Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 13
Role of Sortilin and Matrix Vesicles in Nϵ-Carboxymethyl-Lysine-Induced Diabetic Atherosclerotic Calcification
Authors Jing L, Li L, Ren X, Sun Z , Bao Z, Yuan G, Cai H, Wang L, Shao C, Wang Z
Received 27 July 2020
Accepted for publication 11 September 2020
Published 3 November 2020 Volume 2020:13 Pages 4141—4151
DOI https://doi.org/10.2147/DMSO.S273029
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Konstantinos Tziomalos
Lele Jing,1 Lihua Li,2 Xiaomei Ren,3 Zhen Sun,1 Zhengyang Bao,4 Guoyue Yuan,5 Honghua Cai,6 Lin Wang,1 Chen Shao,1 Zhongqun Wang1
1Department of Cardiology, Affiliated Hospital of Jiangsu University, Zhenjiang 212001, People’s Republic of China; 2Department of Pathology, Affiliated Hospital of Jiangsu University, Zhenjiang 212001, People’s Republic of China; 3Department of Geriatrics, Zhongda Hospital Affiliated of Southeast University, Nanjing, People’s Republic of China; 4Department of Internal Medicine, Affiliated Wuxi Maternity and Child Health Care Hospital of Nanjing Medical University, Wuxi 214000, People’s Republic of China; 5Department of Endocrinology, Affiliated Hospital of Jiangsu University, Zhenjiang 212001, People’s Republic of China; 6Department of Burns and Plastic Surgery, Affiliated Hospital of Jiangsu University, Zhenjiang 212001, People’s Republic of China
Correspondence: Zhongqun Wang
Department of Cardiology, Affiliated Hospital of Jiangsu University, 438 Jiefang Road, Zhenjiang 212001, People’s Republic of China
Tel +86 511 85030586
Email [email protected]
Background and Aims: To investigate the role of Sortilin and matrix vesicles (MVs) in Nϵ-Carboxymethyl-lysine (CML)-induced diabetic atherosclerotic calcification (AC).
Methods: At human level, the correlation between Sortilin and CD9 (marker proteins of MVs) in serum MVs and CML in serum was explored by enzyme-linked immunosorbent assay (ELISA) detection and Pearson correlation analysis. After a diabetic apoE-/- mouse model was constructed, the calcification of aorta and the expressions of related proteins under CML and MVs injection were observed by calcification staining, immunofluorescence staining, and Western blot. MVs levels released by smooth muscle cells (SMCs) under different treatments was detected by nanometer tracking analysis (NTA). After treating SMCs with MVs and Anti-Sortilin, cell calcification was observed by Alizarin red staining.
Results: Serological analysis of patients showed that the concentrations of Sortilin and CD9 in serum MVs were positively correlated with the concentration of CML in serum. Animal experiments showed that CML could promote the progression of diabetic AC and the high expression of Sortilin in plaques. Diabetic apoE-/- mouse tail vein injection of CML-induced SMCs-derived MVs obviously aggravated AC. Cell experiment results showed that a high concentration of CML significantly promoted the release of MVs from SMCs. MVs from this source could markedly worsen cell calcification, while the administration of GW4869 (a widely used extracellular vesicles biogenesis inhibitor) significantly reduced cell calcification. Finally, treatment of high concentrations of CML could also promote the recruitment of Sortilin to MVs, and administration of Anti-Sortilin could markedly reduce cell calcification caused by MVs.
Conclusion: We proved that CML not only affects the release of MVs from SMCs but also affects the recruitment of Sortilin to MVs, thereby promoting diabetic AC. This discovery may provide a new strategy for targeted prevention of vascular calcification in diabetes.
Keywords: Nϵ-Carboxymethyl-lysine, sortilin, matrix vesicles, plaque calcification, diabetes
Introduction
Diabetes is a metabolic disease characterized by chronic hyperglycemia, which can cause a series of metabolic disorders, leading to impaired vascular function. Atherosclerotic calcification (AC) is one of the important complications leading to the malignant evolution of diseases such as acute myocardial infarction and acute heart failure. It was roughly estimated that AC could result in a 1.5-fold increase in mortality rate and a 5.5-fold increase in amputation rate.1
Advanced glycation end products (AGEs) are the most important metabolites of diabetic glucotoxicity, and participate in the formation and evolution of AC.2 An AGEs pool in human body is formed by two major sources. Exogenous AGEs are from the daily diet, especially food processed at high temperatures (such as fried, baked, roasted, etc.). Endogenous AGEs are mainly derived from Maillard reaction in body, and AGEs were more likely to develop in people with high hyperglycemia.3 Previous studies have shown that animal models of diabetes are more likely to form vascular complications such as atherosclerosis and diabetic nephropathy after eating a diet with high AGEs.
Nε-Carboxymethyl-lysine (CML) is a key active component of AGEs in serum. Previous articles have shown that CML and its receptors affect smooth muscle cells (SMCs) calcification from at least four aspects, including inflammatory and oxidative stress, osteogenesis and osteoclast balance, elastin and collagen, and fetuin.3 Studies have shown that CML can play a certain early warning effect on calcification in the anterior tibial artery plaque in diabetic amputated patients.4,5 Studies have also suggested that the receptor of CML may play a role in the pathogenesis of calcified aortic stenosis.6 This evidence suggests that CML plays an important role in the occurrence and development of diabetic AC.
Matrix vesicles (MVs) are membrane-embedded microparticles located in the extracellular matrix, 30–300 nm in diameter.7 Existing research confirmed that MVs are released from mineral-forming cells or SMCs that undergo phenotypic differentiation to the extracellular matrix through exocytosis, and interact with extracellular matrix proteins, causing a large influx of Ca2+ into MVs. This provides a material basis for hydroxyapatite crystallization and initiates the mineralization or ectopic calcification process.8 However, the specific mechanism has not been fully elucidated.
Sortilin is a type I membrane glycoprotein encoded by the SORT1 gene. It belongs to the family of vesicle sorting factor 10 protein (Vps10p) domain receptors. It has a molecular weight of 95 kDa, which plays an important role in protein transportation and signal transmission.9 The earliest research on Sortilin was about Alzheimer’s disease.10 Previous studies have shown that Sortilin is involved in the regulation of liver lipid metabolism.11 Studies on Sortilin in recent years have demonstrated that serum Sortilin levels are involved in the occurrence and development of vascular calcification.12 Further, some researchers have shown that Sortilin can promote calcification by recruiting into extracellular vesicles.13 Therefore, we speculate that Sortilin has a certain effect on the process of CML promoting diabetic AC, with the intention to find ways to inhibit or reverse vascular calcification.
Patients and Methods
Experimental Object
A total of 71 patients who were admitted to the Department of Cardiology of the Affiliated Hospital of Jiangsu University from 2017 to 2020 were selected. According to the inclusion and exclusion criteria, the patients were divided into diabetes (group A, n=22), diabetic atherosclerosis (group B, n=25), and diabetic plaque calcification groups (group C, n=24). The collection of all serum samples was approved by the ethics committee of the Affiliated Hospital of Jiangsu University. The patients signed the informed consent forms, which were filed by the medical department. This research protocol complies with the ethical principles of the 1975 Helsinki Declaration.
Inclusion criteria: 1) Patients were between 40–75 years of age; 2) Patients were diagnosed with Diabetes according to WHO (1999) diabetes diagnostic criteria; 3) Patients with diabetes but without coronary atherosclerosis were included in group A; 4) Patients with diabetes and coronary atherosclerosis were included in group B; 5) Patients with diabetes and coronary plaque calcification were included in group C; and 6) Diagnosis of coronary atherosclerosis and plaque calcification: The patient adopted a supine position for coronary multi-row spiral enhanced CT scan. Coronary lumens were reconstructed and analyzed through post-processing techniques such as multiplanar reconstruction and volume reconstruction. If the wall of the coronary is smooth and there is no different structure from the coronary wall, it is defined as no coronary atherosclerosis; If the CT value is lower than the lumen density, but higher than the surrounding connective tissue, and the structure is visible on at least two different levels, it is defined as coronary atherosclerosis. Patients with at least one coronary atherosclerosis structure are diagnosed with coronary atherosclerosis. If the CT value is higher than 130HU, and the structure can be clearly distinguished from the coronary lumen, and the structure is visible on at least two different levels, it is defined as plaque calcification. Patients with at least one plaque calcification structure were diagnosed with coronary plaque calcification.
Exclusion criteria: 1) Gastric ulcer, gastrectomy; 2) History of taking hormones or metformin; 3) Immunosuppressive therapy; 4) Severe liver and kidney dysfunction; 5) Severe trauma; 6) History of malignant tumors; 7) Patients who are participating in other clinical trials; and 8) Patients with incomplete clinical data.
Collection of Serum Samples
Following an overnight fast (12–14 hours), a 5 mL blood sample was collected from the brachial vein of each patient. After 30 minutes at room temperature, blood samples were centrifuged at 4°C, 3000 rpm for 20 minutes in a high-speed refrigerated centrifuge (Eppendorf, Germany). The serum was taken and stored in a refrigerator (Panasonic, Japan) at −80°C.
Enzyme-Linked Immunosorbent Assay (ELISA)
MVs in serum were separated (Method 11). The concentration of CML in serum, CD9 and Sortilin in serum MVs was measured using an enzyme-linked immunosorbent assay kit (Westang Bio-tech, Shanghai, China; System Biosciences, CA, USA; Aviscera Bioscience, Santa Clara, CA, USA) for quantitative analysis according to the manufacturer’s protocol.
Construction of Diabetic apoE−-/- Mouse Model
Six-week-old male apoE-/- mice (Animal Center, Peking University) were acclimated to the environment for 1 week, and 40 mg/kg/day streptozotocin (STZ) was injected intraperitoneally for 5 days. After 2 weeks, apoE-/- mouse with blood glucose levels exceeding 300 mg/dL were selected to the experiment. Diabetic apoE-/- mice were randomly divided into three groups: the NC group (common diet, n=8), the HFD group (high-fat diet, n=8), and the CML group (high-fat diet+tail vein injection of CML 10mg/kg/day, n=8). After 32 weeks, thoracic aortic tissue 3 cm away from the aortic valve was selected for morphological staining. All animal experiments in this study were conducted in compliance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No.5–23, revised 1996) and approved by the Animal Care and Use Committee of Jiangsu University, China.
Extraction of Mouse Aorta
Cervical dislocation was used to euthanize the mouse, the mouse was sprayed with 75% alcohol until the whole body was wetted, and it was fixed on a constant temperature ultra clean table. The hair was cleaned, the skin cut, and subcutaneous tissue cut, then the chest and abdomen were cut with golden crown scissors to expose organs. After carefully removing excess tissue around the aorta under a general microscope, complete aorta was taken.
Overall Oil-Red Staining
The aorta was flattened out and cut longitudinally along the small curved side of the aortic arch with micro scissors. Then it was immersed in the oil red staining solution and dye for 1 hour at room temperature with a shaker. It was then rinsed with 70% ethanol for 10 minutes, then the aorta was spread into a “Y” shape on the background board. Images were taken by a digital camera, and the ratio of aortic plaque area to aortic area was measured by Image J software.
Vonkossa Staining
The aortic paraffin sections were dewaxed and dehydrated, and then placed in a 2% silver nitrate solution, directly exposed to ultraviolet light for 40 minutes, and rinsed with distilled water 3 times. After absorbing the distilled water, the sections were treated with 5% sodium thiosulfate solution for 2 minutes, and rinsed with distilled water three times. The neutral fuchsin solution was treated for 3 minutes. After dehydration, transparency, and sealing, the aortic calcium salt deposition was observed under a light microscope (Olympus, Japan).
Immunofluorescence Staining
The fresh aortic tissue was snap-frozen, and the OCT embedded gel was completely covered on it, then serial frozen sections were performed. Frozen tissue sections were dried at room temperature for 15 minutes. The sections were then incubated with 1% bovine serum albumin (BSA) for 1 hour at 37°C. The primary antibody was added, which was diluted as required and incubated for 16 hours. After washing the sample in PBS, a secondary fluorescent antibody was added and incubated for 1 hour in the dark. The image was taken under an inverted microscope (Olympus). The antibodies used are: Rabbit polyclonal to Sortilin 1:100 (Abcam Cat# ab16640, RRID: AB_2192606); Rabbit monoclonal to alpha smooth muscle Actin 1:100 (Abcam Cat# ab32575, RRID: AB_722538).
Western Blot
For immunoblot (Bio-Rad, America), cells were lysed with RIPA lysis buffer for total protein. Samples were separated by SDS-PAGE and transferred to a nitrocellulose membrane. After the blockage with 5% nonfat milk, membranes were then incubated with Anti-Sortilin, Mouse monoclonal to CML (Abcam Cat# ab125145, RRID: AB_11127913), Rabbit monoclonal to CD9 (Abcam Cat# ab92726, RRID: AB_10561589), Mouse monoclonal to beta Actin (Abcam Cat# ab8226, RRID: AB_306371), Rabbit polyclonal to Runx2 antibody (Abcam Cat# ab23981, RRID: AB_777785) overnight at 4°C. Then the membranes were washed with TBST, and the diluted secondary antibody (1:5000) was added and incubated at 37°C for 1.5 hours. After enhancement with the ECL detection kit, analysis was performed with a gel imaging system (Amersham Imager 600).
Cell Line
The MOVAS cell line (CRL-2797) was purchased from ATCC company. MOVAS were cultured with high-glucose DMEM containing 10% fetal bovine serum, and were incubated in a cell culture incubator at 37°C and 5% CO2. The cells were passaged when the density reached about 80%. Conditioned medium consisted of DMEM high-glucose medium, 10% FBS, 2.5 mM β-glycerophosphate, and 50 µg/mL ascorbic acid.
Separation of MVs
The method of separating MVs from MOVAs medium and patients serum: samples were centrifuged at 300 g for 5 minutes at room temperature, and then centrifuged at 20,000 g at 4°C for 30 minutes. The supernatant was collected and centrifuged in an ultracentrifuge at 100,000 g for 60 minutes at 4°C (Beckman Coulter, America). After washing the pellet with PBS buffer, the pellet was centrifuged again for 60 minutes under the same conditions. The MVs pellet was diluted with PBS buffer and stored at −20°C.
Nanometer Tracking Analysis (NTA) Detection
The principle of NTA (Zetaview, Germany) is to track and analyze the Brownian motion of each particle, and to calculate the hydrodynamic diameter and concentration of the nano particles by combining the Stokes–Einstein equation. Twenty independent samples from each group were measured and their averages were displayed.
Alizarin Red Staining
The treated MOVAs were gently washed three times with PBS, fixed with 4% paraformaldehyde for 20 minutes, stained with Alizarin red staining solution for 2 hours, observed, and photographed under a microscope.
Statistical Analysis
All data were analyzed using GraphPad Prism. Normally distributed measurement data were expressed as X±SD. Non-normally distributed measurement data were represented by median and quarterback distance. For comparison of two groups of continuous variables with normal distribution and equal variances, two-tailed unpaired Student’s t-tests (with additional Welch’s correction for unequal variances) were performed. For multiple group comparison, we performed one-way or two-way analysis of variance (ANOVA). For comparison of groups of continuous variables with non-normal distribution, nonparametric tests were performed. Correlation between groups was analyzed by Pearson correlation analysis. P<0.05 was considered as statistical significance.
Results
Baseline Data
Baseline information is shown in Supplementary data Table 1). Based on the inclusion and exclusion criteria, 71 serum samples were collected and divided intoa diabetes group (group A, n=22), diabetic atherosclerosis group (group B, n=25), and diabetic plaque calcification group (group C, n=24). There was no significant difference in age and BMI among these three groups. There were significant differences in diabetes duration among three groups. The blood lipid levels in group B and C were significantly higher than those in group A, and there was no statistical difference between groups B and C.
The Concentration of Sortilin and CD9 in Serum MVs of Diabetic Patients Was Positively Correlated
MVs in serum were separated by differential centrifugation. The concentration of CML in serum, CD9 and Sortilin in serum MVs was detected by ELISA. ELISA showed that the concentration of CML, CD9, and Sortilin in group B was 1.38-times, 1.49-times, and 8.18-times that of group A, respectively; the concentration of CML, CD9, and Sortilin in group C was 2.43-times, 1.97-times, and 1.99-times that of group B, respectively (Figure 1A). The results of Pearson correlation analysis showed that the concentration of CD9 and Sortilin in serum MVs was positively correlated with the concentration of CML, respectively (Figure 1B), and the expressions of Sortilin and CD9 in serum MVs were positively correlated (Figure 1C). The difference and correlation between CD9, Sortilin, and CML suggested that they may be involved in the evolution of diabetic AC. Based on this guess, we constructed a diabetic apoE-/-mouse model for further research at the animal level.
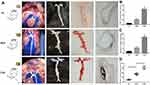
CML Promoted Plaque Calcification in Diabetic apoE-/- Mouse
From the overall perfusion of mouse (second column in Figure 2A), oil red staining (third column in Figure 2A), and vonkossa staining (fourth column in Figure 2A), it could be seen that compared with NC group and HFD group, CML can significantly promote the formation and calcification of atherosclerotic plaques in diabetes apoE-/- mouse. CML concentration in the CML group was significantly increased compared to the NC group and HFD group (Figure 2B). The aortic calcium content of the HFD group was 2.8-times that of the NC group, while that of the CML group was 2.96-times that of the HFD group (Figure 2C). The aortic plaque area was increased in the CML group (Figure 2D).
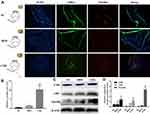
CML Stimulated the Expression of CD9 and Sortilin in Aortic Tissue
CD9 is a marker protein of MVs. Thirty-two weeks after CML injection, the aorta of diabetic apoE-/- mouse was taken for immunofluorescence staining (Figure 3A), relative Sortilin area (Figure 3B), and Western blot (Figure 3C). The results showed that, compared with the NC group and HFD group, the expression of Sortilin and CD9 in the CML group was significantly increased. The expression of CD9 in the CML group was 7.1-times and 8.3-times that of the NC group and HFD group, respectively, and the expression of Sortilin in the CML group was 13.3-times and 46.7-times that of the NC group and HFD group, respectively (Figure 3D). These results showed that CML promoted the expression of CD9 and Sortilin, but it is still unclear whether this effect is related to CML promoting calcification. Further, we conducted cell experiments to solve this problem.
CML Promoted Secretion of MVs from SMCs
MOVAs were treated with different concentrations of CML and GW4869 (a widely-used extracellular vesicles biogenesis inhibitor). After 4 days of calcification induction, MVs in the medium were separated and quantified. As expected, MVs levels released by MOVAs increased with increasing CML concentration (Figure 4A–C). MVs levels were markedly decreased by GW4869. However, compared with the 20 µM CML group, the amount of MVs released by MOVAs was reduced in the 40 µM CML group. We speculated that this may be related to the cytotoxicity of high concentration CML.
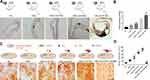
CML-Induced SMCs-Derived MVs Promoted Calcification
Further, we injected MVs released by MOVAs in the 20 µM CML group into the diabetes apoE-/- mouse through the tail vein at concentrations of 2×109 particles/kg/day, 4×109 particles/kg/day, and 8×109 particles/kg/day. Mice were divided into NC group (HFD+STZ), CML group, CML+2×109 MVs group, CML+4×109 MVs group. and CML+8×109 MVs group. After 16 weeks of high-fat diet, the aorta tissue was selected for vonkossa staining (Figure 5A). Calcium content was 1.51-times, 2.45-times, and 3.9-times that of the CML group, respectively (Figure 5B). Results showed that AC became more severe with increasing MVs concentration.
The same treatment was performed on MOVAs, in addition, GW4869 was added. MOVAs were divided into NC group (ox-LDL+calcification induction), CML group, CML+2×109 MVs group, CML+4×109 MVs group, CML+8×109 MVs group, and CML+8×109 MVs+GW4869 group. After 4 days of calcification induction, results showed that CML+MVs administration aggravated cell calcification and GW4869 administration significantly reduced cell calcification (Figure 5C). Relative calcification area under MVs treatment was 1.66-, 2.82-, and 3.55-times that of the CML group, respectively, while there was no statistical difference between the NC group and CML+8×109 MVs+GW4869 group (Figure 5D).
Taken together, these results provided clear evidence that CML promoted atherosclerotic calcification by affecting the release of MVs.
CML Could Up-Regulate the Expression of Sortilin in MVs
In order to explore whether CML could affect the process of Sortilin’s recruitment to MVs, we diluted MVs from the 20 µM CML group to the same concentration for ELISA. The result showed that as the concentration of CML increased, the concentration of Sortilin in MVs also increased (Figure 6).
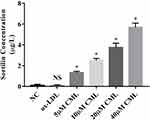 |
Figure 6 Effect of CML on the concentration of Sortilin in MVs. Detection of Sortilin concentration in MVs by ELISA. NS, P>0.05, compared with NC group. *P<0.05, compared with NC group. |
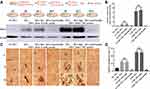
CML-Induced SMCs-Derived MVs Promoting Calcification May Be Achieved by Sortilin
Previous results have shown that CML could up-regulate the expression of Sortilin in MVs. Does this change in Sortilin concentration affect the progression of diabetic AC? Do MVs from different sources have the same effect on calcification? Further, MOVAs were treated with different sources of MVs and Anti-Sortilin, and were divided into NC MVs group, ox-LDL MVs group, ox-LDL MVs+ IgG group, ox-LDL MVs+ Anti-Sortilin group, CML MVs group, CML MVs+ IgG group, and CML MVs+ Anti-Sortilin group.
Results showed that the relative expression of Runx2 in the CML MVs group was 9.45, 233.74-times that of the ox-LDL MVs group and NC MVs group (Figure 7A and B), and relative calcification area in CML MVs group was 2.07, 6.73-times that of the ox-LDL MVs group and NC MVs group (Figure 7C and D). Anti-Sortilin administration significantly reduced Runx2 expression and cell calcification (Figure 7A and C).
These results indicated that, compared with ox-LDL, MVs released by MOVAs treated with CML promoted calcification more significantly, and Anti-Sortilin could reverse this process. Meanwhile, CML-induced SMCs-derived MVs promoting calcification may be achieved by Sortilin (Figure 8).
Discussion
There are about 114 million diabetics in China,14 accounting for about 27% of global diabetics. Diabetes is a chronic disease with less symptoms than many other diseases and is not easily noticed by patients. However, the acute and chronic complications of diabetes are invisible “killers”. Among them, atherosclerosis is one of the late complications of diabetes,15 which brings a great burden to people’s lives and socioeconomics. The previous research found that CML/CD36 may be mediated by cell free cholesterol, reactive oxygen species, p-FAK, Arp2/3 complex and F-actin polymerization, and inhibit foam cell migration, thereby promoting the evolution of diabetic atherosclerosis.16 AC is an advanced stage of diabetic atherosclerosis, and it is closely related to clinical events such as heart failure, acute coronary syndrome, and stroke. AC is an active, highly regulated pathological process.
In the present study, we have made three novel discoveries. First of all, we first detected the serum CML concentration and CD9 and Sortilin in serum MVs in diabetic patients, which were positively correlated with the serum CML concentration. On this basis, we verified that CML promotes diabetic AC and the expression of CD9 and Sortilin in plaques in a diabetic apoE-/- mouse model. Existing studies generally believed that MVs are the starting site of calcification,17 but few studies have involved the role of MVs in diabetic AC. Sortilin is widely distributed in hepatocytes, macrophages, adipocytes, and nerve cells. Studies have shown that high expression of Sortilin can reduce apolipoprotein B100 and participate in the regulation of lipid metabolism.18 Sparks et al19 confirmed that Sortilin and VLDL-B100 secretion may be related to insulin sensitivity. Gautaki et al demonstrated that Sortilin promotes calcification by recruiting into extracellular vesicles. Our current research provides evidence for the involvement of MVs and Sortilin in diabetic AC.
Secondly, we determined the role of MVs and Sortilin in CML-induced diabetic AC through animal experiments and cell experiments. NTA test showed that CML could affect the release of MVs from SMCs. Not under 40 µM CML treatment, but under 20 µM CML treatment, SMCs released the most MVs. This may be due to cell death caused by a high concentration of CML. ELISA results showed that CML could promote Sortilin recruitment into MVs. After a high concentration of CML-induced SMCs-derived MVs were injected into diabetic mouse through the tail vein, the aortic AC of the mouse was significantly exacerbated.
MVs from this source could markedly worsen cell calcification, while the administration of GW4869 significantly reduced cell calcification. Finally, the administration of CML-induced SMCs-derived MVs significantly increased cell calcification, while the administration of Anti-Sortilin could markedly reduce cell calcification. To the best of our knowledge, our study reports the key role of MVs and Sortilin in CML-mediated diabetic AC for the first time. This discovery may provide a new strategy for targeted prevention of vascular calcification in diabetes.
Thirdly, we found that, compared with Ox-LDL treatment, CML treatment is more able to promote the release of MVs from SMCs. We diluted the MVs released by SMCs under different stimulation to the same concentration, then treated receptor SMCs with them. Interestingly, results showed that, compared with ox-LDL, CML-induced SMCs-derived MVs promoted calcification more significantly. This result may imply that the diabetic diet has a higher priority than the low-fat diet in the clinic.
Our future research will solve several important issues. In order to target Sortilin to prevent diabetic AC, the core molecular mechanism of CML affecting Sortilin recruitment to MVs deserves further study. By consulting relevant literature, we speculate that the histone methylation level of Sortilin plays a regulatory role in CML-induced diabetic AC, and this guess requires further research at the animal level and the cellular level. In addition, this study only selected SMCs for research. Diabetic AC involves a variety of tissue cells, and further experiments are needed to verify whether MVs and Sortilin play the same pathological role in different tissue cells.
In summary, we proved that CML not only affects the release of MVs from SMCs, but also affects the recruitment of Sortilin to MVs, thereby promoting diabetic AC. These results suggested that preventing the release of MVs and the recruitment of Sortilin to MVs may be a new effective treatment for preventing diabetic AC.
Abbreviations
AC, atherosclerotic calcification; AGEs, advanced glycation end products; CML, Nε-Carboxymethyl-lysine; CVD, cardiovascular disease; MVs, matrix vesicles; SMCs, smooth muscle cells.
Data Sharing Statement
All datasets supporting the conclusions of this article are included in the article/Supplementary Data.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported as follows: National Natural Science Foundation of China (81770450, 81370408, 81801664); the related Foundation of Jiangsu Province (WSN-044, LGY2018092, QNRC2016836, 19GLD001); Zhenjiang Cardiovascular Clinical Research Center Project (SS2018008); Postgraduate Research&Practice Innovation Program of Jiangsu Province (KYCX20_2881).
Disclosure
The authors declare that there is no conflict of interest regarding the publication of this article. The authors report no conflicts of interest for this work.
References
1. Detrano R, Guerci A, Carr J, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358(13):1336–1345. doi:10.1056/NEJMoa072100
2. Yamagishi S, Nakamura N, Suematsu M, Kaseda K, Matsui T. Advanced glycation end products: a molecular target for vascular complications in diabetes. Mol Med. 2015;21(Suppl1):S32–S40. doi:10.2119/molmed.2015.00067
3. Wang Z, Jing L, Yan J, et al. Role of AGEs in the progression and regression of atherosclerotic plaques. Glycoconj J. 2018;35(5):443–450. doi:10.1007/s10719-018-9831-x
4. Li L, Ye F, Fu X, et al. Chinese guidelines for the management of hypertension. Zhonghua Xin Xue Guan Bing Za Zhi. 2017;45(11):958–962.
5. Wang Z, Li L, Du R, et al. CML/RAGE signal induces calcification cascade in diabetes. Diabetol Metab Syndr. 2016;8(1):83. doi:10.1186/s13098-016-0196-7
6. Saku K, Tahara N, Takaseya T, et al. Pathological role of receptor for advanced glycation end products in calcified aortic valve stenosis. J Am Heart Assoc. 2020;9(13):e015261. doi:10.1161/JAHA.119.015261
7. Bottini M, Mebarek S, Anderson K, et al. MVs from chondrocytes and osteoblasts: their biogenesis, properties, functions and biomimetic models. Biochim Biophys Acta. 2018;1862(3):532–546. doi:10.1016/j.bbagen.2017.11.005
8. Hutcheson JD, Goettsch C, Bertazzo S, et al. Genesis and growth of extracellular-vesicle-derived microcalcification in atherosclerotic plaques. Nat Mater. 2016;15(3):335–343. doi:10.1038/nmat4519
9. Goettsch C, Kjolby M, Aikawa E. Sortilin and its multiple roles in cardiovascular and metabolic diseases. Arterioscler Thromb Vasc Biol. 2018;38(1):19–25. doi:10.1161/ATVBAHA.117.310292
10. Fleitas C, Piñol-Ripoll G, Marfull P, et al. proBDNF is modified by advanced glycation end products in Alzheimer’s disease and causes neuronal apoptosis by inducing p75 neurotrophin receptor processing. Mol Brain. 2018;11(1):68. doi:10.1186/s13041-018-0411-6
11. Gao A, Cayabyab FS, Chen X, et al. Implications of sortilin in lipid metabolism and lipid disorder diseases. DNA Cell Biol. 2017;36(12):1050–1061. doi:10.1089/dna.2017.3853
12. Goettsch C, Iwata H, Hutcheson JD, et al. Serum sortilin associates with aortic calcification and cardiovascular risk in men. Arterioscler Thromb Vasc Biol. 2017;37(5):1005–1011. doi:10.1161/ATVBAHA.116.308932
13. Goettsch C, Hutcheson JD, Aikawa M, et al. Sortilin mediates vascular calcification via its recruitment into extracellular vesicles. J Clin Invest. 2016;126(4):1323–1336. doi:10.1172/JCI80851
14. Zhou X, Ni Y, Xie G, et al. Analysis of the health information needs of diabetics in China. Stud Health Technol Inform. 2019;264:487–491. doi:10.3233/SHTI190269
15. Xiao C, Dash S, Morgantini C, Hegele RA, Lewis GF. Pharmacological targeting of the atherogenic dyslipidemia complex: the next frontier in CVD prevention beyond lowering LDL cholesterol. Diabetes. 2016;65(7):1767–1778. doi:10.2337/db16-0046
16. Xu S, Li L, Yan J, et al. CML/CD36 accelerates atherosclerotic progression via inhibiting foam cell migration. Biomed Pharmacother. 2018;97:1020–1031. doi:10.1016/j.biopha.2017.11.041
17. Jing L, Li L, Sun Z, et al. Role of matrix vesicles in bone–vascular cross-talk. J Cardiovasc Pharmacol. 2019;74(5):372–378. doi:10.1097/FJC.0000000000000720
18. Amengual J, Guo L, Strong A, et al. Autophagy is required for sortilin-mediated degradation of apolipoprotein B100. Circ Res. 2018;122(4):568–582. doi:10.1161/CIRCRESAHA.117.311240
19. Sparks RP, Guida WC, Sowden MP, et al. Sortilin facilitates VLDL-B100 secretion by insulin sensitive McArdle RH7777 cells. Biochem Biophys Res Commun. 2016;478(2):546–552. doi:10.1016/j.bbrc.2016.07.096
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.