS-stimulated HUVECs, supporting the anti-ED role of RUS.24,25
To provide a preliminary explanation of the mechanism underlying the effect of RUS, the current study focused on the role of miRs during treatment. The dysregulation of miRs is involved in the progression of ED. For example, miR-181a inhibited activity of NF-κB signaling and suppressed vascular inflammation,31 and miR-31 down-regulated expression of E-selectin and ICAM-1 in TNF-α-treated cells.32 In addition, the dysregulation of miRs is also generally observed during the progression of sepsis: miR-25 was involved in the anti-sepsis effect of ginkgolide A,13 and miR-22-3p was an early diagnostic biomarker for sepsis.14 Thus, the abnormal expression of miRs might connect the initiation of ED with the progression of sepsis. Based on our microarray data, the induced level of miR-146a-5p in LPS-stimulated HUVECs confirmed the pro-ED function of this miR.17 To further explain the mechanism underlying the pro-ED function of miR-146a-5p, the expression of NRP2, a downstream effector of miR-146a-5p, was also detected. It was shown that levels of NRP2 and SSH1 were firstly inhibited by LPS-induced sepsis and then restored by RUS. Functional NRP2 deficiency promotes aortic ED,18 and NRP2-mediated activation of SSH1 also protects the vascular system by triggering PNET.5 Thus, the inhibition of miR-146a-5p by RUS restored the activity of the NRP2/SSH1 axis and ultimately alleviated ED symptoms associated with LPS-induced sepsis. Afterwards, the level of miR-146a-5p was induced to verify its inhibition in the anti-ED effects of RUS. The data showed that the re-induction of miR-146a-5p impaired the effects of RUS on LPS-induced septic ED symptoms.
In conclusion, the protective effects of RUS against LPS-induced septic ED preliminarily explain the mechanism by connecting the function of RUS with the miR-146a-5p/NRP2/SSH1 axis. Since multiple effectors of miR-146a-5p exist, the mechanism mediating anti-sepsis effects of RUS may be complicated. Moreover, based on the microarray detection, levels of multiple miRs were influenced by RUS, but our study only focused on the function of miR-146a-5p. Thus, the current study only provided a preliminary explanation on the mechanism underlying the function of RUS, and further assays and analyses should be performed to promote the understanding of the pathways promoting treatment effects of RUS against disorders associated with LPS-induced sepsis.
Disclosure
The authors disclose no conflicts of interest in this work.
References
1. Shane AL, Stoll BJ. Neonatal sepsis: progress towards improved outcomes. J Infect. 2014;68(Suppl 1):S24–S32. doi:10.1016/j.jinf.2013.09.011
2. Burnham JP, Lane MA, Kollef MH. Impact of sepsis classification and multidrug-resistance status on outcome among patients treated with appropriate therapy. Crit Care Med. 2015;43(8):1580–1586. doi:10.1097/ccm.0000000000001013
3. Lv D, Luo M, Yan J, Yang X, Luo S. Protective effect of sirtuin 3 on CLP-induced endothelial dysfunction of early sepsis by inhibiting NF-κB and NLRP3 signaling pathways. Inflammation. 2021;44:1782–1792. doi:10.1007/s10753-021-01454-7
4. Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43(3):304–377. doi:10.1007/s00134-017-4683-6
5. Levy B, Collin S, Sennoun N, et al. Vascular hyporesponsiveness to vasopressors in septic shock: from bench to bedside. Intensive Care Med. 2010;36(12):2019–2029. doi:10.1007/s00134-010-2045-8
6. Quyyumi AA. Endothelial function in health and disease: new insights into the genesis of cardiovascular disease. Am J Med. 1998;105:32S–39S. doi:10.1016/S0002-9343(98)00209-5
7. Tohru M, Hideyuki M, Toshihiko Y, Issei K. Endothelial cell senescence in human atherosclerosis: role of telomeres in endothelial dysfunction. J Cardiol. 2003;41(1):39–40.
8. Barton M. Obesity and aging: determinants of endothelial cell dysfunction and atherosclerosis. Pflügers Archiv. 2010;460(5):825–837. doi:10.1007/s00424-010-0860-y
9. Oveia J, Stapor P, Carmeliet P. Principles of targeting endothelial cell metabolism to treat angiogenesis and endothelial cell dysfunction in disease. EMBO Mol Med. 2014;6(9):1105–1120. doi:10.15252/emmm.201404156
10. Hainsworth AH, Oommen AT, Bridges LR. Endothelial cells and human cerebral small vessel disease. Brain Pathol. 2015;25(1):44–50. doi:10.1111/bpa.12224
11. Michael G, Guillermo G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ Res. 2016;118:620–636.
12. Vaccarezza M, Balla C, Rizzo P. Atherosclerosis as an inflammatory disease: doubts? No more. IJC Heart Vasculature. 2018;19:1–2. doi:10.1016/j.ijcha.2018.03.003
13. Li J, Chen J, Yang Y, Ding R, Wang M, Gu Z. Ginkgolide A attenuates sepsis-associated kidney damage via upregulating microRNA-25 with NADPH oxidase 4 as the target. Int Immunopharmacol. 2021;95:107514. doi:10.1016/j.intimp.2021.107514
14. Zhang H, Che L, Wang Y, et al. Deregulated microRNA-22-3p in patients with sepsis-induced acute kidney injury serves as a new biomarker to predict disease occurrence and 28-day survival outcomes. Int Urol Nephrol. 2021;53:2107–2116. doi:10.1007/s11255-021-02784-z
15. Poliseno L, Tuccoli A, Mariani L, et al. MicroRNAs modulate the angiogenic properties of HUVECs. Blood. 2006;108(9):3068–3071. doi:10.1182/blood-2006-01-012369
16. Suárez Y, Fernández-Hernando C, Pober JS, Sessa WC. Dicer dependent microRNAs regulate gene expression and functions in human endothelial cells. Circ Res. 2007;100(8):1164–1173. doi:10.1161/01.Res.0000265065.26744.17
17. Shimada BK, Yang Y, Zhu J, et al. Extracellular miR-146a-5p induces cardiac innate immune response and cardiomyocyte dysfunction. Immunohorizons. 2020;4(9):561–572. doi:10.4049/immunohorizons.2000075
18. Mucka P, Levonyak N, Geretti E, et al. Inflammation and lymphedema are exacerbated and prolonged by neuropilin 2 deficiency. Am J Pathol. 2016;186(11):2803–2812. doi:10.1016/j.ajpath.2016.07.022
19. Wu F, Cao J, Jiang J, Yu B, Xu Q. Ruscogenin glycoside (Lm-3) isolated from Liriope muscari improves liver injury by dysfunctioning liver-infiltrating lymphocytes. J Pharm Pharmacol. 2001;53(5):681–688. doi:10.1211/0022357011775802
20. Kou J, Sun Y, Lin Y, et al. Anti-inflammatory activities of aqueous extract from Radix Ophiopogon japonicus and its two constituents. Biol Pharm Bull. 2005;28(7):1234–1238. doi:10.1248/bpb.28.1234
21. Kou J, Tian Y, Tang Y, Yan J, Yu B. Antithrombotic activities of aqueous extract from Radix Ophiopogon japonicus and its two constituents. Biol Pharm Bull. 2006;29(6):1267–1270. doi:10.1248/bpb.29.1267
22. Sun Q, Chen L, Gao M, et al. Ruscogenin inhibits lipopolysaccharide-induced acute lung injury in mice: involvement of tissue factor, inducible NO synthase and nuclear factor (NF)-κB. Int Immunopharmacol. 2012;12(1):88–93. doi:10.1016/j.intimp.2011.10.018
23. Wang Y, Xue L, Wu Y, et al. Ruscogenin attenuates sepsis-induced acute lung injury and pulmonary endothelial barrier dysfunction via TLR4/Src/p120-catenin/VE-cadherin signalling pathway. J Pharm Pharmacol. 2021;73:893–900. doi:10.1093/jpp/rgaa039
24. Cao G, Jiang N, Hu Y, et al. Ruscogenin attenuates cerebral ischemia-induced blood-brain barrier dysfunction by suppressing TXNIP/NLRP3 inflammasome activation and the MAPK pathway. Int J Mol Sci. 2016;17(9):1418. doi:10.3390/ijms17091418
25. Bi LQ, Zhu R, Kong H, et al. Ruscogenin attenuates monocrotaline-induced pulmonary hypertension in rats. Int Immunopharmacol. 2013;16(1):7–16. doi:10.1016/j.intimp.2013.03.010
26. Zhan L, Pu J, Zheng J, et al. Tetrastigma hemsleyanum Diels et Gilg ameliorates lipopolysaccharide induced sepsis via repairing the intestinal mucosal barrier. Biomed Pharmacother. 2022;148:112741. doi:10.1016/j.biopha.2022.112741
27. Chekanov V. Low frequency electrical impulses reduce atherosclerosis in cholesterol fed rabbits. Med Sci Monit. 2003;9(8):Br302–9.
28. Libby P, Bornfeldt KE, Tall AR. Atherosclerosis: successes, surprises, and future challenges. Circ Res. 2016;118:531–534.
29. Wang TZ, Chen Y, He YM, et al. Effects of Chinese herbal medicine Yiqi Huaju Qingli Formula in metabolic syndrome patients with microalbuminuria: a randomized placebo-controlled trial. J Integr Med. 2013;11(3):175–183. doi:10.3736/jintegrmed2013032
30. Yang TY, Wei JC, Lee MY, Chen CB, Ueng KC. A randomized, double-blind, placebo-controlled study to evaluate the efficacy and tolerability of Fufang Danshen (Salvia miltiorrhiza) as add-on antihypertensive therapy in Taiwanese patients with uncontrolled hypertension. Phytother Res. 2011;26:291–298.
31. Su Y, Yuan J, Zhang F, et al. MicroRNA-181a-5p and microRNA-181a-3p cooperatively restrict vascular inflammation and atherosclerosis. Cell Death Dis. 2019;10(5):365. doi:10.1038/s41419-019-1599-9
32. Yin Y, Li F, Shi J, Li S, Cai J, Jiang Y. MiR-146a regulates inflammatory infiltration by macrophages in polymyositis/dermatomyositis by targeting TRAF6 and affecting IL-17/ICAM-1 pathway. Cell Physiol Biochem. 2016;40(3–4):486–498. doi:10.1159/000452563
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.