Back to Journals » Infection and Drug Resistance » Volume 13
Role Of Vitamin-D Supplementation In TB/HIV Co-Infected Patients
Authors Ayelign B , Workneh M , Molla MD , Dessie G
Received 22 August 2019
Accepted for publication 28 October 2019
Published 10 January 2020 Volume 2020:13 Pages 111—118
DOI https://doi.org/10.2147/IDR.S228336
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Eric Nulens
Birhanu Ayelign, 1 Meseret Workneh, 1 Meseret Derbew Molla, 2 Gashaw Dessie 2
1Department of Immunology and Molecular Biology, School of Biomedical And Laboratory Sciences, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia; 2Department of Biochemistry, School of Medicine, College of Medicine And Health Sciences, University of Gondar, Gondar, Ethiopia
Correspondence: Birhanu Ayelign Email [email protected]
Objective: This review aimed to assess the role of vitamin D supplementation on the decrement of mortality and morbidity rate among tuberculosis (TB)/human immune deficiency virus (HIV) co-infected clients.
Method: Pub Med, google scholar and google search were accessed to find out all document to describe this review article.
Results: Nowadays TB/HIV co-infection has become a major global concern, particularly in low and middle-income countries. Mycobacterium tuberculosis and HIV infections are co-endemic and more susceptible to the progression of TB. Immunosuppression associated with HIV is a strong risk factor for the reactivation of latent TB to the active form. Immune cells like macrophages recognized Mycobacterium tuberculosis through TLR2/1, and it increases the expression of the vitamin D receptor (VDR) and CYP27B1. The synthesis of 1,25-dihydroxy vitamin D promotes VDR-mediated transactivation of the antimicrobial peptide cathelicidin and the killing of intracellular Mycobacterium tuberculosis. Cathelicidins have a direct antimicrobial effect through membrane disruption. Besides, it has also antiviral effects via inhibition of retrovirus (HIV) replication. In fact, as some studies showed, there was a lower induction of cathelicidin in monocytes who have low vitamin D levels.
Conclusion: Therefore, vitamin D supplementation can be directly involved in the reduction of TB/HIV co-infection and its progression.
Keywords: vitamin D, tuberculosis, HIV
Background
Human immunodeficiency virus (HIV) disease is one of the main risks of developing tuberculosis (TB) disease.1,2 Tuberculosis is the leading cause of death in HIV-infected individuals in Africa.3 Globally in 2017, the best estimate is that 10.0 million people (9.0–11.1 million) developed TB disease, 9% having TB/HIV co-infection. In 2017, TB caused an estimated 1.6 million deaths and there were 300 000 deaths from TB/HIV co-infection.4 TB/HIV co-infection accelerates the sudden deterioration of the immune system that causes premature death.
 |
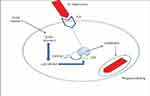
Figure 1 The metabolism of vitamin D. |
 |
Table 1 Sputum Conversions By Vit-D And Placebo Administration. |
In sub-Saharan African countries up to 70% of HIV-positive patients are estimated to be co-infected with Mycobacterium tuberculosis(Mtb). The immune suppression associated with HIV infection is an aggravating factor for the development of latent tuberculosis to vigorous disease and finally leads to death.5 Human immunodeficiency virus infection has contributed to a doubling of the number of tuberculosis cases in some African countries during the past decade. Thus, the double burden due to co-infection increased difficulties in tuberculosis diagnosis, an increased frequency of the treatment side-effects, and higher relapse and reinfection rates.6 Even though we are unable to understand the exact mechanism of TB/HIV co-infection, they have a positive correlation to one another.7
The management of HIV and TB co-infection is challenging and usually associated with favorable treatment outcome. The emergency and dissemination of drug-resistant Mtb strains contribute to its epidemicity. In addition to drug resistance strains, both clinical and public health management problems are other reasons. Some studies showed that HIV testing and drug susceptibility (DST) capacity for MtB is robust, 40–80% of TB patients with multidrug-resistant and extensive drug resistance TB are HIV infected.8
As a result, vitamin D (Vit-D) supplementation can overcome the challenge and inhibit HIV and replication and growth. Cathelicidin, which is part of the innate immune system, plays a critical role in the fight against TB. Mycobacterium tuberculosis binds to toll-like receptors (TLR2/1) on macrophages and leads to upregulation of 1α-hydroxylase gene expression. Intracellular 1,25(OH)2D binds to the vitamin D receptor to activate production of cathelicidin that has antimicrobial effects. In the same way, Vit-D also activates T helper cells through activation of VDR gene-encoded PLC-y, which also results in upregulation of PLC-y1 by 75-fold for good activation of the TCR.9
The Immunological Interaction Between Mycobacterium Tuberculosis And HIV Infection
Immunocompetent individuals have a 5–10% chance to develop active TB in a lifetime, but patients with HIV have a chance to develop TB of up to 15% per year. Epidemiological studies showed that the risk of TB increases in HIV-1-infected people due to a decline in CD4 count and advanced clinical stages of disease (WHO stages 3/4). Although unsuppressed HIV-1 viral load during ART treatment has been linked with increased risk of TB in a large retrospective study, others have not shown it to be an independent risk factor.10–12
The initial cellular targets of HIV-1 infection are the mucosal CD4 T cells, dendritic cells, and local macrophages; final results are the depletion of these cells. The exhaustion of CD4+ T cells, which is the most important characteristic of HIV/AIDS, is entirely a significant contributor to the increased risk of reactivation of latent TB and vulnerability to new infection. The other mechanisms are also that in HIV-positive individuals there is upregulation of Mtb receptors on the surface of macrophages,19 inhibition of the macrophage bacterial killing pathway, deregulated chemotaxis, and a polarization of the Th1/Th2 balance facilitating Mtb infection.13 In the same way, HIV inhibits tumor necrosis factor (TNF)-mediated macrophage apoptotic response to Mtb and results in survival of the bacteria. Activation of infection results from an alteration of protection and/or crosstalk between adaptive and innate immune responses.13
CD4+ T cells and TNF are essential for granuloma organization maintenance. In people with a compromised immune system, the formation of granuloma may decrease. This was connected with the killing of CD4+ cells in the granuloma, which may lead to a direct disturbance of the granuloma structure and may boost the risk of disease dissemination.14,15
The immune protection against viral load becomes weak following infection with Mtb; rather, it accelerates progression to an advanced stage of AIDS. Unless it is treated, the probability of death is very high as compared to HIV clients who are not infected with TB.1 This was also supported by researchers; the replication of HIV in immune cells (lung cells and macrophages) for co-infected patients becomes fast resulting in elevation of plasma viral loads. This shows that mycobacteria aggravate the coping of HIV.16,17 Other researchers also revealed that the replication and transmission of HIV toward non-infected cells is positively controlled by Mtb in monocyte-derived macrophages (MDMs). Mainly, it facilitates the copying of X4 HIV by upregulation of CXCR4.18 Moreover, in vitro, the maturation of DCs and MDMs is compromised because of impairment of monocyte response to TLR ligands and viral proteins in co-infected patients.19,20 The TNF produced from infection is necessary to inhibit bacterial growth and activate HIV replication in macrophages.19
Moreover, researchers proposed that TB patients have a micro-environment that promotes HIV infection through increased expression of co-receptors CXCR4 and CCR5. In addition, increased pro-inflammatory cytokines, mainly TNF-α and CCL5 downregulation, enhance the replication and progression of HIV/AIDS.18 It has also been demonstrated that Mtb and its lipoarabinomannan (LAM) cell wall element can activate HIV provirus-carrying cell replication through the induction of TNF and IL-6 production. Tumor necrosis factor and IL-6 are generated via the NF-κB pathway, which in turn causes the long terminal repeat (LTR) promoter transcriptional activation and promotes HIV replication.19
Role Of Vitamin D On TB/HIV Co-Infection
Before the foundation of antibiotics, skin TB was treated effectively with UV light. It was also true that by the 1920s, repeated sunlight exposure was used as a main option to treat pulmonary TB. Thus, conditions give insight for the researchers to investigate the role of Vit-D on the antimicrobial effect, including Mtb. Vitamin D has a direct effect on both calcium and phosphorus homeostasis.21 Furthermore, it has a critical role in the innate immune response through the synthesis of host defense peptide, particularly cathelicidin.22,23 Sunlight exposure is the predominant factor in the activation of pre-vitamin D3 (7-dehydrocholesterol) found in skin into its active form. In addition to sunlight, Vit-D can also be gained from foods such as fish oil (Figure 1).22,23
The ability of Vit-D to manage infections and the autoimmune system is becoming a novel thought in the management of disease.22 The biochemical mechanisms of the immune-regulatory effect of Vit-D against microorganisms are not still clearly investigated. However, the impact of Vit-D as an anti-infective agent through modulation of host immune responses has been basically studied in vitro.24
The immunomodulatory consequences of Vit-D have been widely investigated, even before the detection of the cell-mediated antimicrobial mechanism of Vit-D.22 The molecular link between Vit-D and the immune system was initially recognized in the early 1980s. Vitamin D receptors on the surface of monocytes and macrophages synthesize 1,25(OH)2D3 and 1,25(OH)2D3, which in turn enhance separation of monocytes into macrophage-like cells having phagocytic, lysozyme, and migration activity.25,26
Even if various studies have been done to assess the effect of Vit-D on infection clearance through mouse models, there is no clear decision on it.24 For IFN-γ stimulated reaction in monocytic cells, such as conversion of 25(OH)D to active 1,25(OH)2D, VDR activation, and induction of host defense peptides including cathelicidin and defensins, Vit-D has been shown to be crucial to the control of TB/HIV co-infection.27
Mycobacterium tuberculosis-derived macrophage stimulation induces VDR expression and CYP27B1 enzyme, and inhibits Mtb in vitro mainly by inducing innate immune responses that cause cathelicidin LL-37 synthetic antibacterial peptide. A notable feature of the cathelicidin family is that the number of different genes varies greatly among species; however, humans express only one cathelicidin-hCAP.28,29
The activation of TLRs on macrophages by mycobacterial lipoprotein augments cathelicidin synthesis in cells treated with 25-dihydroxy vitamin D through the increased expression of vitamin D receptors (VDRs), and the enzyme that converted circulating 25-hydroxyvitamin D into biologically active 1,25-dihydroxy vitamin D.30,31
The binding of PAMPs to TLRs increases the number of VDRs within the cell nucleus and activates the enzyme cytochrome P450, CYP27B1, which converts circulating 25-hydroxyvitamin D into the biologically active 1,25-dihydroxy vitamin D. As a result, supplementation increases cathelicidin production by monocytes.32 This showed that Vit-D supplementation can have a direct effect on TB/HIV co-infection and initiated us to review it (Figure 2).
Effects Of Vitamin D Deficiency On TB/HIV Co-Infection
Currently, Vit-D deficiency becomes a global public health problem.33 Although there are no clear internationally confirmed definitions of Vit-D deficiency, 25(OH)D concentration 50 nmol/L, or 20 mg/mL, is an indication of Vat-D deficiency.33,34 Low Vit-D levels in HIV-1-infected persons are associated with more rapid disease progression and increased risk of getting opportunistic infection including TB.35 TB/HIV co-infection patients frequently have lower Vit-D levels than the general population. A study conducted in Spain indicated that the majority of TB/HIV co-infected patients had lower serum 25(OH)D levels, which indicates that sufficient 25(OH)D levels protect against infection and vitamin D deficiency is considered a risk factor for TB/HIV co-infection.36
50 nmol/L, or 20 mg/mL, is an indication of Vat-D deficiency.33,34 Low Vit-D levels in HIV-1-infected persons are associated with more rapid disease progression and increased risk of getting opportunistic infection including TB.35 TB/HIV co-infection patients frequently have lower Vit-D levels than the general population. A study conducted in Spain indicated that the majority of TB/HIV co-infected patients had lower serum 25(OH)D levels, which indicates that sufficient 25(OH)D levels protect against infection and vitamin D deficiency is considered a risk factor for TB/HIV co-infection.36
Effects Of Vitamin D Supplementation On TB/HIV Co-Infection
Vitamin D has been attributed to host immune defense against TB/HIV co-infection. The addition of Vit-D in the treatment of moderately advanced PTB and HIV has confirmed the presence of significant difference in sputum conversion as compared to the placebo group. The study done in Jakarta found the two groups treated with Vit-D with greater sputum conversion and radiological enhancement (100%) compared to the placebo group (76.7%), which was statistically important (p=0.002) (Table 1).24
Vitamin D supplementation and anti-TB treatment among black American women having Vit-D deficiency and unmanageable drug-susceptible pulmonary TB may have a positive effect. At 13 months of complete treatment, the patient showed a substantial improvement in radiography with negative sputum cultures.37 An Egyptian study of the administration of Vit-D in children with TB showed that clinical improvement was seen among patients who take Vit-D as compared to those who received treatment alone. Moreover, the study concluded that Vit-D therapy may be very effective in addition to anti-tuberculous drugs in the treatment of TB children.38
Another study showed that the human cathelicidin antimicrobial peptide (CAMP) is required for both 1,25D3-mediated antimycobacterial activity and 1,25D3-mediated autophagy in human macrophages; hence, the role of CAMP in 1,25D3-induced antimicrobial activity was investigated. Moreover, related to our conclusion, autophagy is necessary in favor of the 1,25D3 restriction of HIV replication and antimycobacterial activities. On the other hand, CAMP silencing also reduces the 1,25D3-mediated inhibition of HIV and Mtb in co-infected cells. Collectively, those conditions showed that physiological concentrations of 1,25D3 can act as a potent stimulator of innate antimicrobial responses that can induce autophagy and overcome the HIV-imposed autophagosome maturation block by a CAMP-dependent system that constrains HIV replication and causes mycobacterial destruction (Figure 3).
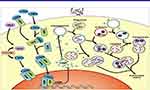 |
Figure 3 The role of autophagy and CAMP in 1,25D3-mediated inhibition of Mycobacterium tuberculosis (Mtb) and HIV. HIV budding occurs into the multivesicular endosomes of macrophages. Mtb enters through phagocytosis. Cytochrome p450, family 27, subfamily B, polypeptide 1 (CYP27B1) 1α-hydroxyls the inactive 25D3 into the active 1,25D3. 1,25D3 induces the expression of camp, presumably by binding to the Vit-D (1,25D3) receptor (VDR), which heterodimerizes with the retinoid X receptor (RXR) and directly regulates transcription by binding to the vitamin D response element (VDRE) consensus sequence located upstream of the Camp gene. The expression of camp is required both for autophagosome and phagolysosome biogenesis, which leads to killing of the microbial pathogens through autophagy. Adapted from Jo EK. Innate immunity to mycobacteria: vitamin D and autophagy. Cell Microbiol. 2010;12(8):1026-1035. © 2010 Blackwell Publishing Ltd.39 |
HIV budding occurs into the multivesicular endosomes of macrophages. Mycobacterium tuberculosis enters through phagocytosis. Cytochrome P450, family 27, subfamily B, polypeptide 1 (CYP27B1) 1α-hydroxyls the 25D3 inactive form into the 1,25D3 active form. It induces the expression of CAMP presumably through binding to the Vit-D (1,25D3) receptor (VDR) that heterodimerizes with the retinoid X receptor (RXR) and directly regulates transcription by binding to the vitamin D response element (VDRE) consensus sequence located upstream of the Camp gene. The expression of CAMP is required both for autophagosome and phagolysosome biogenesis, which leads to killing of the microbial pathogens through autophagy.39
Moreover, HIV transcription will be regulated through Vit-D supplementation. Cytokine and chemokine regulation by Vit-D has an effect not only on the inflammation of TB, but also on the replication of HIV-1. As some researchers indicated that the possible autophagy-mediated antimicrobial action of Vit-D on HIV-1 replication, the inhibition of TNF, IL-6, and CCL2 secretion by Vat-D and its metabolites both in vivo and in vitro diminishes HIV-1replication, through inhibiting NF-κB-mediated HIV-1 transcription.38,40 Recently, 1,25(OH)2D3 has also been discovered to control the expression of microRNA to induce miRNA-22, which targets NFAT5.40 Since this transcription factor has been regulated in macrophages for HIV-1 LTR transcription, miRNA-22 induction may be an additional mechanism of Vit-D to inhibit HIV-1 replication.41
Anti-retroviral therapy is effective in reducing HIV viral load and improving the count of CD4 cells, these findings indicate that Vit-D supplementation may be capable of targeting the tissue viral reservoirs. Thus, ARV is unable to eliminate and decline the risk of TB in HIV-1-infected persons. In line with this, a recent prospective study of TB occurrence in HIV-1-infected individuals also demonstrates that Vit-D deficiency was significantly related to increased all-cause fatality compared to Vit-D insufficiency.34
Vitamin D And TB/HIV Immune Reconstitution Inflammatory Syndrome
The complications related to antiretroviral (ARV) initiation in people infected with HIV-1 along with other opportunistic infections are Mtb and Pneumocystis carinii, which cause the formation of immune reconstitution inflammatory syndrome (IRIS).42 Paradoxical TB-IRIS happens in patients with TB who are clinically improved with anti-TB treatment and start to create ARV paradoxically.43
A cross-sectional study showed that patients with TB-IRIS have a greater frequency of hyper-inflammatory profiles derived from myeloid such as TNF, IL-1β, IL-6, IL-8, IL-2, IL-10, IL-12p40, IFN-γ, and GM-CSF. Similarly, high numbers of MMP-1, MMP-3, MMP-7, and MMP-10-secreting T cells and macrophages recognize antigens, but the frequency of cells is lower in patients who did not develop IRIS and were treated with ART and antituberculosis.44 However, in the longitudinal assessment of ARV initiation over the last 8 weeks, regardless of the development of IRIS all patients have significant changes in the frequency of pathogen-specific IFN-γ-secreting T cells, implying that modifications are not contributory.44 This indicates they develop due to overstimulation of innate immune cells with antigens, which may be inhibited through Vit-D.45 Therefore, Vit-D can have a positive effect on the reduction of bacterial and viral load through improvement of regulatory T-cell function. Thus, Vit-D inhibits a wide range of proinflammatory cytokines, the production of MMP, and could be a more effective therapy for IRIS prevention and adverse effects.44,46,47
In conclusion, deficiency of Vit-D has been associated with increased risk of active tuberculosis and HIV disease progression and susceptibility. Vitamin D supplementation in TB/HIV co-infection inhibits the growth and replications of Mtb and HIV through production of cathelicidin, which also induces autophagic flux. This review may help clinicians consider Vit-D supplementation to the treatment protocols which may improve outcome. It will also form a basis for clinical trials to establish the role of Vit-D in HIV/TB co-infection. It will also open avenues of research on vitamin D in other infectious diseases.
Abbreviations
AIDS, Acquired Immune Deficiency Syndrome; ARV, Antiretroviral; CAMP, Cathelicidin Antimicrobial Peptide; CD, Cluster Differentiation; HIV, Human Immunodeficiency Virus; MDM, Monocyte-Derived Macrophage; Mtb, Mycobacterium tuberculosis; Vit-D, Vitamin D.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests in this work.
References
1. WHO. Global Tuberculosis Control: Surveillance, Planning, Financing: WHO Report 2008. World Health Organization; 2008.
2. Amelio P, Portevin D, Hella J, et al. HIV infection functionally impairs Mycobacterium tuberculosis-specific CD4 and CD8 T-cell responses. J Virol. 2019;93(5):e01728–e017218. doi:10.1128/JVI.01728-18
3. Campbell GR, Spector SA. Autophagy induction by vitamin D inhibits both Mycobacterium tuberculosis and human immunodeficiency virus type 1. Autophagy. 2012;8(10):1523–1525. doi:10.4161/auto.21154
4. WHO. Global Tuberculosis Report 2018; 2018. Geneva:World Health Organization. Licence: CC BY-NC-SA 3.0. IGO. WHO/CDS/TB/2018.20. Available from. http://apps.who.int/iris/bitstream.
5. Harries A, Zachariah R, Lawn S. Providing HIV care for co-infected tuberculosis patients: a perspective from sub-Saharan Africa [State of the art series. Tuberculosis. Edited by ID Rusen. Number 3 in the series]. Int J Tuberculosis Lung Dis. 2009;13(1):6–16.
6. Godfrey-Faussett P, Ayles H. The impact of HIV on tuberculosis control–towards concerted action. Lepr Rev. 2002;73(4):376–385.
7. Modjarrad K, Vermund SH. Effect of treating co-infections on HIV-1 viral load: a systematic review. Lancet Infect Dis. 2010;10(7):455–463. doi:10.1016/S1473-3099(10)70093-1
8. O’Donnell MR, Daftary A, Frick M, et al. Re-inventing adherence: toward a patient-centered model of care for drug-resistant tuberculosis and HIV. Int J Tuberculosis Lung Dis. 2016;20(4):430–434. doi:10.5588/ijtld.15.0360
9. Nansera D, Graziano F, Friedman D, Bobbs M, Jones A, Hansen K. Vitamin D and calcium levels in Ugandan adults with human immunodeficiency virus and tuberculosis. Int J Tuberculosis Lung Dis. 2011;15(11):1522–1528. doi:10.5588/ijtld.10.0701
10. Houben R, Crampin A, Ndhlovu R, et al. Human immunodeficiency virus associated tuberculosis more often due to recent infection than reactivation of latent infection. Int J Tuberculosis Lung Dis. 2011;15(1):24–31.
11. Crampin AC, Mwaungulu JN, Mwaungulu FD, et al. Recurrent TB: relapse or reinfection? The effect of HIV in a general population cohort in Malawi. AIDS. 2010;24(3):417. doi:10.1097/QAD.0b013e32832f51cf
12. Gupta A, Wood R, Kaplan R, Bekker L-G, Lawn SD. Tuberculosis incidence rates during 8 years of follow-up of an antiretroviral treatment cohort in South Africa: comparison with rates in the community. PLoS One. 2012;7(3):e34156. doi:10.1371/journal.pone.0034156
13. Patel NR, Zhu J, Tachado SD, et al. HIV impairs TNF-α mediated macrophage apoptotic response to Mycobacterium tuberculosis. J Immunol. 2007;179(10):6973–6980. doi:10.4049/jimmunol.179.10.6973
14. Ignatowicz L. Studies on the Host Immune Response during Pulmonary TB and during M. Tuberculosis/HIV Co-Infection: Inst För Mikrobiologi, Tumör-Och Cellbiologi/Dept of Microbiology, Tumor and Molecular biology; 2012.
15. Diedrich CR, Flynn JL. HIV-1/mycobacterium tuberculosis coinfection immunology: how does HIV-1 exacerbate tuberculosis? Infect Immun. 2011;79(4):1407–1417. doi:10.1128/IAI.01126-10
16. Singh SK, Larsson M, Schön T, Stendahl O, Blomgran R. HIV interferes with the dendritic cell–T cell axis of macrophage activation by shifting Mycobacterium tuberculosis–specific CD4 T cells into a dysfunctional phenotype. J Immunol. 2019;202(3):816–826. doi:10.4049/jimmunol.1800523
17. Dezzutti CS, Hladik F. Use of human mucosal tissue to study HIV-1 pathogenesis and evaluate HIV-1 prevention modalities. Curr HIV/AIDS Rep. 2013;10(1):12–20. doi:10.1007/s11904-012-0148-2
18. Jiang W, Lederman MM, Salkowitz JR, Rodriguez B, Harding CV, Sieg SF. Impaired monocyte maturation in response to CpG oligodeoxynucleotide is related to viral RNA levels in human immunodeficiency virus disease and is at least partially mediated by deficiencies in alpha/beta interferon responsiveness and production. J Virol. 2005;79(7):4109–4119. doi:10.1128/JVI.79.7.4109-4119.2005
19. Muthumani K, Hwang DS, Choo AY, et al. HIV-1 Vpr inhibits the maturation and activation of macrophages and dendritic cells in vitro. Int Immunol. 2004;17(2):103–116. doi:10.1093/intimm/dxh190
20. Bell LC, Noursadeghi M. Pathogenesis of HIV-1 and Mycobacterium tuberculosis co-infection. Nature Rev Microbiol. 2018;16(2):80. doi:10.1038/nrmicro.2017.128
21. Wimalawansa S. Biology of vitamin D. J Steroids Horm Sci. 2019;10(198):2.
22. Bartley J. Vitamin D: emerging roles in infection and immunity. Expert Rev Anti Infect Ther. 2010;8(12):1359–1369. doi:10.1586/eri.10.102
23. Cannell J, Hollis B, Zasloff M, Heaney R. Diagnosis and treatment of vitamin D deficiency. Expert Opin Pharmacother. 2008;9(1):107–118. doi:10.1517/14656566.9.1.107
24. Larcombe L, Orr P, Turner-Brannen E, Slivinski CR, Nickerson PW, Mookherjee N. Effect of vitamin D supplementation on Mycobacterium tuberculosis-induced innate immune responses in a Canadian Dene First Nations cohort. PLoS One. 2012;7(7):e40692. doi:10.1371/journal.pone.0040692
25. Mangelsdorf DJ, Koeffler HP, Donaldson CA, Pike JW, Haussler MR. 1, 25-Dihydroxyvitamin D3-induced differentiation in a human promyelocytic leukemia cell line (HL-60): receptor-mediated maturation to macrophage-like cells. J Cell Biol. 1984;98(2):391–398. doi:10.1083/jcb.98.2.391
26. Jones G, Prosser DE, Kaufmann M. The activating enzymes of vitamin D metabolism (25-and 1α-hydroxylases). Vitamin D. 2018;57–79.
27. Fabri M, Stenger S, Shin D-M, et al. Vitamin D is required for IFN-γ–mediated antimicrobial activity of human macrophages. Sci Transl Med. 2011;3(104):104ra2–ra2. doi:10.1126/scitranslmed.3003045
28. Zhao C, Liu L, Lehrer RI. Identification of a new member of the protegrin family by cDNA cloning. FEBS Lett. 1994;346(2–3):285–288.
29. Wang J, Wong ES, Whitley JC, et al. Ancient antimicrobial peptides kill antibiotic-resistant pathogens: Australian mammals provide new options. PLoS One. 2011;6(8):e24030. doi:10.1371/journal.pone.0024030
30. Fiske CT, Blackman A, Maruri F, et al. Increased vitamin D receptor expression from macrophages after stimulation with M. tuberculosis among persons who have recovered from extrapulmonary tuberculosis. BMC Infect Dis. 2019;19(1):366. doi:10.1186/s12879-019-3958-7
31. White JH. Vitamin D as an inducer of cathelicidin antimicrobial peptide expression: past, present and future. J Steroid Biochem Mol Biol. 2010;121(1–2):234–238. doi:10.1016/j.jsbmb.2010.03.034
32. Liu PT, Stenger S, Tang DH, Modlin RL. Cutting edge: vitamin D-mediated human antimicrobial activity against Mycobacterium tuberculosis is dependent on the induction of cathelicidin. J Immunol. 2007;179(4):2060–2063. doi:10.4049/jimmunol.179.4.2060
33. Palacios C, Gonzalez L. Is vitamin D deficiency a major global public health problem? J Steroid Biochem Mol Biol. 2014;144:138–145. doi:10.1016/j.jsbmb.2013.11.003
34. Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr. 2008;87(4):1080S–1086S. doi:10.1093/ajcn/87.4.1080S
35. Campbell GR, Spector SA, Deretic V. Vitamin D inhibits human immunodeficiency virus type 1 and Mycobacterium tuberculosis infection in macrophages through the induction of autophagy. PLoS Pathog. 2012;8(5):e1002689. doi:10.1371/journal.ppat.1002689
36. Dini C, Bianchi A. The potential role of vitamin D for prevention and treatment of tuberculosis and infectious diseases. Annali dell’Istituto Superiore Di Sanita. 2012;48:319–327. doi:10.4415/ANN_12_03_13
37. Yamshchikov AV, Oladele A, Leonard MK
38. Morcos M, Gabr A, Samuel S, Kamel M, Michail R. Vitamin D administration to tuberculous children and its value. Boll Chim Farm. 1998;137(5):157–164.
39. Jo EK. Innate immunity to mycobacteria: vitamin D and autophagy. Cell Microbiol. 2010;12(8):1026–1035. doi:10.1111/j.1462-5822.2010.01491.x
40. Coussens AK, Martineau AR, Wilkinson RJ. Anti-inflammatory and antimicrobial actions of vitamin D in combating TB/HIV. Scientifica. 2014;2014.
41. Ranjbar S, Jasenosky LD, Chow N, Goldfeld AE. Regulation of Mycobacterium tuberculosis-dependent HIV-1 transcription reveals a new role for NFAT5 in the toll-like receptor pathway. PLoS Pathog. 2012;8(4):e1002620. doi:10.1371/journal.ppat.1002620
42. Narendran G, Andrade BB, Porter BO, et al. Paradoxical tuberculosis immune reconstitution inflammatory syndrome (TB-IRIS) in HIV patients with culture confirmed pulmonary tuberculosis in India and the potential role of IL-6 in prediction. PLoS One. 2013;8(5):e63541. doi:10.1371/journal.pone.0063541
43. Meintjes G, Lawn SD, Scano F, et al. Tuberculosis-associated immune reconstitution inflammatory syndrome: case definitions for use in resource-limited settings. Lancet Infect Dis. 2008;8(8):516–523. doi:10.1016/S1473-3099(08)70184-1
44. Meintjes G, Wilkinson KA, Rangaka MX, et al. Type 1 helper T cells and FoxP3-positive T cells in HIV–tuberculosis-associated immune reconstitution inflammatory syndrome. Am J Respir Crit Care Med. 2008;178(10):1083–1089. doi:10.1164/rccm.200806-858OC
45. Conesa-Botella A, Meintjes G, Coussens AK, et al. Corticosteroid therapy, vitamin D status, and inflammatory cytokine profile in the HIV-tuberculosis immune reconstitution inflammatory syndrome. Clin Infect Dis. 2012;55(7):1004–1011. doi:10.1093/cid/cis577
46. Tadokera R, Meintjes G, Skolimowska KH, et al. Hypercytokinaemia accompanies HIV–tuberculosis immune reconstitution inflammatory syndrome. Eur Respir J. 2011;37(5):1248–1259. doi:10.1183/09031936.00091010
47. Tadokera R, Meintjes GA, Wilkinson KA, et al. Matrix metalloproteinases and tissue damage in HIV‐tuberculosis immune reconstitution inflammatory syndrome. Eur J Immunol. 2014;44(1):127–136. doi:10.1002/eji.201343593
48. Nursym EW, Amin Z, Rumende CM. The effect of Vitamin D as supplmentary treatment in patients with moderately advanced pulmonary tuberclosis lesion. Acta Med Indones. 2006;38(1):3–5.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.