Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 16
Role of Increased miR-222-3p Expression in Peripheral Blood and Wound Marginal Tissues of Type 2 Diabetes Mellitus Patients with Diabetic Foot Ulcer
Authors Jie R , Qian J, Tang Y, Li Y , Xu M , Zhao X , Chen M
Received 27 April 2023
Accepted for publication 28 July 2023
Published 15 August 2023 Volume 2023:16 Pages 2419—2432
DOI https://doi.org/10.2147/DMSO.S410986
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Antonio Brunetti
Ruyan Jie,* Jing Qian,* Ying Tang,* Yutong Li, Murong Xu, Xiaotong Zhao, Mingwei Chen
Department of Endocrinology, The First Affiliated Hospital of Anhui Medical University, Hefei, Anhui, 230032, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiaotong Zhao; Mingwei Chen, Department of Endocrinology, The First Affiliated Hospital of Anhui Medical University, No. 218 Jixi Road, Hefei, Anhui, 230032, People’s Republic of China, Tel +86-551-2923631, Fax +86-551-2922160, Email [email protected]; [email protected]
Purpose: To study the correlations of miR-222-3p expression in the peripheral blood and wound marginal tissues of type 2 diabetes mellitus (T2DM) patients with the onset of diabetic foot ulcer (DFU), as well as explore the clinical value possessed by miR-222-3p in the diagnosis and treatment outcomes of DFU.
Methods: The study included 70 T2DM patients who did not suffer foot ulcers (T2DM group), 146 T2DM patients who suffered foot ulcers (DFU group), as well as 70 normal controls (NC group). Quantitative real-time PCR determined the MiR-222-3p relative expression. Clinical features and risk factors regarding DFU were assessed. Multiple stepwise logistic regression analysis assisted in confirming whether miR-222-3p expression could serve for independently predicting the risk factors for DFU. ROC curve analysis evaluated the diagnostic value exhibited by miR-222-3p level against DFU.
Results: T2DM group exhibited an obviously higher MiR-222-3p expression relative to NC group [1.98 (0.98, 3.62) vs 0.92 (0.61, 1.87)] (P < 0.01), but DFU group exhibited an obviously higher miR-222-3p expression relative to T2DM group [5.61 (1.98, 10.24) vs 1.98 (0.98, 3.62)] (P < 0.01). Besides, miR-222-3p expression presented a negative correlation with DFU healing rate (P < 0.05). According to Kaplan–Meier survival curve analysis, the group with high miR-222-3p expression showed higher unhealed DFU cumulative rate relative to the group with low expression (log-rank, P = 0.011, 0.001, respectively). Multivariate logistic regression analysis confirmed that high miR-222-3p expressions could independently predict DFU risk (OR=3.85, 95% CI 1.18~12.37, P = 0.008). According to the ROC curve analysis, the AUC of miR-222-3p specific to DFU diagnosis reached 0.803, with the best sensitivity of 95.93% and best specificity of 96.27%.
Conclusion: The increased expression of miR-222-3p in the peripheral blood of T2DM patients is closely related to the occurrence of DFU. MiR-222-3p is a biomarker with potential clinical value in diagnosing and evaluating the prognosis of DFU.
Keywords: miR-222-3p, diabetic foot ulcer, type 2 diabetes mellitus, microRNA, biomarker
Introduction
Diabetic foot (DF), commonly manifested as diabetic foot ulcer (DFU), is the primary cause of disability and death for diabetic patients. It threatens the public health and is costly to treat. According to a meta-analysis, DFU has a global prevalence of 6.3%.1 DFU has a poor prognosis and a high risk of disability and death. More than 85% of amputations in diabetic patients are due to DFU, and the mortality rate after DFU occurrence is estimated to be 5% in the first 12 months and 42% in 5 years.2 DFU has a quite complex pathogenesis, which has not been well understood.
MicroRNA (miRNA), a kind of endogenous non-coding small RNA, has the function of regulating gene expression by hindering the protein translation process of target mRNA or directly degrading the mRNA.3 According to more and more recent studies, abnormal miRNA expression exhibits a close association with DFU occurrence and prognosis.4
MiR222, which is located on the X chromosome p11.3 of the human genome, belongs to the miR221/222 family. In the mature miR222 sequence, there is a hairpin precursor, which has two arms, referred to as 5p and 3p, respectively. MiR-222-3p is a highly conserved miRNA mainly expressed in bladder, prostate, lung, ovary, adipose tissue, thyroid, bronchus, islet, skin, and other tissues.5 Studies have found an increasing miR-222-3p expression in the peripheral blood (PB) of patients suffering type 2 diabetes mellitus (T2DM),6 obesity,7 polycystic ovary syndrome,8 and gestational diabetes mellitus.9 According to a cohort follow-up study, miR-222-3p overexpression in the mononuclear cells of PB raises the risk of T2DM.10 In addition, high miR-222-3p expression impacts the atherosclerosis progression in patients with subclinical hypothyroidism.11 Many studies have confirmed the crucial role played by miR-222-3p in oncogenesis, tumor cell multiplication, apoptosis, distant invasion and metastasis, and tumor microenvironment.12 Some scholars believe that there are similar molecular and functional mechanisms between the wound healing and tumor.13 Insufficient blood supply and dysangiogenesis of wound surface are important causes of difficulty in DFU healing. Currently, miR-222-3p is a type of anti-angiogenic miRNA closely related to arterial atherosclerosis.14,15 In a vitro study, miR-222-3p can reduce the survival, migration, and angiogenesis ability possessed by human umbilical vein endothelial cells through directly hindering the expression of c-Kit.16 Downregulation of miR-222-3p expression can alleviate the damage to human brain microvascular endothelial cells triggered by oxygen-glucose deprivation/reoxygenation and increase the cyclic adenosine monophosphate level in endothelial cells by reducing the expression of phosphodiesterase 3 (PDE3), thereby improving vascular endothelial dysfunction.17,18 Previous studies have confirmed that endothelial progenitor cell-derived exosome is conducive to the healing of skin wounds.19 According to high-throughput sequencing, miR-222-3p and miR-221-3p are the second and third most abundant miRNAs, respectively, in endothelial progenitor cell-derived exosomes. Animal experiments and in vitro studies have shown that miRNA-221-3p, belonging to the same gene cluster with miR-222-3p, can promote angiogenesis in the skin wound granulation tissue of diabetic mice through the AGE (advanced glycosylation end products)-RAGE (receptor of AGEs) signaling pathway and then promote wound healing. These studies indicated that miR-222-3p may participate in chronic skin wound healing.
Present clinical studies have not paid attention to the impact of miR-222-3p on the DFU pathogenesis. Hence, we conducted this study to understand miR-222-3p expression change in DFU patients’ PB and wound marginal tissue (WMT) as well as its relevance to DFU pathogenesis and treatment. In addition, we evaluated the clinical value possessed by miR-222-3p for DFU diagnosis and treatment as a useful biomarker.
Materials and Methods
Study Subjects
146 T2DM patients with DFU (DFU group) hospitalized in the Department of Endocrinology of the First Affiliated Hospital of Anhui Medical University during January 2020 and June 2022 were selected. The foot ulcer course was ≥4 weeks, the Wagner classification grade was II–IV, and the ABI was 0.7–1.3. During the same period, 70 newly diagnosed T2DM patients were recruited as the T2DM group. These diabetics had neither lower extremity atherosclerotic disease nor diabetic peripheral neuropathy. The NC group included another 70 healthy individuals receiving physical examinations at our hospital in the same period, and all of them received a 75 g oral glucose tolerance test and were confirmed to have normal glucose tolerance. Participants with serious heart, kidney, or liver dysfunction; autoimmune disease; cancerous ulcer wounds; or severe sepsis were excluded. The study completed with the approval of the Medical Ethics Committee of the First Affiliated Hospital of Anhui Medical University (Ethics batch number P20210039) and received written informed consent from all participants.
Research Methods
DFU Treatment
After admission, all DFU patients received conventional systemic treatment, including anti-infection therapy, hypertension management, hypoglycemic therapy, hypoproteinemia correction, nerve nutrition, and lower limb wound blood supply improvement. Wound debridement served for cleaning up necrotic tissues. Other treatments were given depending on the specific condition of patients, such as decompression or negative pressure wound treatment. During debridement, a skilled surgeon took charge of cutting full-thickness skin tissue within 0.5 cm of the wound margin as per the sampling protocol, followed by storing them in a −80°C refrigerator. Follow up was conducted on all DFU patients until the complete wound healing, together with the recording of the healing time. During follow-up, a multidisciplinary diabetes foot team decided whether to amputate based on the patient’s condition. Ulcer healing after eight weeks referred to spontaneous complete wound closure after eight weeks of DFU treatment (100% re-epithelialization), and recorded 8 weeks after the treatment.20
Detection of Clinical Indicators
All participants fasted for 10 hours and the next morning from 8:00 to 8:30, venous blood was extracted in the fasting state to determine albumin (ALB), blood glucose, blood lipid composition, glycosylated hemoglobin A1c (HbA1c), white blood cell (WBC) count, hemoglobin (Hb), C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), interleukin-6 (P-IL-6), interleukin-10 (P-IL-10), and other indicators. In addition, tools were used to measure the skin ulcer area, ABI, and transcutaneous oxygen partial pressure (TcPO2). The specific detection methods for each index are shown in Table S1.
Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
The miRcute miRNA Extraction and Isolation kit served for extracting RNA from EDTA anticoagulant blood samples (2 mL) or WMT (50 mg). The miRcute miRNA cDNA Synthesis Kit served for the synthesis of the cDNA. At last, the miRcute miRNA fluorescence quantitative detection kit served for the qRT-PCR (Table S2). The above reagent kits were all purchased from Tianjin Biochemical Technology Co., Ltd., Beijing, China. U6 or GAPDH served as internal controls. We calculated the miR-222-3p expression in the PB (P-miR-222-3p) and WMT (T-miR-222-3p), and the mRNA expression of VEGF, CD31, IL-6, and IL-10 in the WMT by the 2−ΔΔCt method. We repeated each sample three times and took the mean value as the final result.
Statistical Analysis
Statistical analysis was carried out with the assistance of SPSS 19.0 software (SPSS Inc., Chicago, IL). Normal and non-normal measurement data were in the form of mean ± standard deviation and median (interquartile range) [M (P25, P75)], respectively. Chi-square test or t-test was used for comparison between two groups. As for the multi-group comparison, the analysis of variance test was used if the data followed a normal distribution, and the LSD-t test served for deeper pairwise comparisons; a non-parametric test (Kruskal–Wallis rank sum test) was conducted if the normal distribution was not followed, and Dunn’s test served for deeper pairwise comparison. Spearman correlation analysis assisted in evaluating the relevance of miR-222-3p expressions to other clinical variables. Multiple stepwise logistic regression analysis confirmed whether miR-222-3p in PB could independently predict DFU risk. Kaplan–Meier survival curve analysis assisted in investigating the association between DFU wound healing and miR-222-3p expression in PB and WMTs. ROC curve analysis assisted in exploring whether miR-222-3p in the PB could serve for the DFU diagnosis as a potential biomarker. All tests were two-sided. P <0.05 reported statistical significance.
Results
Clinical Parameter Comparison Among the Three Groups
Among the three groups, the sex composition, age, TCH, LDL-C, and P-IL-10 levels were not significantly different (P > 0.05). The T2DM and DFU groups showed higher FPG, HbA1c, TG, and P-miR-222-3p expressions relative to the NC group. In contrast, the T2DM and DFU groups exhibited lower HDL-C levels relative to the NC group. The differences presented statistical significance (P < 0.05). Besides, the NC and T2DM groups did not present significantly different in TcPO2, ABI, CRP, ESR, ALB, WBC count, Hb, and P-IL-6 (P > 0.05). Furthermore, the DFU group had higher FPG, HbA1c, TG, CRP, ESR, WBC count, P-IL-6, diabetes duration, and P-miR-222-3p expression levels, but lower HDL-C, TcPO2, ABI, ALB, and Hb levels relative to the T2DM group. These differences indicated statistical significance (P < 0.05) (Table 1).
 |
Table 1 Comparisons of Clinical Parameters Among the Three Groups [n (%), ( |
Relevance of miR-222-3p Expressions in PB and WMT to Clinical Features of DFU
For more deeply studying the clinical implications of miR-222-3p expression change, we took the median expression in DFU patients’ PB and WMT as the cut point for grouping, classifying those with lower miR-222-3p expression than the median into the group with low expression, and those whose miR-222-3p expression was higher than or equal to the median into group with high expression. We compared the clinical characteristics of foot ulcers in the two groups, finding the positive relevance of miR-222-3p expression in PB to the ulcer duration, Wagner grade, and severity of IDSA infection (P = 0.039, 0.023, 0.046, respectively). Similarly, miR-222-3p expression in WMT showed a positive relevance to ulcer duration, Wagner grade, and severity of IDSA infection (P = 0.023, 0.024, and 0.016, respectively). In addition, the miR-222-3p expression in both PB and WMT was negatively related to the ulcer healing rate after 8 weeks (P = 0.030, 0.028, respectively). The miR-222-3p expression (either in PB or WMT) showed no relevance to other clinical characteristics of foot ulcers (Table 2 and Table 3). Kaplan–Meier survival curve analysis more deeply explored how miR-222-3p expression change in PB and WMT affected the wound healing. According to the analysis, in PB, the median estimated wound healing time in groups with high and low miR-222-3p expression was 9.64 weeks and 8.70 weeks, respectively. Also, in WMT, the median estimated wound healing time in the two groups was 9.76 weeks and 8.71 weeks, respectively. In both PB and WMT, group with high miR-222-3p expression showed higher unhealed DFU cumulative rate relative to low expression group (log-rank, P = 0.011, 0.001, respectively). Group with high expression exhibited longer wound healing time relative to group with low expression (P < 0.05) (Figure 1A and B).
 |
Table 2 Relationship Between miR-222-3p Expression Levels in Peripheral Blood and Clinical Characteristics of DFU [n (%)] |
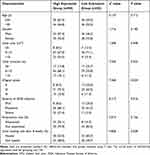 |
Table 3 Relationship Between miR-222-3p Expression Levels in Wound Margin Tissue and Clinical Characteristics of DFU [n (%)] |
Relevance of miR-222-3p Expression in PB and WMT to Other Clinical Parameters in the Three Groups
The NC group did not exhibit obvious relevance of the miR-222-3p expression in the PB to other clinical parameters (P > 0.05). The T2DM group revealed the positive relevance of the miR-222-3p expression in the PB to FPG and HbA1c levels, negative relevance to HDL-C (P < 0.05), and no obvious relevance to the other parameters (P > 0.05). The DFU group presented the positive relevance of the miR-222-3p expression in PB and WMT to the foot ulcer duration, Wagner grade of foot ulcer, IDSA infection severity, CRP, WBC count, P-IL-6, T-IL-6, and T-miR-222-3p expression, the negative relevance to HDL-C, TcPO2, T-VEGF, and T-CD31 expressions (P < 0.05), and no obvious relevance to the other parameters (P > 0.05) (Table 4 and Table 5).
 |
Table 4 Correlations Between miR-222-3p Expression in Peripheral Blood and Other Clinical Parameters in NC Group, T2DM Group, and DFU Group (r) |
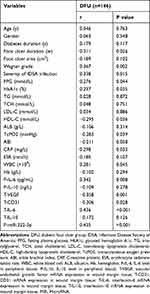 |
Table 5 Correlations Between miR-222-3p Expression in Wound Margin Tissue and Other Clinical Parameters in the DFU Group (r) |
DFU Risk Factor Analysis
We used DFU as the dependent variable, and used age, gender, and all variables (P < 0.1) from the univariate logistic regression analysis (diabetes duration, FPG, HbA1c, TG, LDL-C, HDL-C, ALB, TcPO2, ABI, CRP, WBC, Hb, ESR, P-IL-6, P-IL-10, and P-miR-222-3p) in diabetic patients as independent variables to conduct the multiple stepwise logistic regression analysis. As found, the diabetes duration, HbA1c, CRP, low TcPO2, low ALB, and high P-miR-222-3p expression could be used for independently predicting the DFU risk (Table 6).
 |
Table 6 The Multivariate Stepwise Logistic Regression Analysis of Risk Factors for Diabetic Foot Ulcer |
Mark Verification
For more deeply exploring the diagnostic efficacy of miR-222-3p for DFU in clinical practice, our study focused on detecting the P-miR-222-3p expression in 216 subjects of T2DM and DFU groups, and plotted the receiver operating characteristic (ROC) curve. Accordingly, the AUC of P-miR-222-3p specific to DFU diagnosis reached 0.803 (95% CI 0.713–0.884, P < 0.001), with the optimal cutoff point of 2.52, the sensitivity of 95.93%, and the specificity of 96.27% (Figure 2).
Discussion
This study found that the DFU patients’ PB exhibited obviously increased miR-222-3p expression relative to those without DFU. According to multivariate logistic regression analysis, elevated miR-222-3p expression in PB could independently predict the DFU risk. Furthermore, the miR-222-3p expression in DFU patients’ PB and WMT showed an obvious relevance to Wagner grade, foot ulcer infection severity, and foot ulcer healing rate. For patients whose PB and WMT had high miR-222-3p expression, DFU status was more serious, the ulcer healing rate was lower, and the ulcer healing time was longer. Hence, high miR-222-3p expression strongly indicates the DFU onset on the one hand, and is an underlying biomarker for DFU assessment, therapy, and prognosis on the other hand. Per our knowledge, this study is the first one that focuses on the relevance of miR-222-3p expression change to DFU onset and treatment in T2DM patients. However, this study still has some shortcomings: (1) the study covered a single center; (2) there may be a selection bias due to small sample size; (3) the existence of the miR-222-3p 3’ isoform was not considered in this study when analyzing miR-222-3p, which may have led to misjudgment of the results. Notably, small endogenous RNA (miRNA) usually exists in the form of multiple isoforms and may have different functional roles.21 Further studies shall be conducted for confirming our findings.
According to the study results, T2DM patients presented obviously increased miR-222-3p levels in PB relative to normal-glucose-tolerance controls. Correlation analysis revealed the positive relevance of miR-222-3p expression to FPG and HbA1c in T2DM and DFU patients. In addition, miR-222-3p expression exhibited a negative relevance to HDL-C in T2DM patients. In previous studies, miR-222-3p can not only target and regulate the genetic expression closely associated with glycometabolism in the PI3K-Akt pathway,22 but also regulate genes related to cholesterol metabolism (such as MYLIP),10 thus affecting glycolipid metabolism. Furthermore, high miR-222-3p expression exhibits a positive relation to FPG in patients with GDM, T2DM, and PCOS.8–10 Consistently, in T2DM patients treated with metformin, reduced miR-222 in the PB positively correlates with the reduction of FPG and HbA1c.6 Nevertheless, in patients suffering T2DM and PCOS, high miR-222-3p expressions report lower HDL-C.10 All these conformed to our study results. Our study was unable to explain the upregulated miR-222-3p expression in T2DM patients. According to one previous study, hyperglycemia may regulate miR-24 expression through stimulating c-Myc to be activated.23 However, the role of hyperglycemia in directly upregulating miR-222-3p expression has not be explored. Hence, more studies must be conducted to elucidate the mechanisms of miR-222-3p expression change in environments with high glucose.
Moreover, our study also revealed the positive relevance of miR-222-3p expression in PB and WMT to inflammatory indicators (CRP, WBC count, and IL-6), Wagner grade, and IDSA infection severity in the DFU group. Accordingly, high miR-222-3p expression in DFU patients’ PB and WMT is possibly relevant to the infectious inflammatory state. A study reveals obviously increased miR-222-3p expression in septic mice’ plasma which can promote the production of IL-6, TNF-α, IL-1β, and MIP-2, and is related to sepsis severity.24 Mycoplasma pneumoniae pneumonia (MPP) patients’ PB exhibits obviously higher miR-222-3p level relative to healthy people.25 In vitro studies confirm the two-fold upregulation of miR-222-3p expression in mouse bone marrow-derived macrophages treated with bacterial lipopolysaccharide (LPS).21 MiR-222-3p can increase the expression of proinflammatory cytokines such as IL-1β, IL-6, and TNF-α by targeted regulation of downstream gene functions.25–27 Inflammatory cytokines, such as TNF-α and IFN-γ can also induce the upregulation of miR-222-3p expression.15 This suggests that there may be a bidirectional regulatory effect between miR-222-3p expression and inflammatory factors, forming a vicious circle that aggravates the inflammatory response. All these conformed to our study results. In addition, despite that the DFU and T2DM groups presented obviously different diabetes courses, according to deeper analysis, miR-222-3p showed no correlation with diabetes course in neither of them. Hence, the obviously different disease course between the two groups may impact their miR-222-3p expression difference to a very little extent.
The DFU group had a foot ulcer course of at least four weeks, which is what we call chronic refractory ulcers. According to multivariate regression analysis, the diabetes duration, HbA1c, TcPO2, CRP, and hypoalbuminemia could independently affect foot ulcer occurrence, which conformed to previous study results.20,28 Further, the DFU group presented obviously higher miR-222-3p level in PB and WMT relative to the T2DM group. In the DFU group, the miR-222-3p expression in PB and WMT exhibited a positive relevance to the foot ulcer duration, Wagner grade, and severity of IDSA infection, and a negative relevance to foot ulcer healing rate following eight weeks of treatment. Higher miR-222-3p expression means longer healing time and larger difficulty in complete DFU wound healing. This study did not observe a correlation between amputation rate and the miR-222-3p expression in PB and WMT in the DFU group, which may be due to insufficient follow-up time to accurately track the final results of each participant. According to multiple regression analysis, high miR-222-3p expression in PB and WMT could independently predict foot ulcers risk. Hence, miR-222-3p impacts DFU development, and can evaluate DFU severity and prognosis as a useful marker.
Researches have not well elucidated the action mechanism of miR-222-3p in the wound healing at present. Generally, persistent and excessive inflammation and oxidative stress in the wound, deficient wound angiogenesis, and damaged function of epidermal cells involved in wound healing primarily lead to DFU healing difficulty.29 A study has found that in a liver injury model induced by staphylococcal enterotoxin B (SEB), inhibition of miRNA-222-3p expression can reduce SEB-induced liver inflammatory injury.30 According to in vitro studies, miR-222-3p expression downregulation improves H2O2-induced human lens epithelial HLE-B3 cell damage.31 Studies have found the close relevance of the elevated miR-222-3p expression to the expression of genes related to chronic inflammation and oxidative stress in tissues, and reveals the ability of miR-222-3p to elevate proinflammatory cytokines expression and enhance oxidative stress conditions.32,33 However, there is still no evidence proving the direct involvement of miR-222-3p in inflammation and oxidative stress in diabetic skin chronic wounds. Further studies are needed in this respect. As reported, miR-222-3p, as a kind of miRNA, ranks second in human fibroblasts in terms of the abundance. After human fibroblasts are treated with LPS, the miR-222-3p expression underwent an obvious upregulation,21 which may have adverse effects on the biological functions of fibroblasts. Therefore, we speculated that miR-222-3p may affect wound epithelialization. In addition, angiogenesis in wound tissues vitally impacts the DFU wound healing. In studies, high miR-222-3p expression results in dysfunction of vascular endothelial cells and reduce angiogenesis.16,18 In a rat model of abdominal aortic aneurysm, downregulation of miR-222-3p expression promoted the migration, invasion, and recruitment regarding endothelial progenitor cells; repaired vascular endothelial damage; and maintained vascular integrity.34 In human atherosclerotic vascular samples, the vascular intima observes increased miR-222-3p expression, and according to in vitro studies, miR-222-3p overexpression in human aortic endothelial cells (HAEC) can lead to intracellular ROS accumulation and apoptosis and participate in the occurrence of atherosclerosis.15 The TcPO2 level and VEGF and CD31 expression levels in the WMTs can reflect circulatory perfusion and angiogenesis in the wound healing process.35,36 In our study, in the DFU group, miR-222-3p expression in PB and WMTs exhibited a negative relevance to the expression of TcPO2, VEGF, and CD31. Hence, the way miR-222-3p impacts angiogenesis may be one of the important mechanisms of miR-222-3p involvement in wound healing. This needs to be confirmed by further studies.
The miR-222-3p expression in PB is capable of predicting the MPP diagnosis and prognosis from biological perspective, and also serves for prostate cancer detection as a biomarker without invasiveness.37,38 In this study, based on ROC curve analysis results, miR-222-3p expression in T2DM patients’ PB could predict DFU risk as a useful biomarker. In addition, miR-222-3p expression in PB and WMT showed a positive relevance to DFU duration, complete healing time of DFU and a negative relevance to the wound healing rate after eight weeks of treatment. Hence, miR-222-3p expressions in PB and WMT are useful for DFU diagnosis and prognosis. Despite this, why miR-222-3p expression elevated in DFU patients’ PB and WMT shall be determined in further studies. Notably, our study determined the good agreement of miR-222-3p regarding its expression in PB and WMT and its value for wound healing prediction. The PB sampling has a low trauma risk, and it is easy and convenient to determine miR-222-3p in PB. On that account, combining our study with previous studies,20,39 we recommend detecting miR-222-3p expression in PB to predict the therapeutic outcome of DFU.
Conclusion
According to our study, the increasing miR-222-3p expression in T2DM patients’ PB exhibited a close relation to DFU occurrence, which could be used as a biomarker for DFU diagnosis (Figure 3). Besides, elevated miR-222-3p expression in the PB and WMT reported poor DFU prognosis. Nevertheless, the present study could not elucidate the causal relationship between miR-222-3p and DFU, nor could it explain why the miR-222-3p expression increased in DFU patients’ PB and WMT. Therefore, in the future, more research is required for more deeply exploring the action mechanism of miR-222-3p as well as assessing whether miR-222-3p can be a new treatment target for DFU.
 |
Figure 3 MiR-222-3p effectively served for DFU diagnosis and prognosis as a useful biomarker. ↑: increase; ↓: decrease. |
Abbreviations
T2DM, type 2 diabetes mellitus; DFU, diabetic foot ulcer; FPG, fasting plasma glucose; HbA1c, glycosylated hemoglobin A1c; TCH, total cholesterol; TG, triacylglycerol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; ALB, serum albumin; Hb, hemoglobin; WBC, white blood cell; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; P-IL-6, IL-6 level in peripheral blood; P-IL-10, IL-10 level in peripheral blood; TcPO2, transcutaneous oxygen pressure; ABI, ankle brachial index; qRT-PCR, Real-time quantitative PCR assays; T-VEGF, vascular endothelial growth factor mRNA expression in wound margin tissue; T-CD31, CD31 mRNA expression in wound margin tissue; T-IL-6, interleukin-6 mRNA expression in wound margin tissue; T-IL-10, interleukin-10 mRNA expression in wound margin tissue; MiR, MicroRNA; LPS, lipopolysaccharide; ROC, Receiver operating characteristic; AUC, area under the curves; IDSA, Infectious Disease Society of America; MPP, Mycoplasma pneumoniae pneumonia.
Data Sharing Statement
The datasets used and/or analyzed in the study can be obtained from the corresponding author on reasonable request. Inquiries for data access may be sent to: [email protected].
Ethical Approval
The study completed with the approval of the Medical Ethics Committee of the First Affiliated Hospital of Anhui Medical University (Ethics batch number P20210039), and received written informed consent from all participants. In studies involving human participants, all procedures were performed following the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Acknowledgments
We want to express our appreciation to all patients for their participation, and to all medical staff and researchers, including doctors, nurses, and researchers from the Department of Endocrinology and the Burns Department in the First Affiliated Hospital of Anhui Medical University.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was completed with the support from the Natural Science Foundation of Anhui Province in China (2108085MH269) and the Natural Science Research Project of Colleges and Universities in Anhui Province (KJ2021A0274). The funding body did not participate or impact the study design, data collection, analysis, and interpretation, or the manuscript writing.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Zhang P, Lu J, Jing Y, Tang S, Zhu D, Bi Y. Global epidemiology of diabetic foot ulceration: a systematic review and meta-analysis. Ann Med. 2017;49(2):106–116. doi:10.1080/07853890.2016.1231932
2. Everett E, Mathioudakis N. Update on management of diabetic foot ulcers. Ann N Y Acad Sci. 2018;1411(1):153–165. doi:10.1111/nyas.13569
3. Saliminejad K, Khorram Khorshid HR, Soleymani Fard S, Ghaffari SH. An overview of microRNAs: biology, functions, therapeutics, and analysis methods. J Cell Physiol. 2019;234(5):5451–5465. doi:10.1002/jcp.27486
4. Goodarzi G, Maniati M, Qujeq D. The role of microRNAs in the healing of diabetic ulcers. Int Wound J. 2019;16(3):621–633. doi:10.1111/iwj.13070
5. Erener S, Marwaha A, Tan R, Panagiotopoulos C, Kieffer TJ. Profiling of circulating microRNAs in children with recent onset of type 1 diabetes. JCI Insight. 2017;2(4):e89656. doi:10.1172/jci.insight.89656
6. Ortega FJ, Mercader JM, Moreno-Navarrete JM, et al. Profiling of circulating microRNAs reveals common microRNAs linked to type 2 diabetes that change with insulin sensitization. Diabetes Care. 2014;37(5):1375–1383. doi:10.2337/dc13-1847
7. Ortega FJ, Mercader JM, Catalán V, et al. Targeting the circulating microRNA signature of obesity. Clin Chem. 2013;59(5):781–792. doi:10.1373/clinchem.2012.195776
8. Wang Q, Fang C, Zhao Y, Liu Z. Correlation study on serum miR-222-3p and glucose and lipid metabolism in patients with polycystic ovary syndrome. BMC Womens Health. 2022;22(1):398. doi:10.1186/s12905-022-01912-w
9. Tagoma A, Alnek K, Kirss A, Uibo R, Haller-Kikkatalo K. MicroRNA profiling of second trimester maternal plasma shows upregulation of miR-195-5p in patients with gestational diabetes. Gene. 2018;672:137–142. doi:10.1016/j.gene.2018.06.004
10. Parker DC, Wan M, Lohman K, et al. Monocyte miRNAs are associated with type 2 diabetes. Diabetes. 2022;71(4):853–861. doi:10.2337/db21-0704
11. Yao X, Wang Y, Wang L, Cao M, Chen A, Zhang X. Expression patterns of serum MicroRNAs related to endothelial dysfunction in patients with subclinical hypothyroidism. Front Endocrinol. 2022;13:981622. doi:10.3389/fendo.2022.981622
12. Wang D, Sang Y, Sun T, et al. Emerging roles and mechanisms of microRNA-222-3p in human cancer (Review). Diabetes J Oncol. 2021;58(5):20.
13. Byun JS, Gardner K. Wounds that will not heal: pervasive cellular reprogramming in cancer. Am J Pathol. 2013;182(4):1055–1064. doi:10.1016/j.ajpath.2013.01.009
14. Suárez Y, Sessa WC. MicroRNAs as novel regulators of angiogenesis [published correction appears in Circ Res. 2009 Apr 10;104(7):e55]. Circ Res. 2009;104(4):442–454. doi:10.1161/CIRCRESAHA.108.191270
15. Xue Y, Wei Z, Ding H, et al. MicroRNA-19b/221/222 induces endothelial cell dysfunction via suppression of PGC-1α in the progression of atherosclerosis. Atherosclerosis. 2015;241(2):671–681. doi:10.1016/j.atherosclerosis.2015.06.031
16. Poliseno L, Tuccoli A, Mariani L, et al. MicroRNAs modulate the angiogenic properties of HUVECs. Blood. 2006;108(9):3068–3071. doi:10.1182/blood-2006-01-012369
17. Li J, Wang J, Wang Z. Circ_0006768 upregulation attenuates oxygen-glucose deprivation/reoxygenation-induced human brain microvascular endothelial cell injuries by upregulating VEZF1 via miR-222-3p inhibition. Metab Brain Dis. 2021;36(8):2521–2534. doi:10.1007/s11011-021-00775-8
18. Yasmeen S, Kaur S, Mirza AH, Brodin B, Pociot F, Kruuse C. miRNA-27a-3p and miRNA-222-3p as novel modulators of phosphodiesterase 3a (PDE3A) in cerebral microvascular endothelial cells. Mol Neurobiol. 2019;56(8):5304–5314. doi:10.1007/s12035-018-1446-5
19. Xu J, Bai S, Cao Y, et al. miRNA-221-3p in endothelial progenitor cell-derived exosomes accelerates skin wound healing in diabetic mice. Diabetes Metab Syndr Obes. 2020;13:1259–1270. doi:10.2147/DMSO.S243549
20. Xu M, Li Y, Tang Y, Zhao X, Xie D, Chen M. Increased expression of miR-155 in peripheral blood and wound margin tissue of type 2 diabetes mellitus patients associated with diabetic foot ulcer. Diabetes Metab Syndr Obes. 2022;15:3415–3428. doi:10.2147/DMSO.S376292
21. Nejad C, Pillman KA, Siddle KJ, et al. miR-222 isoforms are differentially regulated by type-I interferon. RNA. 2018;24(3):332–341. doi:10.1261/rna.064550.117
22. Filardi T, Catanzaro G, Grieco GE, et al. Identification and validation of miR-222-3p and miR-409-3p as plasma biomarkers in gestational diabetes mellitus sharing validated target genes involved in metabolic homeostasis. Int J Mol Sci. 2022;23(8):4276. doi:10.3390/ijms23084276
23. Xiang Y, Cheng J, Wang D, et al. Hyperglycemia repression of miR-24 coordinately upregulates endothelial cell expression and secretion of von Willebrand factor. Blood. 2015;125(22):3377–3387. doi:10.1182/blood-2015-01-620278
24. Xu J, Feng Y, Jeyaram A, Jay SM, Zou L, Chao W. Circulating plasma extracellular vesicles from septic mice induce inflammation via microRNA- and TLR7-dependent mechanisms. J Immunol. 2018;201(11):3392–3400. doi:10.4049/jimmunol.1801008
25. Liu XX, Wang MJ, Kan QN, et al. Kukoamine A improves mycoplasma pneumoniae pneumonia by regulating miR-222-3p/superoxide dismutase 2. Biomed Res Int. 2022;2022:2064013. doi:10.1155/2022/2064013
26. Yang L, Zhang X, Liu X. Long non-coding RNA GAS5 protects against Mycoplasma pneumoniae pneumonia by regulating the microRNA-222-3p/TIMP3 axis. Mol Med Rep. 2021;23(5):380. doi:10.3892/mmr.2021.12019
27. Xia F, Bo W, Ding J, Yu Y, Wang J. MiR-222-3p aggravates the inflammatory response by targeting SOCS1 to activate STAT3 signaling in ulcerative colitis. Turk J Gastroenterol. 2022;33(11):934–944. doi:10.5152/tjg.2022.21769
28. Shi L, Wei H, Zhang T, et al. A potent weighted risk model for evaluating the occurrence and severity of diabetic foot ulcers. Diabetol Metab Syndr. 2021;13(1):92. doi:10.1186/s13098-021-00711-x
29. Wang N, Ju S. Research advances on the mechanism of refractory healing of diabetic foot ulcer. Zhonghua Shao Shang Za Zhi. 2022;38(11):1085–1089. doi:10.3760/cma.j.cn501225-20220227-00038
30. Zhang P, Yu J, Gui Y, Sun C, Han W. Inhibition of miRNA-222-3p relieves staphylococcal enterotoxin B-induced liver inflammatory injury by upregulating suppressors of cytokine signaling 1. Yonsei Med J. 2019;60(11):1093–1102. doi:10.3349/ymj.2019.60.11.1093
31. Xu X, Gao R, Li S, et al. Circular RNA circZNF292 regulates H2 O2 -induced injury in human lens epithelial HLE-B3 cells depending on the regulation of the miR-222-3p/E2F3 axis. Cell Biol Int. 2021;45(8):1757–1767. doi:10.1002/cbin.11615
32. Wijayatunga NN, Pahlavani M, Kalupahana NS, et al. An integrative transcriptomic approach to identify depot differences in genes and microRNAs in adipose tissues from high fat fed mice. Oncotarget. 2018;9(10):9246–9261. doi:10.18632/oncotarget.24226
33. Wu C, Liu Z, Ma L, et al. MiRNAs regulate oxidative stress related genes via binding to the 3’ UTR and TATA-box regions: a new hypothesis for cataract pathogenesis. BMC Ophthalmol. 2017;17(1):142. doi:10.1186/s12886-017-0537-9
34. Gao L, Chen M, Li F. MiR-222-3p downregulation prompted the migration, invasion and recruitment of endothelial progenitor cells via ADIPOR1 expression increase-induced AMKP activation. Microvasc Res. 2021;135:104134. doi:10.1016/j.mvr.2021.104134
35. Singer AJ, Choi Y, Rashel M, Toussaint J, McClain SA. The effects of topical nitric oxide on healing of partial thickness porcine burns. Burns. 2018;44(2):423–428. doi:10.1016/j.burns.2017.07.017
36. DiPietro LA. Angiogenesis and wound repair: when enough is enough. J Leukoc Biol. 2016;100(5):979–984. doi:10.1189/jlb.4MR0316-102R
37. Chu C, Lei X, Li Y, et al. High expression of miR-222-3p in children with Mycoplasma pneumoniae pneumonia. Ital J Pediatr. 2019;45(1):163. doi:10.1186/s13052-019-0750-7
38. Heydari Z, Moudi E, Sadeghi F, et al. Circulating plasma miR222-3P status and its potential diagnostic performance in prostate cancer. J Gene Med. 2022;24(12):e3459. doi:10.1002/jgm.3459
39. Liu L, Chen R, Jia Z, et al. Downregulation of hsa-miR-203 in peripheral blood and wound margin tissue by negative pressure wound therapy contributes to wound healing of diabetic foot ulcers. Microvasc Res. 2022;139:104275. doi:10.1016/j.mvr.2021.104275
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



