Back to Journals » Cancer Management and Research » Volume 15
Role of Serum CYFRA 21-1 in Diagnosis and Prognostic in Colorectal Liver Metastases
Authors Li S, Wei W, Feng Z, Bian Y, Pan J, Mai J, Ning S, Huang J, Gao X, Zhang L
Received 17 March 2023
Accepted for publication 30 June 2023
Published 6 July 2023 Volume 2023:15 Pages 601—614
DOI https://doi.org/10.2147/CMAR.S410477
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjeev K. Srivastava
Shirong Li,1,* Wene Wei,1,* Zhaorong Feng,1,* Yingzhen Bian,1 Jinmiao Pan,1 Jinling Mai,1 Shufang Ning,1,2 Jinglei Huang,1 Xiangyang Gao,3,* Litu Zhang1,2,*
1Department of Research, Guangxi Medical University Cancer Hospital, Nanning, People’s Republic of China; 2Department of Research, Guangxi Cancer Molecular Medicine Engineering Research Center, Nanning, People’s Republic of China; 3Health Management Institute, The Second Medical Center & National Clinical Research Center for Geriatric Diseases, Chinese PLA General Hospital, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiangyang Gao; Litu Zhang, Email [email protected]; [email protected]
Purpose: In current studies, the role of serum Cytokeratin-19 fragments (CYFRA 21-1) in colorectal cancer (CRC) remains unclear. This study aimed to clarify the diagnostic and prognostic value of CYFRA 21-1 in CRC.
Patients and Methods: Data were collected for 196 stage I–III CRC patients and 50 colorectal liver metastases (CRLM) patients between January 2018 and December 2019. The serum CYFRA 21-1 levels were measured using the chemiluminescent particle immunoassay (CMIA) kit in all objects and common biomarkers such as CA19-9, CEA, HSP90α, and AFP were measured in all colorectal cancer patients. We investigated the association between CYFRA 21-1 level and clinicopathological features. In addition, we evaluated the ability of serum CRFRA21-1 to differentiate CRLM from CRC. To assess the potential prognostic value, we used Cox proportional hazard model for univariate or multivariate analyses.
Results: Serum CYFRA 21-1 was significantly elevated in CRLM patients compared to stage I–III CRC patients (5.85 ng/mL vs 2.29 ng/mL, p < 0.001). For all CRC patients cohort, stage I–III CRC patients cohort and CRLM patients cohort, the optimal cutoff levels of CYFRA 21-1 for overall survival (OS) were 3.47 ng/mL, 2.14 ng/mL and 7.63 ng/mL, respectively, and the optimal cutoff levels for progression-free survival (PFS) were 3.47 ng/mL, 2.56 ng/mL and 7.63 ng/mL, respectively. For CRLM patients, Kaplan–Meier analysis showed that patients with high CYFRA 21-1 level had poor OS. Multivariate analysis indicated that the CYFRA 21-1 level was an independent prognostic factor for PFS in stage I–III patients. And CYFRA 21-1 levels and age were independent prognostic factors for OS and PFS in CRLM patients.
Conclusion: CYFRA 21-1 can better differentiate CRLM patients from the whole CRC patients and has unique prognostic value for CRLM patients.
Keywords: colorectal cancer, colorectal liver metastases, CRLM, prognosis, cytokeratin-19 fragments, CYFRA21-1, overall survival, progression-free survival
Introduction
The third highest incidence of cancer in the world is colorectal cancer (CRC), which has a mortality rate second only to lung cancer. In 2020 alone, more than 1.9 million people were first diagnosed with CRC, While 10 million deaths occurred solely due to the fact of cancer of which 9.4% showed direct relevance to CRC, over 930,000.1 Metastasis is not uncommon in cancer, especially colorectal cancer, about half of colorectal cancer patients prove to show a much higher tendency of metastasis, of which liver metastasis is more than frequently, and it is also a contributing factor in the mortality of colorectal cancer patients.2,3 With the continuous updating of medical technology, a broader spectrum of treatment options are available as of now, including radiation therapy, immunotherapy, palliative chemotherapy, targeted therapy, and local ablation therapy, surgery is still the main treatment method.
Despite a growing number of tumor biomarkers reported in recent years, there is no consensus on reliable biomarkers to identify and predict the prognosis of CRLM. Some studies believe that the IL-8 is related to CRLM, but its predictive value is still unclear.4 Some people believe that serum carboxypeptidase a4 is closely related to CRLM and has high predictive value for patients with colorectal cancer. Yet, the prognosis of patients with liver metastasis has not been studied.5 Recently, many scholars have studied the application of circulating tumor cells (CTCs) in predicting the prognosis of CRC patients, and found that CTCs have high prognostic value in CRLM patients.6 However, it has high requirements for hospital equipment and technology, which is difficult to popularize in subordinate hospitals, and its high cost makes it rather hard for average working class to afford. It is difficult to popularize clinically. Therefore, there is still an urgent need to find more reliable and popular markers in clinical practice to obtain better clinical treatment and a more accurate prognosis.
Compared with flexible sigmoidoscopy, low-cost and noninvasive blood tests can be utilized to determine biomarkers which make said tests a more suitable option as a screening tool. Besides widely recognized tumor biomarkers such as CA19-9 and CEA, CYFRA 21-1 is a relatively new tumor biomarker discovered in recent years. Recent studies indicate that CYFRA 21-1 can be used not only for the differential diagnosis and prognosis of lung, laryngeal, and esophageal cancer but also for CRC.7–10 However, some studies have shown that CYFRA21-1 does not significantly distinguish CRC from benign cases and non-cancer controls and is not sensitive enough to be used as a screening marker alone.11,12 The European group on tumor markers guidelines pointed out that continuous CEA determination is more sensitive to patients with colorectal cancer liver metastasis, but it relies on the same CEA detection method and has a long time span, which has higher requirements for patients’ medical compliance.13 Here, we will investigate serum CYFRA 21-1 levels in CRLM and stage I–III patients and explore its diagnostic and prognostic value.
Materials and Methods
Collection of Patients
Data from 246 patients with colorectal cancer admitted to The Cancer Hospital of Guangxi Medical University from January 2018 and December 2019 were included in our retrospective study. The retrospective analysis consists of these data. The enrollment criteria are as follows: (1) The diagnosis was confirmed by histopathology; (2) To evaluate the type of distal metastasis; (3) No tumor-related treatment had been performed at the time of diagnosis; (4) All test indicators, such as CEA, CA19-9, AFP, HSP90α and CYFRA 21-1, were obtained before treatment; (5) Complete clinical information. The followings are excluded from the study cohort: (1) Concomitant with other primary cancers; (2) Experienced other malignant solid tumors; (3) Received tumor-related treatment before admission; (4) The type of distant metastasis cannot be assessed; (5) Unavailable and incomplete clinical information. Patients were first diagnosed with CRC and received their first tumor-related treatment, after which full follow-up begins. Follow-up time was defined as the time interval from the date of diagnosis until the date of the last known follow-up visit or date of death. Patient selection and research methods are shown in Figure 1. All methods were carried out in accordance with the Declaration of Helsinki. Ethics approval number: LW2023062.
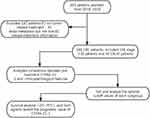 |
Figure 1 Study design and workflow diagram. |
Methods
Venous blood samples were drawn from subjects who had completed an 8-hour or above fast, which happened upon said subjects’ arrival the ward, followed by a series of serum sample preparation process. Serum CEA, CA19-9, AFP, and CYFRA 21-1 levels in centrifuged blood specimens were measured by chemiluminescent particle immunoassay (CMIA). These operations were performed utilizing the Architect Alinity I analyzer and matching kits (American Architect Diagnostics, USA). All processes were guided by the instruction manual of the analyzer and the kit. The content of plasma-free HSP90α in centrifuged blood samples was determined using the ELISA kit. Operate under manufacturer’s instructions. Serum samples were mixed with paramagnetic particles coated with CYFRA 21-1 specific monoclonal antibody KS. The sample’s CYFRA 21-1 antigen binds to a CYFRA 21-1 antibody-coated particle. After rinsing, the second step was followed by adding another CYFRA 21-1 specific monoclonal antibody BM labeled with acridine ester to form a reactant intermixture. When another rinse was completed, the pre-starter and excitation solutions were dropped in the reaction intermixture. The chemiluminescence reaction was surveyed in relative luminescence units (RLUs), and the content of CYFRA 21-1 antigen in the specimen was proportional to the RLUs value tested by the optical inspection system.
Statistical Analysis
SPSS (version 25.0) was used to analyze the tumor biomarker indexes and clinical characteristics statistically. Continuous data are expressed in the median and interquartile range. The optimal cutoff values of CYFRA 21-1 among each subgroup are determined by reference to the X-tile software. The diagnostic values were analyzed according to the area under the curve (AUC) obtained by the Receiver Operating Characteristics (ROC) curve analysis. The primary outcomes of survival analysis were overall survival (OS) and progression-free survival (PFS). The survival rate of patients was analyzed by Kaplan Meier method and Log rank test. Prognostic factors associated with OS and PFS were assessed by univariate and multivariate analysis using Cox proportional hazard model. Nomogram was constructed using R 4.1.2, and independent risk factors in multivariate analysis and CYFRA 21-1 were selected for inclusion in the nomogram. A p-value less than 0.05 was supported to statistically different.
Results
Add up to 246 CRC patients were amassed in this study, incorporating 196 patients with stage I–III and 50 with CRLM (Table 1). All CRC patients with a median age of 60 years were composed of 96 female and 150 male. Routine tumor markers were evaluated in all CRC patients. The levels of CEA, CA19-9, AFP, HSP90α and CYFRA 21-1 in all CRC patients were 4.36 (2.19, 15.40) ng/mL, 7.15 (3.18, 26.25)U/mL, 2.57 (1.90, 3.38) ng/mL, 46.60 (30.79, 70.53) ng/mL and 2.47 (1.77, 4.07) ng/mL, respectively (Table 1). In subgroup analysis, it was observed that the expression levels of CEA, CA19-9, HSP90α and CYFRA 21-1 were obviously higher in patients with CRLM than in patients with stage I–III (all p < 0.05, Figure 2 and Table 1).
 |
Table 1 Basic Characteristics of CRC Patients with Stage I–III and CRLM |
Relevance Between CYFRA 21-1 and Clinicopathological Features
The relevance between pre-treatment CYFRA 21-1 level and clinicopathological features in all CRC patients and subgroups were shown in Table 2 and Table 3. Serum level of pre-treatment CYFRA 21-1 had strong relevance of primary tumor, pre-treatment CEA, CA19-9 and HSP90α, lymphatic invasion, and stage in all CRC patients (all p < 0.05). For subgroups, the pre-treatment CYFRA 21-1 level showed no significant correlations with any clinicopathological features in the CRC patients with stage I–III. However, the pre-treatment CYFRA 21-1 levels were visibly relevant with the primary tumor’s location, pre-treatment CA19-9 and HSP90α in the CRLM patients subgroup (all p < 0.05).
 |
Table 2 Association of Pre-Treatment CYFRA21-1 Levels with Clinicopathological Features in CRC Patients and Their Subgroups |
 |
Table 3 Association of Pre-Treatment CYFRA21-1 Levels with Other Markers in CRC Patients and Their Subgroups |
The Ability of CYFRA 21-1 to Differentiate CRLM Patients
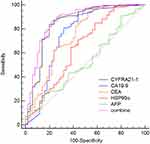
To evaluate the ability of CYFRA21-1 to differentiate patients with stage I–III and CRLM, we also included common tumor markers, for instance CA19-9, CEA, AFP, and the emerging marker HSP90α for comparison. We appraise the ability of CYFRA21-1 to distinguish patients with stage I–III and CRLM according to the constructed ROC curve (Figure 3). In all CRC patients, the AUC of CYFRA 21-1, CA19-9, CEA, HSP90α, and AFP were 0.844 (p < 0.001), 0.747 (p < 0.001), 0.743 (p < 0.001), 0.644 (p = 0.0031) and 0.525 (p = 0.579). We found that the AUC values of CYFRA 21-1, CA19-9, and CEA were all higher than 0.7. Using these three indexes for combined detection, the AUC was 0.868, the sensitivity was 86.22%, and the specificity was 78%, which was not a big improvement compared to the 83.67% sensitivity and 80% specificity of CYFRA 21-1. ROC analysis pointed out that the ability of CYFRA 21-1 to identify CRLM patients and patients with stage I–III was better than other indicators.
Pre-Treatment CYFRA21-1 Levels Correlate with Survival in Stage I–III and CRLM CRC Patients
For all CRC patients, the optimal cutoff values of CYFRA 21-1 for OS and PFS were 3.47 ng/mL. Kaplan Meier survival analysis noted that the patients with higher CYFRA 21-1 levels (>3.47 ng/mL) had lower OS and PFS than those with lower CYFRA 21-1 (≤3.47 ng/mL) (all p < 0.05, Figure 4A and B). In stage I–III CRC patients, the pre-treatment CYFRA21-1 levels showed no significant difference with OS under the optimal cutoff values of CYFRA 21-1 2.14 ng/mL (p > 0.05, Figure 4C). However, in the stage I–III CRC patients, patients with higher CYFRA21-1 levels had significantly shorter PFS, with an optimal cutoff level of 2.56 ng/mL (p = 0.019, Figure 4D). For CRLM patients, the optimal CYFRA 21-1 for OS and PFS was 7.63 ng/mL. Kaplan Meier survival analysis proved that CRLM patients with higher CYFRA 21-1 levels (>7.63 ng/mL) had significantly poorer OS (p = 0.018, Figure 4E), but no statistical difference in PFS (p = 0.06, Figure 4F).
Prognostic Value of Outcome Prediction for CYFRA21-1
According to the ROC curve of the survival rate of patients who were followed up for 12, 24, 36 and 48 months, the AUC values were compared to assess the predictive ability of CRLM patients with the assistance of CYFRA 21-1 (Figures 5 and 6). These results showed that the long-term prognostic function of CYFRA 21-1 in the CRLM patients cohort was superior to the stage I–III CRC patients cohort, regardless of OS or PFS.
Independent Factors Affect the Long-Term Prognostic of CRC Patients
In order to define independent prognostic risk factors in patients with stage I–III and CRLM, univariate analysis was performed and the results of P <0.1 were recruited into multivariate analysis. Univariate analysis of OS in CRLM patients showed that age (HR 2.744, 95% CI 1.409–5.345, p = 0.003) and pre-treatment CYFRA 21-1 (HR 2.218, 95% CI 1.108–4.408, p = 0.023) were significant prognostic factors, and univariate analysis of PFS in CRLM patients showed that age (HR 1.923, 95% CI 1.026–3.605, p = 0.041) were significant prognostic factors (Table 4). Then, the results of univariate analysis’s variable of P < 0.1 were recruited into multivariate analysis. In a multivariate analysis incorporated with these variables, age (OS, HR 3.304, 95% CI 1.653–6.605, p = 0.001; PFS, HR 2.154, 95% CI 1.134–4.093, p = 0.019) and pretreatment CYFRA 21-1 (OS, HR 5.442, 95% CI 2.701–5.362, p = 0.004; PFS, HR 2.038, 95% CI 1.069–3.883, p = 0.031) were independent prognostic factor of a poor prognosis in CRLM patients (Table 4).
 |
Table 4 The Correlations between OS, PFS, and various Clinicopathological Factors in CRLM Patients |
Univariate analysis of OS in the stage I–III patients showed that pre-treatment CEA (HR 4.338, 95% CI 1.681–11.193, p = 0.002) and pre-treatment CA19-9 (HR 3.434, 95% CI 1.331–8.858, p = 0.011) were significant prognostic factors, and univariate analysis of PFS in the stage I–III patients showed that lymphatic invasion (HR 2.167, 95% CI 1.066–4.406, p = 0.033), pre-treatment CEA (HR 2.245, 95% CI 1.163–4.335, p = 0.016) and pre-treatment CYFRA 21-1 (HR 1.797, 95% CI 0.653–4.947, p = 0.022) were significant prognostic factors (Table 5). Then, the results of univariate analysis’s variable of P < 0.1 were recruited into multivariate analysis. In a multivariate analysis incorporated with these variables, pre-treatment CEA (OS, HR 3.660, 95% CI 1.363–9.825, p = 0.010; PFS, HR 2.066, 95% CI 1.053–4.055, p = 0.035) were an independent prognostic factor of a poor evolution in patients with stage I–III (Table 5). Meanwhile, CYFRA21-1 also independently affects the PFS (HR 2.167, 95% CI 1.121–4.188, p = 0.021). Univariate and multivariate analyses of OS and PFS in all CRC patients are shown in Supplementary Table 1.
 |
Table 5 The Correlations between OS, PFS, and various Clinicopathological Factors in Stage I–III CRC Patients |
Establishing Prognostic Nomograms
After incorporating significant independent prognostic risk factors from multivariate analysis and pre-treatment CYFRA 21-1, we built nomograms to predict survival in CRC patients. The prognostic nomogram for OS and PFS in all CRC patients is displayed in Figure 7A and B. The C-index for OS and PFS prediction were 0.78 and 0.75. Figure 7C and D revealed the prognostic nomogram for OS and PFS in the stage I–III CRC patients, with C-index for OS and PFS prediction of 0.71 and 0.65. For the CRLM patients, the prognostic nomogram for OS and PFS is displayed in Figure 7E and F. The C-index for OS and PFS prediction were 0.68 and 0.60. The calibration curves supported the prediction results of nomogram to some extent (Supplementary Figures 1 and 2).
Discussion
Over the past few decades, colorectal cancer screening has significantly reduced morbidity and mortality. Effective clinical screening can detect primary lesions before they become cancerous and early cancers spread beyond the intestinal wall.14 The survival rate in 5 years for early localized cases is close to 90%, and the survival rate for patients diagnosed with end-rage colorectal cancer is only 13.1%, which is associated with the spread of distant organs, the most common of which are liver metastases.15 Liver metastases represent a worse prognosis and were among the most momentous prognostic risk factors for colorectal cancer.16 Although screening and treatment methods have improved and developed in recent years, survival rates in patients with distant metastases have not been effectively improved.17 Identifying a reliable prognostic factor is key to improving survival in patients with colorectal cancer liver metastases. Few studies on reliable and easily popularized tumor biomarkers can distinguish the stage I–III and CRLM patients.
Cytokeratin has been universally used for a cancer diagnosis or prognosis monitoring circulating tumor markers, especially in lung cancer and breast cancer.18–20 Cytokeratin circulates in blood and tumors as a complex and is partially degraded.21 CYFRA 21-1, tissue polypeptide antigen (TPA), and tissue polypeptide-specific antigen (TPS) are the three most used cytokeratins at present. The theory that these markers are associated with disease progression of various malignant tumors is recognized by more and more scholars.22–24 CYFRA 21-1 is a low molecular weight (40 kDa) acidic (type 1) subunit scientifically known as a soluble fragment of cytokeratin 19.21 Although reports have brought to light that serum CYFRA 21-1 indicates a rise in CRC patients, generally uninformed about the worth of serum CYFRA 21-1 in colorectal cancer liver metastasis.25
In recent decades, studies have found that cancer patients with distal metastases have higher levels of CYFRA 21-1, such as prostate cancer, nasopharyngeal carcinoma, lung cancer and thyroid cancer.26–30 In this study, patients with CRLM have higher levels of CYFRA 21-1, which supports this view to a certain extent. ROC curve analysis pointed that CYFRA 21-1 had higher diagnostic efficiency than common tumor markers such as CEA, AFP, CA19-9, HSP90α in distinguishing the stage I–III and CRLM patients. When we combined the three indicators of CYFRA 21-1, CA19-9, and CEA with higher AUC, compared with CYFRA 21-1 alone, we found that the sensitivity of 86.22% of the combined index was slightly higher than that of CYFRA 21-1 of 83.67%, but its 78% specificity decreased for 80% of CYFRA 21-1. Perhaps the combined detection of these three indicators does not significantly improve its sensitivity and specificity.
There are researches indicating that CYFRA 21-1 has prognostic value in patients with lung cancer, hepatocellular carcinoma, oropharyngeal squamous cell carcinoma, cervical cancer, esophageal Squamous cell carcinoma and gastric cancer.7,31–35
In all CRC patients, it is not difficult to conclude that the prognosis of patients with high CYFRA 21-1 level is worse through KM survival analysis, regardless of OS or PFS. However, liver metastasis, an important prognostic factor, may produce a biased result. Therefore, we continued the analysis of the subgroup. In patients with CRLM, KM survival analysis showed that patients with a high level of CYFRA 21-1 also underwent conspicuously shorter OS compared with patients with low levels of CYFRA 21-1. Although no difference was observed in the statistics of PFS, we can see a clear trend from the figure and speculate that patients with high CYFRA 21-1 level may have worse prognoses in PFS. Similar results have been observed in the stages I–III patients, and KM survival analysis announced that patients with low CYFRA 21-1 levels have a better prognosis than patients with opposite levels for PFS. However, we did not find similar results in OS.
We continued to analyze the survival rates of CRLM patients at 12, 24, 36, and 48 months, and the ROC analysis unveiled that the AUC of CYFRA 21-1 was relatively high at 36 and 48 months. One study found that patients with low three-year survival rates tended to have higher CYFRA 21-1 in CRC patients.36 Similar to the results we got. The evidence above proves that CRC patients with high CYFRA 21-1 have a worse prognosis. However, they did not distinguish between stage I–III and CRLM patients and only looked at three-year survival rates.
A study on gastric cancer pointed out that patients with high levels of CYFRA 21-1 had significantly poorer prognosis, in which CYFRA 21-1 could independently affect prognosis, while CEA and CA19-9 had no predictive value.35 We observed similar results in colorectal cancer. In our research, the optimal cutoff value of CYFRA 21-1 in CRLM patients is higher than in patients with stage I–III CRC. The constructed Cox proportional hazard model indicated that CYFRA 21-1 and age can independently influence the prognosis of patients with CRLM, whether OS or PFS. The above results prove that CYFRA 21-1 may be an independent prognostic factor in gastrointestinal tumors. Some studies have pointed out that the preoperative CEA level may be normal even for advanced colorectal cancer, and it is not uncommon.37 One study had pointed out that age can independently influence the prognosis of patients with CRLM, and this result was consistent with the results we obtained.38 In this paper, the patients with stage I–III CRC, CYFRA 21-1 were only independent prognostic factors for PFS in Cox proportional hazard model, not for OS. We observed that CEA independently affected OS and PFS in patients with stage I–III CRC, but there was no obvious link between OS or PFS in CRLM patients. And in our analysis, the difference between survival rates at 12, 24, 36 and 48 months for patients with CRC was not significant. The above evidence demonstrates that CYFRA 21-1 has a unique prognostic ability for OS in patients with colorectal cancer liver metastases and PFS in patients with stage I–III.
We developed survival nomograms based on the CYFRA 21-1 in the hope of increasing the reliability of prognostic studies. With predictions supported by the C-index, nomogram does a decent job of predicting survival.
Although CYFRA 21-1 in this paper revealed high diagnostic and prognostic value, there are some deficiencies to close attention and more work can be done in the future. First, we conducted only single-centre retrospective study. Secondly, that may be considered a contributing factor of suspected bias is our insufficient sample size. Finally, in this study, we only focused on liver metastases, and more studies can be done on metastases in other parts in the future, such as lung metastases. We will continue to refine this research, and the next step may be to conduct a multi-center prospective study.
Conclusions
In summary, serum CYFRA 21-1 was significantly elevated in colorectal cancer liver metastasis, which can distinguish stage I–III and CRLM colorectal cancer patients well. Elevated serum CYFRA 21-1 was correlated with shorter OS of patients with CRLM. As a cost-effective and reliable pre-treatment serum biomarker, serum CYFRA21-1 can be used as a diagnostic prognostic marker for colorectal liver metastases patients, in which crucial clinical significance has shown.
Data Sharing Statement
The original contributions involved in this study are included in the article/Supplementary Material. Further enquiries can be directed to the corresponding authors.
Ethics Statement
The studies involving human participants were reviewed and approved by the Local Ethics Committee of Guangxi Medical University Cancer Hospital. The patients/participants provided their written informed consent to participate in this study. All methods were carried out in accordance with the Declaration of Helsinki. Ethics approval number: LW2023062.
Author Contributions
XY Gao, and LT Zhang designed the study. ZR Feng, JL Huang performed the experiments. YZ Bian, JL Mai, JM Pan and SF Ning analyzed the data. SR Li and WE Wei wrote the manuscript. All authors approved the final version of the manuscript. All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work.
Funding
This study was supported by grants from the Key R&D Program of Scientific Research and Technology Development Project of Guangxi (Grant No. 2022AB11046), the Key R&D Program of Scientific Research and Technical Development Project of Qingxiu District, Nanning, Guangxi (Grant No. 2021015), the Key R&D Program of Scientific Research and Technology Development Project of Nanning, Guangxi (Grant No. ZC20213009) and the Technology Development Program Project, and the Self-funded scientific research project of Guangxi Zhuang Autonomous Region Health Committee (Grant No. 20191022), Guangxi Medical and health key discipline construction project.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Goodla L, Xue X. The role of inflammatory mediators in colorectal cancer hepatic metastasis. Cells. 2022;11(15):2313. doi:10.3390/cells11152313
3. Zeng X, Ward SE, Zhou J, Cheng ASL. Liver immune microenvironment and metastasis from colorectal cancer‐pathogenesis and therapeutic perspectives. Cancers. 2021;13(10):1–22. doi:10.3390/cancers13102418
4. Bie Y, Ge W, Yang Z, et al. The crucial role of CXCL8 and its receptors in colorectal liver metastasis. Dis Markers. 2019;2019:1–12. doi:10.1155/2019/8023460
5. Sun L, Guo C, Burnett J, Yang Z, Ran Y, Sun D. Serum carboxypeptidaseA4 levels predict liver metastasis in colorectal carcinoma. Oncotarget. 2016;7(48):78688–78697. doi:10.18632/oncotarget.12798
6. Huang M, Cheng L, Mo SS, Ru H, Mo X, Yan L. Evaluation of colorectal cancer liver metastases based on liquid biopsy combined with folate receptor– positive circulating tumor cells and HSP90. Front Oncol. 2022;12:1–14. doi:10.3389/fonc.2022.912016
7. Yuan J, Sun Y, Wang K, et al. Development and validation of reassigned CEA, CYFRA21-1 and NSE-based models for lung cancer diagnosis and prognosis prediction. BMC Cancer. 2022;22(1):686. doi:10.1186/S12885-022-09728-5
8. Ji M, Zhang LJ. Expression levels of SCCA and CYFRA 21-1 in serum of patients with laryngeal squamous cell carcinoma and their correlation with tumorigenesis and progression. Clin Transl Oncol. 2021;23(2):289–295. doi:10.1007/S12094-020-02417-4
9. Qiao Y, Ma M, Zhang H, Yu Z, Tang P. Prognostic significance of the combination of fibrinogen and tumor marker index in esophageal squamous cell carcinoma patients. Onco Targets Ther. 2021;14:1101–1111. doi:10.2147/OTT.S278831
10. Lee JH. Clinical usefulness of serum CYFRA 21-1 in patients with colorectal cancer. Nucl Med Mol Imaging. 2013;47(3):181–187. doi:10.1007/S13139-013-0218-4
11. Thomas DS, Fourkala EO, Apostolidou S, et al. Evaluation of serum CEA, CYFRA21-1 and CA125 for the early detection of colorectal cancer using longitudinal preclinical samples. Br J Cancer. 2015;113(2):268–274. doi:10.1038/bjc.2015.202
12. Wild N, Andres H, Rollinger W, et al. A combination of serum markers for the early detection of colorectal cancer. Clin Cancer Res. 2010;16(24):6111–6121. doi:10.1158/1078-0432.CCR-10-0119
13. Duffy MJ, Lamerz R, Haglund C, et al. Tumor markers in colorectal cancer, gastric cancer and gastrointestinal stromal cancers: European group on tumor markers 2014 guidelines update. Int J Cancer. 2014;134(11):2513–2522. doi:10.1002/ijc.28384
14. Li N, Lu B, Luo C, et al. Incidence, mortality, survival, risk factor and screening of colorectal cancer: a comparison among China, Europe, and Northern America. Cancer Lett. 2021;522:255–268. doi:10.1016/j.canlet.2021.09.034
15. Doll KM, Rademaker A, Sosa JA. Practical guide to surgical data sets: Surveillance, Epidemiology, and End Results (SEER) database. JAMA Surg. 2018;153(6):588–589. doi:10.1001/JAMASURG.2018.0501
16. Miller KD, Nogueira L, Devasia T, et al. Cancer treatment and survivorship statistics, 2022. CA Cancer J Clin. 2022;72(5):409–436. doi:10.3322/CAAC.21731
17. Allemani C, Matsuda T, Di Carlo V, et al. Global surveillance of trends in cancer survival 2000–14 (Concord-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391(10125):1023–1075. doi:10.1016/S0140-6736(17)33326-3
18. Fahrmann JF, Marsh T, Irajizad E, et al. Blood-based biomarker panel for personalized lung cancer risk assessment. J Clin Oncol. 2022;40(8):876–883. doi:10.1200/JCO.21.01460
19. Wang X, Wang M, Feng L, et al. Four-protein model for predicting prognostic risk of lung cancer. Front Med. 2022;16(4):618–626. doi:10.1007/S11684-021-0867-0
20. Yin L, Li Q, Mrdenovic S, et al. KRT13 promotes stemness and drives metastasis in breast cancer through a plakoglobin/c-Myc signaling pathway. Breast Cancer Res. 2022;24(1):7. doi:10.1186/S13058-022-01502-6
21. Werner S, Keller L, Pantel K. Epithelial keratins: biology and implications as diagnostic markers for liquid biopsies. Mol Aspects Med. 2020;72:1. doi:10.1016/j.mam.2019.09.001
22. Shukla P, Dange P, Mohanty BS, Gadewal N, Chaudhari P, Sarin R. ARID2 suppression promotes tumor progression and upregulates cytokeratin 8, 18 and β-4 integrin expression in TP53-mutated tobacco-related oral cancer and has prognostic implications. Cancer Gene Ther. 2022;29(12):1908–1917. doi:10.1038/S41417-022-00505-X
23. Nowak E, Bednarek I. Aspects of the epigenetic regulation of EMT related to cancer metastasis. Cells. 2021;10(12):3435. doi:10.3390/CELLS10123435
24. Urano-Takaoka M, Sumida H, Miyagawa T, et al. Serum cytokeratin 18 as a metastatic and therapeutic marker for extramammary paget’s disease. Acta Derm Venereol. 2022;102:adv00636. doi:10.2340/ACTADV.V101.866
25. Lim DH, Lee JH, Kim JW. Feasibility of CYFRA 21-1 as a serum biomarker for the detection of colorectal adenoma and advanced colorectal adenoma in people over the age of 45. J Clin Lab Anal. 2018;32(1):e22163. doi:10.1002/JCLA.22163
26. Yu M, Yang C, Wang S, et al. Serum ProGRP as a novel biomarker of bone metastasis in prostate cancer. Clin Chim Acta. 2020;510:437–441. doi:10.1016/J.CCA.2020.08.007
27. Peng SJ, Wang CF, Yu YJ, et al. CYFRA21-1/TG ratio as an accurate risk factor to predict eye metastasis in nasopharyngeal carcinoma: a STROBE-compliant article. Medicine. 2020;99(46):e22773. doi:10.1097/MD.0000000000022773
28. Lin Q, Chen XY, Liu WF, et al. Diagnostic value of CA-153 and CYFRA 21-1 in predicting intraocular metastasis in patients with metastatic lung cancer. Cancer Med. 2020;9(4):1279–1286. doi:10.1002/CAM4.2354
29. Jiang C, Zhao M, Hou S, et al. The indicative value of serum tumor markers for metastasis and stage of non-small cell lung cancer. Cancers. 2022;14(20):5064. doi:10.3390/CANCERS14205064
30. Jeong C, Lee J, Yoon H, et al. Serum CYFRA 21.1 level predicts disease course in thyroid cancer with distant metastasis. Cancers. 2021;13(4):1–13. doi:10.3390/CANCERS13040811
31. Caviglia GP, Ciruolo M, Olivero A, et al. Prognostic role of serum cytokeratin-19 fragment (CYFRA 21-1) in patients with hepatocellular carcinoma. Cancers. 2020;12(10):1–10. doi:10.3390/cancers12102776
32. Li L, Liu G, Jin K, et al. Prognostic significance of pre-treatment serum Cyfra21-1 as a tumor marker in patients with oropharyngeal squamous cell carcinoma treated with concurrent chemoradiotherapy. Ann Transl Med. 2020;8(20):1302. doi:10.21037/ATM-20-6124
33. Pedraza S, Seiffert AP, Sarandeses P, et al. The value of metabolic parameters and textural analysis in predicting prognosis in locally advanced cervical cancer treated with chemoradiotherapy. Strahlenther Onkol. 2022;198(9):792–801. doi:10.1007/S00066-022-01900-X
34. Ju M, Ge X, Di X, Zhang Y, Liang L, Shi Y. Diagnostic, prognostic, and recurrence monitoring value of plasma CYFRA21-1 and NSE levels in patients with esophageal squamous cell carcinoma. Front Oncol. 2022;11:789312. doi:10.3389/FONC.2021.789312/PDF
35. Nakata B, Chung YS, Kato Y, et al. Clinical significance of serum CYFRA 21-1 in gastric cancer. Br J Cancer. 1996;73(12):1529–1532. doi:10.1038/BJC.1996.288
36. Holdenrieder S, Stieber P, Liska V, et al. Cytokeratin serum biomarkers in patients with colorectal cancer. Anticancer Res. 2012;32(5):1971–1976.
37. Menclová K, Pudil J, Benešová L, et al. Circulating tumor DNA as a biomarker in metastatic colorectal carcinoma case report. Rozhl Chir. 2020;99(4):179–182. doi:10.33699/PIS.2020.99.4.179-182
38. Tang M, Wang H, Cao Y, Zeng Z, Shan X, Wang L. Nomogram for predicting occurrence and prognosis of liver metastasis in colorectal cancer: a population-based study. Int J Colorectal Dis. 2021;36(2):271–282. doi:10.1007/s00384-020-03722-8
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.