Back to Journals » Cancer Management and Research » Volume 11
Role of the pretreatment neutrophil-to-lymphocyte ratio in the survival of primary parotid cancer patients
Authors Cheng G, Liu F, Niu X, Fang Q
Received 21 November 2018
Accepted for publication 4 February 2019
Published 21 March 2019 Volume 2019:11 Pages 2281—2286
DOI https://doi.org/10.2147/CMAR.S195413
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Harikrishna Nakshatri
Guangyan Cheng,1 Fei Liu,1 Xinyu Niu,1 Qigen Fang2
1Department of Oral Medicine, Stomatology Center, The First affiliated hospital of Zhengzhou University, Zhengzhou, People’s Republic of China; 2Department of Head Neck and Thyroid, The Affiliated Cancer Hospital of Zhengzhou University, Henan Cancer Hospital, Zhengzhou, People’s Republic of China
Background: To analyze the value of the pretreatment neutrophil-to-lymphocyte ratio (NLR) in the survival of patients with parotid cancer.
Methods: In total, 249 patients were enrolled. Information including age, sex, pretreatment NLR, and pathologic variables such as, tumor stage, intraparotid node (IPN) metastasis, and follow-up findings was extracted and analyzed.
Results: IPN metastasis was noted in 45 (18.1%) patients, and the mean NLR was 2.48, with a range from 1.5 to 6.1. The NLR was significantly associated with tumor stage, disease stage, and disease grade. A total of 73 patients died of the disease, and the 10 -year disease-specific survival (DSS) rate was 62%. In patients with an NLR<2.48, the 10 -year DSS rate was 68%; in patients with an NLR≥2.48, the 10 -year DSS rate was 58%, and the difference was significant (P=0.006). Cox model analysis showed that the NLR was an independent prognostic factor for DSS.
Conclusion: The long-term survival of primary parotid cancer patients is relatively favorable, and the pretreatment NLR is significantly associated with prognosis.
Keywords: parotid cancer, intraparotid node metastasis, neutrophil-to-lymphocyte ratio, prognosis
Introduction
Interactions between the tumor microenvironment and tumor cells have important roles in cancer progression, and the microenvironment includes, metabolic, inflammatory, and immune responses to stimuli from the surrounding tissue. Recent basic studies have showed that tumor metastasis, microvascular regeneration, and tumor cell proliferation abilities are promoted by the systemic inflammatory response.1–3 The peripheral neutrophil-to-lymphocyte ratio (NLR) is a well-known inflammatory marker. Several authors have previously reported that a high NLR is significantly associated with poor survival in solid cancers,4–14 including head and neck squamous cell carcinoma and breast cancer. However, whether the NLR affects prognosis in patients with parotid cancer remains unknown; therefore, the current study aimed to clarify this question.
Patients and methods
The Zhengzhou University Institutional Research Committee approved our study. All participants signed an informed consent agreement for medical research before initial treatment, and all experiments were performed in accordance with relevant guidelines and regulations. This study was conducted in accordance with the Declaration of Helsinki.
From January 2002 to December 2016, the surgical medical records of all patients (>18 years old) diagnosed with primary parotid cancer were retrospectively included. All related data, including age, sex, tumor stage, neck stage, pretreatment NLR, postoperative pathological report, operation record, adjuvant treatment, and follow-up information, of the enrolled patients were extracted and analyzed. All pathologic sections were re-reviewed. The tumor grade was defined according to the WHO 2017 classification, and the tumor stage was defined based on the AJCC eighth Edition staging system. In our cancer center, preoperative ultrasound and CT or MRI were routinely performed. In addition, frozen sectioning of the primary tumor was routinely performed; if the pathology was malignant, a total parotidectomy was performed.
The NLR was defined as the absolute neutrophil count divided by the absolute lymphocyte count within 2 weeks before the initial treatment.4–14 The cutoff value calculated from the ROC curve, mean, tertile, or median in previous studies varied from 1.98–5;4–11 thus, the standard cutoff value remains unknown. In the current study, the cutoff value was defined as the mean value of the NLR.
The chi-squared test was used to assess the association between the NLR and the clinicopathologic variables. The Kaplan–Meier method was used to calculate the disease-specific survival (DSS) rate. The Cox proportional hazards method was used to determine the independent risk factors for DSS. All statistical analyses were conducted with the help of SPSS 20.0 (IBM Corporation, Armonk, NY, USA). A P<0.05 was considered significant.
Results
In total, 249 patients (147 female and 102 male) were enrolled with a mean age of 47.7 (range: 19–73) years. Tumor stage was distributed as follows: T1 in 53 (21.2%) patients, T2 in 89 (35.7%) patients, T3 in 61 (24.5%) patients, and T4 in 46 (18.5%) patients. Perineural invasion and lymphovascular invasion were noted in 31 (12.4%) and 25 (10.0%) patients, respectively. Intraparotid node metastasis (IPN) was noted in 45 (18.1%) patients. The disease grade was distributed as follows: low grade in 123 (49.4%) patients, moderate grade in 89 (35.7%) patients, and high grade in 37 (14.9%) patients (Table 1). A negative margin was achieved in 237 (95.2%) patients. The mean NLR was 2.48, with a range from 1.5 to 6.1.
  | Table 1 Distribution of parotid gland cancers |
In total, 189 (75.9%) patients were classified as cN0, and 57 patients underwent neck dissection; positive neck disease was reported in 18 patients. In total, 60 patients were classified as cN+ and underwent neck dissection; positive neck disease was reported in 50 patients.
When analyzing the association between the NLR and clinicopathologic variables, it was noted that the NLR was significantly associated with tumor stage, disease stage, and disease grade (Table 2).
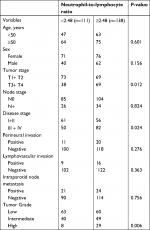  | Table 2 Association between neutrophil-to-lymphocyte ratio and clinical pathologic variables |
During our follow up with a mean time of 89.4 (range: 8–190) months, 132 patients had received radiotherapy, 27 patients received adjuvant chemotherapy, and 10 patients received herceptin treatment; in these 10 patients, staining for Her2 was 2+ in 6 cases and 3+ in four cases. A total of 73 patients died of the disease, and the 10-year DSS rate was 62%. When analyzing the predictors for DSS, it was noted that a high NLR was associated with a poorer prognosis in patients with an NLR <2.48 the 10-year DSS rate was 68% and in patients with an NLR ≥2.48, the 10-year DSS rate was 58%; the difference was significant (P=0.006, Figure 1). Further, the Cox model proved that the NLR was an independent predictor of DSS (Table 3).
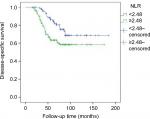  | Figure 1 Disease-specific survival in patients with different NLRs. Note: P=0.006. Abbreviation: NLR, neutrophil-to-lymphocyte ratio. |
  | Table 3 Univariate and multivariate analysis of the predictors for disease-specific survival in patients with parotid cancer Abbreviation: NLR, neutrophil-to-lymphocyte ratio. |
Discussion
The importance of the NLR in patients with parotid cancer has rarely been analyzed, and to the best of the authors’ knowledge, to date, only two papers have focused on this question.15,16 Damar et al15 found that the NLR was significantly lower in patients with benign salivary tumors than that in patients with malignant salivary gland tumors, and the NLR also significantly differed with disease grade. Similar findings were also noted in the current study. Moreover, we also noted that a higher NLR was related to more advanced stage disease. Kawakita et al16 described an NLR >2.5 in patients with salivary duct carcinoma, indicating that there was a nearly 2-fold risk for death in overall survival compared to baseline levels. The current study also found that a high pretreatment NLR was associated with an increased mortality risk. A similar finding was also reported for other solid malignancies. Yu et al6 described that head and neck cancer patients with elevated pretreatment NLRs in peripheral blood were prone to local invasion and distant recurrence and had poor prognosis. Kano et al4 analyzed the data for 285 patients with head and neck cancer treated with concurrent chemotherapy and found that there were significant relationships between a high NLR and hypopharyngeal or oropharyngeal cancer; N2b to N3; T3 to T4; and clinical stage III to IV. In further survival outcomes, a high NLR was significantly associated with decreases in disease-free survival and overall survival. Recently, Dell’Aquila et al7 also proved the prognostic value of the pretreatment NLR in metastatic colorectal cancer.
The exact mechanism underlying the associations between clinical pathologic variables as well as prognosis and the NLR remains unknown, and some possible explanations can be mentioned based on previous evidence. The pretreatment NLR reflects the status of the immune system and systemic inflammation. The elevation of neutrophils is a sign of local and systemic inflammatory responses. Several cytokines and angiogenic factors are produced by neutrophils, and these agents play important roles in promoting tumor development.17 Additionally, hematological markers might be surrogate markers of cancer cachexia, which is related to poor survival.15,18 On the other hand, lymphocytes are associated with immune surveillance and act by eliminating cancer cells.19 Therefore, a high NLR is considered to predict worse prognosis.
Another interesting finding was that IPN metastasis was associated with poor survival. Little data is available on this topic. The reported IPN metastasis rate varied from 15% to 38%, and it was significantly associated with tumor stage, disease grade, and neck node stage.20–27 With regard to survival analysis, only four papers have aimed to clarify this question. Lim et al22 might be the first to find that, compared to patients without IPN metastasis, patients with a cN0 neck tumor and IPN metastasis were more likely to develop locoregional recurrence. In a study published by Klussmann et al,20 univariate analysis reported that the involvement of IPN was an additional, significant, risk factor for tumor recurrence in 55 patients with pN+ tumors. Recently, Nisa et al23 described that decreased disease-free survival could be expected in patients with IPN involvement. Our previous research showed that patients with metastasis in more than 2 IPNs had the worst prognosis.28 Our current finding is consistent with that of previous reports. The N parameter in the TNM classification refers to regional, cervical lymph nodes, and IPN metastasis was not included in any of the groups of neck lymph nodes. Therefore, IPN seems to be a local factor, not a regional factor.27 Therefore, the presence of more metastatic nodes may decrease survival, mimicking advanced tumor stage. A similar finding was also reported by O’Brien et al29 after analyzing 87 patients with cutaneous squamous cell carcinoma of the parotid gland.
Herceptin has been described in the treatment of Her2+ parotid cancer, and most previous studies have reported that the application of herceptin could increase loco-regional control and prolong survival.30–32 Unfortunately, a significant association between good prognosis and herceptin was not achieved in the current study. We did not expect this result, and possible explanations may include the following: first, there were only 10 patients receiving herceptin treatment; and second, unlike previous case reports, most of the 10 patients did not have strong positivity for Her2, which might be the most likely cause of this variation.
Limitations
Limitations of the current study must be acknowledged. Firtsly, this was a retrospective study, and there is inherent bias that might decease the statistical power; and second, it should be recognized that neutrophil and lymphocyte counts are nonspecific parameters because they can be influenced by concomitant conditions, such as infections or inflammation.4–10
Conclusion
The long-term survival of primary parotid cancer patients is relatively favorable, and the pretreatment NLR is significantly associated with prognosis.
Data sharing statement
All data generated or analyzed during this study are included in this published article. The primary data can be obtained from the corresponding author.
Acknowledgment
The authors would like to thank Baixia Zhang and Kang Gan from Zhengzhou University for their assistance in this work.
Author contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 2005;7(3):211–217. | ||
Baniyash M, Sade-Feldman M, Kanterman J. Chronic inflammation and cancer: suppressing the suppressors. Cancer Immunol Immunother. 2014;63(1):11–20. | ||
Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21(3):309–322. | ||
Kano S, Homma A, Hatakeyama H, et al. Pretreatment lymphocyte-to-monocyte ratio as an independent prognostic factor for head and neck cancer. Head Neck. 2017;39(2):247–253. | ||
Kapoor S. Neutrophil to lymphocyte ratio and its association with tumor prognosis in systemic malignancies. J Surg Oncol. 2013;107(5):560. | ||
Yu Y, Wang H, Yan A, et al. Pretreatment neutrophil to lymphocyte ratio in determining the prognosis of head and neck cancer: a meta-analysis. BMC Cancer. 2018;18(1):383. | ||
dell’aquila E, Cremolini C, Zeppola T, et al. Prognostic and predictive role of neutrophil/lymphocytes ratio in metastatic colorectal cancer: a retrospective analysis of the tribe study by GONO. Ann Oncol. 2018;29(4):924–930. | ||
Fang H-Y, Huang XY, Chien H-T, et al. Refining the role of preoperative C-reactive protein by neutrophil/lymphocyte ratio in oral cavity squamous cell carcinoma. Laryngoscope. 2013;123(11):2690–2699. | ||
Ethier J-L, Desautels D, Templeton A, Shah PS, Amir E. Prognostic role of neutrophil-to-lymphocyte ratio in breast cancer: a systematic review and meta-analysis. Breast Cancer Res. 2017;19(1):2. | ||
Ethier J-L, Desautels DN, Templeton AJ, Oza A, Amir E, Lheureux S. Is the neutrophil-to-lymphocyte ratio prognostic of survival outcomes in gynecologic cancers? A systematic review and meta-analysis. Gynecologic Oncology. 2017;145(3):584–594. | ||
Fang Q, Liu F, Seng D. Oncologic outcome of parotid mucoepidermoid carcinoma in pediatric patients. Cancer Manag Res. 2019;11:1081–1085. | ||
Zhang X, Li J, Peng Q, et al. Association of markers of systemic and local inflammation with prognosis of patients with rectal cancer who received neoadjuvant radiotherapy. Cancer Manag Res. 2018;11:191–199. | ||
Sun Y, Zhang L. The clinical use of pretreatment NLR, PLR, and LMR in patients with esophageal squamous cell carcinoma: evidence from a meta-analysis. Cancer Manag Res. 2018;10:6167–6179. | ||
Jonska-Gmyrek J, Gmyrek L, Zolciak-Siwinska A, Kowalska M, Fuksiewicz M, Kotowicz B. Pretreatment neutrophil to lymphocyte and platelet to lymphocyte ratios as predictive factors for the survival of cervical adenocarcinoma patients. Cancer Manag Res. 2018;10:6029–6038. | ||
Damar M, Dinç AE, Erdem D, et al. Pretreatment neutrophil-lymphocyte ratio in salivary gland tumors is associated with malignancy. Otolaryngol Head Neck Surg. 2016;155(6):988–996. | ||
Kawakita D, Tada Y, Imanishi Y, et al. Impact of hematological inflammatory markers on clinical outcome in patients with salivary duct carcinoma: a multi-institutional study in Japan. Oncotarget. 2017;8(1):1083–1091. | ||
Tecchio C, Scapini P, Pizzolo G, Cassatella MA. On the cytokines produced by human neutrophils in tumors. Semin Cancer Biol. 2013;23(3):159–170. | ||
Liu F, Yuan S, Fang Q, Sun Q. Natural history of untreated squamous cell carcinoma of the head and neck. Clin Otolaryngol. 2018;38. | ||
Mohammed ZMA, Going JJ, Edwards J, Elsberger B, Doughty JC, McMillan DC. The relationship between components of tumour inflammatory cell infiltrate and clinicopathological factors and survival in patients with primary operable invasive ductal breast cancer. Br J Cancer. 2012;107(5):864–873. | ||
Klussmann JP, Ponert T, Mueller RP, Dienes HP, Guntinas-Lichius O. Patterns of lymph node spread and its influence on outcome in resectable parotid cancer. Eur J Surg Oncol. 2008;34(8):932–937. | ||
Lau VH, Aouad R, Farwell DG, Donald PJ, Chen AM. Patterns of nodal involvement for clinically N0 salivary gland carcinoma: Refining the role of elective neck irradiation. Head Neck. 2014;36(10):1435–1439. | ||
Lim CM, Gilbert MR, Johnson JT, Kim S. Clinical significance of intraparotid lymph node metastasis in primary parotid cancer. Head Neck. 2014;36(11):1634–1637. | ||
Nisa L, Salmina C, Dettmer MS, et al. Implications of intraglandular lymph node metastases in primary carcinomas of the parotid gland. The Laryngoscope. 2015;125(9):2099–2106. | ||
Lima RA, Tavares MR, Dias FL, et al. Clinical prognostic factors in malignant parotid gland tumors. Otolaryngol Head Neck Surg. 2005;133(5):702–708. | ||
Shinomiya H, Otsuki N, Yamashita D, Nibu K-Ichi, Nibu K. Patterns of lymph node metastasis of parotid cancer. Auris Nasus Larynx. 2016;43(4):446–450. | ||
Stenner M, Molls C, Luers JC, Beutner D, Klussmann JP, Huettenbrink K-B. Occurrence of lymph node metastasis in early-stage parotid gland cancer. Eur Arch Otorhinolaryngol. 2012;269(2):643–648. | ||
Stodulski D, Mikaszewski B, Majewska H, Wiśniewski P, Stankiewicz C. Probability and pattern of occult cervical lymph node metastases in primary parotid carcinoma. Eur Arch Otorhinolaryngol. 2017;274(3):1659–1664. | ||
Feng Y, Liu F, Cheng G, Fang Q, Niu X, He W. Significance of intraparotid node metastasis in predicting local control in primary parotid cancer. Laryngoscope. Epub 2018 Dec 14. | ||
O’Brien CJ, Mcneil EB, McMahon JD, Pathak I, Lauer CS, Jackson MA. Significance of clinical stage, extent of surgery, and pathologic findings in metastatic cutaneous squamous carcinoma of the parotid gland. Head Neck. 2002;24(5):417–422. | ||
Limaye SA, Posner MR, Krane JF, et al. Trastuzumab for the treatment of salivary duct carcinoma. Oncologist. 2013;18(3):294–300. | ||
Nabili V, Tan JW, Bhuta S, Sercarz JA, Head CS. Salivary duct carcinoma: a clinical and histologic review with implications for trastuzumab therapy. Head Neck. 2007;29(10):907–912. | ||
Krishnamurthy J, Krishnamurty DM, Baker JJ, Zhen W, Lydiatt D, Ganti AK. Salivary duct carcinoma responding to trastuzumab-based therapy: case report and review of the literature. Head Neck. 2013;35(12):E372–E375. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
