Back to Journals » Pediatric Health, Medicine and Therapeutics » Volume 14
Role of Anti-Vascular Endothelial Growth Factor (Anti-VEGF) in the Treatment of Retinopathy of Prematurity: A Narrative Review in the Context of Middle-Income Countries
Received 28 September 2022
Accepted for publication 31 January 2023
Published 15 February 2023 Volume 2023:14 Pages 59—69
DOI https://doi.org/10.2147/PHMT.S391591
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Roosy Aulakh
Mangat Ram Dogra,1 Anand Vinekar2
1Grewal Eye Institute, Chandigarh, India; 2Narayana Nethralaya Eye Institute, Bangalore, India
Correspondence: Anand Vinekar, Narayana Nethralaya Eye Institute, Bangalore, India, Email [email protected]
Abstract: The rise in preterm births and higher survival rates of premature infants have led to a global increase in retinopathy of prematurity (ROP), a vasoproliferative retinal disorder common in premature infants. ROP is one of the leading causes of childhood blindness. Clinical manifestation of ROP ranges from mild abnormal retinal neovascularization to bilateral retinal detachment and vision loss. The incidence of ROP is higher in middle income countries, including India, which has the highest number of global preterm births. Low birth weight and low gestational age are the primary risk factors for ROP; however, anemia, cardiac defects, blood transfusion, apnea, sepsis, respiratory distress syndrome, high exposure to oxygen and poor postnatal weight gain may also contribute to its development. India has stringent ROP screening guidelines revised in 2018, and screening of infants with either birth weight < 2000 grams or gestational age < 34 weeks is mandated. With an improved understanding of the pathogenesis of ROP in the past decades and advances in clinical research, treatment for ROP has evolved from cryotherapy to laser retinal ablation. Most recently, anti-vascular endothelial growth factor (anti-VEGF) drugs have emerged as a favorable treatment option for zone-I and II ROP. This article reviews the current approaches for ROP treatment in India with a particular focus on anti-VEGF drugs. The article also integrates the understanding of safety and risk-benefit evaluation of the current approaches in ROP management. The review concluded that there is a need to increase the ROP screening not only for preterm and low birth weight but also for optimal gestational age infants with healthy birth weight. Anti-VEGF therapies have shown improved efficacy, although studies are required to establish the long-term safety.
Keywords: retinal disorder, childhood blindness, ROP management, anti-VEGF therapy, laser retinal ablation
Introduction
Retinopathy of prematurity (ROP) is a vasoproliferative retinal disorder and a common complication in extremely premature infants.1,2 The World Health Organization’s vision program has identified ROP as one of the principal causes of childhood blindness.3,4 Furthermore, ROP is predominant in infants with low birth weight (≤1500 grams) and/or low gestational age at birth (≤32 weeks).5,6 It was estimated that 32,200 infants worldwide developed vision impairment due to ROP in 2010, with 65% of the cases from middle-income countries.7,8
India has the highest number of global preterm births-3.5 million per year, and is currently facing the third epidemic of ROP.9 Studies conducted across India have reported the incidence of ROP varying between 2.3% and 47.2%.10–14 India has accounted for about 10% of the estimated global visual impairment due to ROP in 2010.8 Considering that 1 in 6 preterm births in India occurs at a gestational age of <32 weeks and 40% of which require neonatal intensive care unit (NICU) admission, with an 80% survival rate, it can be inferred that about 190,000 neonates are at risk of developing ROP. Assuming that 10% of the at-risk infants require intervention, about 20,000 infants in India would require ROP treatment each year.15 However, as per a multicenter study report, more number of infants require ROP treatment.16
Infants born prematurely have delayed retinal vascularization, leading to the formation of a peripheral avascular zone initially.2,17 As the infant matures, the non-vascularization in the retina, coupled with its high metabolic demands, causes hypoxia, which induces local secretion of vascular endothelial growth factor (VEGF), those are endothelial cell-specific mitogen and regulates angiogenesis. An increase in VEGF induces pathologic angiogenesis, leading to abnormal retinal neovascularization, and lead to ROP.2,17–20 In some cases, the vascular growth observed in ROP is mild and may resolve over time. However, there are instances where the vascular changes are severe and, if left untreated, these changes may lead to macular dragging, retinal scarring, and retinal detachment, which are the primary causes of vision impairment and blindness in ROP.7,21
Lack of awareness, especially among the rural population, and limited specialized ROP professionals are the key contributors to increase in at-risk population of ROP.9,22 In addition to birth weight and age, supplemental oxygen, anemia, blood transfusions, intraventricular hemorrhage, breathing difficulties, infant health and poor postnatal weight gain contribute to ROP risk. Contrary, some studies highlight the incidence of ROP among gestationally older infants in middle income countries compared to western countries.10,23 Additionally, there is a report from India showing an increasing trend towards ROP occurrence among newborns with optimal birth weight (>1500 to 2000 grams) and gestational age.23
The International Classification of ROP (ICROP) classifies ROP based on the disease location and retinal involvement (zone I–III), the severity of the disease (stages 1–5), and the presence of concomitant disease (a complicating factor at any stage associated with increased dilatation and tortuosity of the retinal vessels). A severe form of ROP, not representing the classical stages 1 to 5, is termed aggressive posterior ROP (APROP), which rapidly progresses to stage 5 ROP if left untreated.6,18,24 However, the third edition of ICROP (ICROP3) consensus statement puts forth refined classification metrics (eg, posterior zone II, notch, subcategorization of stage 5) that divides ROP into acute and retinal detachment stages. The committee also recommended a new terminology, aggressive ROP (AROP), in place of APROP. The update was in line with increasing instances where ROP may occur beyond the posterior retina and in preterm infants. The rapid development of pathologic neovascularization and severe concomitant disease without progression being observed through the typical stages of ROP is the cornerstone for AROP.25
Severe ROP may manifest as white pupils (leukocoria), abnormal eye movements (nystagmus), and crossed eyes. The operational guidelines for ROP in India and the screening guidelines by Rashtriya Bal Swasthya Karyakram (RBSK) mandate screening for all infants with birth weight ≤2000 grams, gestational age ≤34 weeks, or gestational age between 34 and 36 weeks, with any of the risk factors such as respiratory distress syndrome, cardio-respiratory support, prolonged oxygen therapy, chronic lung disease, fetal hemorrhage, intraventricular hemorrhage, blood transfusion, exchange transfusion, neonatal sepsis, apneas, or poor postnatal weight gain.15,18,24,26 In preterm delivery cases, the initial retinal screening needs to be conducted after discharge from NICU.26
The overall goal of ROP management is to prevent retinal detachment, reduce the incidence of subsequent blindness, and promote optimized visual outcomes.17,24 For decades, the standard of care followed for ROP has been peripheral retinal ablation with either cryotherapy or laser photocoagulation therapy.27 Further, in the 1990s, laser photocoagulation therapy gained widespread acceptance and almost replaced cryotherapy as the standard of care for ROP.27 Laser therapy is associated with better long-term structural, visual, and refractive outcomes compared to cryotherapy.28
An increase in VEGF induces retinal angiogenesis, and subsequent abnormal neovascularization leads to ROP. Therefore, intravitreal anti-VEGF agents have emerged as a promising treatment alternative for ROP management in premature infants.20,28 Anti-VEGF agents suppress the excessive levels of VEGF within the retina and vitreous, along with targeting the abnormal vascularization observed in ROP.20,28 This article consolidates the current knowledge on the available anti-VEGF drugs used in managing ROP in India and provides a comprehensive review of the ROP treatment in the country. It also highlights the safety and risk-benefit evaluation of the current approaches in ROP management and aims to integrate key recommendations for ROP-treating ophthalmologists.
Management of ROP
The ROP disease can be classified based on treatment requirements. Type 1 ROP requires treatment and includes zone I, any stage ROP with plus disease or stage 3 with or without plus disease, and zone II, stage 2 or 3 ROP with plus disease. An AROP in any zone required treatment as needed for ROP.18 Previously, the multicenter trial of Cryotherapy for ROP (CRYO ROP)30 and the Early Treatment for Retinopathy of Prematurity Randomized Trial (ETROP)31 established the criteria for threshold and pre-threshold disease to describe type 1 ROP. Type 2 ROP that requires close follow-up includes zone I, stage 1 or 2 ROP without concomitant disease, and zone II, stage 3 ROP without the concomitant disease.18,24
Laser Photocoagulation Therapy
The current standard of treatment for ROP is laser photocoagulation of the non-vascularized retina. Laser therapy is associated with better long-term structural, visual, and refractive outcomes compared with cryotherapy.29 While both cryotherapy and laser therapy ablate the majority of retinal cells that produce VEGF, these therapies do not directly reduce all the elevated levels of VEGF, and when they do, it is a less rapid response.32
Certain studies have shown a high success rate (92–95%) after laser therapy, depicting it as a primary modality in ROP and AROP.33–35 However, the success rate of laser therapy was significantly lower (p=0.01) in the referred category compared to the non-referred category, attributing to the potential delay in laser therapy and disease progression to the advanced stage at the time of treatment initiation.36 Laser therapy also minimizes systemic side effects and recurrent/persistent avascular zones.33 Recent evidence shows that a two-session laser procedure in AROP is associated with fewer and smaller hemorrhages and no fibrosis compared with a single session.37 However, certain complications, such as new‑onset hemorrhage, anterior segment ischemia, cataract, intraprocedural bradycardia, and vitreous hemorrhage are observed after laser therapy.34,35
Anti-VEGF Therapy in ROP
The need for alternate treatment options has incited a rapid increase in worldwide off-label use of anti-VEGF therapy for ROP management, especially for zone I disease.28,38 A 2016 survey of the Indian ROP society reported that around 2% of members claimed anti-VEGF agents as the first choice of ROP treatment. Whereas 57% of members recommended the selective use of anti-VEGF and maintained laser therapy as the first choice.9 In an another recent survey conducted among the Indian ROP (iROP) society members, bevacizumab was the preferred choice by more than half (54%).39 Although, most of the respondents practicing in public institutions preferred ranibizumab. This is attributable to the government advisory on the limited use of bevacizumab. Irrespective of the agent of choice, most respondents (77.9%) confirmed using half the adult dose. However, more than 96% of the members suggested a need for specific guidelines on anti-VEGF use in ROP.39
Bevacizumab
Bevacizumab is a humanized anti-VEGF monoclonal antibody that inhibits all VEGF isoforms and commonly used anti-VEGF agent with the half-life of 20 days.40 The National Neonatology Forum (NNF), India, guidelines recommend bevacizumab for treating type 1 ROP involving zone I, although recommendations are weak and conditional due to insufficient evidence.18 The most frequently used dose of bevacizumab in ROP is 0.625 mg (range, 0.03 mg–1.25 mg).41,42 The BEAT-ROP study demonstrated a significant benefit of bevacizumab monotherapy (0.625 mg) over laser therapy for the treatment of zone I stage 3+ disease in terms of absolute difference in the risk of recurrence.32 However, the benefit was not observed for zone II posterior disease, which may be related to insufficient sample size.28,32 Treatment with bevacizumab allowed continued development of peripheral retinal vessel, whereas treatment with laser resulted in permanent destruction. Although the outcome of the BEAT-ROP study cannot be generalized since a larger proportion of infants enrolled in the study were largely hispanic.32
The pharmacokinetics of bevacizumab is not established in infants, though a study reported significant reduction in VEGF concentration in systemic circulation following bevacizumab administration.43 A lower effective dose of bevacizumab must be considered while administering to infants.44
Ocular complications such as vitreous hemorrhage, retinal detachment are reported with intravitreal (2.5 mm) bevacizumab injection.43 The SAFER-ROP study described a protocol for intravitreal injections of anti-VEGF to mitigate avoidable complications. The findings of study suggested that 0.75 mm to 1.0 mm from the limbus is safe to inject anti-VEGF.43
Ranibizumab
Ranibizumab is a humanized anti-VEGF monoclonal antibody fragment with a binding affinity toward all VEGF-A isoforms.20 It has a shorter serum half-life of 2 hours and is considered safe for preterm infants. The recommended dose for ranibizumab is 0.2 mg (range, 0.15–0.3 mg), and it can be administered bilaterally on the same day.45–48 The recommended minimum interval between 2 doses is 4 weeks, and a total of up to 3 injections per eye may be administered within 6 months of treatment.49
Efficacy of ranibizumab was first evaluated in a pilot study CARE ROP.50 This study reported control of ROP without any rescue therapy in 87.5% infants with 0.12 or 0.20 mg of ranibizumab. Peripheral normal vascularization developed more rapidly and frequently with 0.12 mg dose of ranibizumab. Further the 2 year RAINBOW study conducted across 26 countries on very–low birth weight infants with bilateral ROP highlighted the superiority of 0.2 mg ranibizumab over laser therapy (80% vs 66% treatment success).51 Ranibizumab treatment resulted in fewer eyes with unfavorable structural outcomes than laser therapy. Systemic safety profile in a preterm population, and ocular adverse events is consistent with the established safety profile of ranibizumab in adults. RAINBOW is the first study to measure ranibizumab pharmacokinetics in preterm infants. Based on RAINBOW study findings, ranibizumab was approved by the European Union and India for treating ROP with zone I stage 1+, 2+, 3 or 3+, zone II (stage 3+), or AROP disease.47 A RAINBOW Extension Study to evaluate the long-term efficacy and safety of ranibizumab compared with laser therapy is ongoing and results are awaited.52
Aflibercept
Aflibercept is a recombinant fusion protein with binding affinity to VEGF-A, PIGF isoforms.46 Studies have demonstrated aflibercept to be effective in type 1 ROP, however significant reduction of systemic VEGF levels was observed with aflibercept compared with ranibizumab.53 The serum half-life of aflibercept is 5 to 6 days, which is lesser than bevacizumab but higher than ranibizumab.54
A consecutive case series of 46 Indian eyes reported the effectiveness of aflibercept 1 mg (0.025 mL) in regression of all classes of ROP.55 Recently, the FIREFLEYE randomized clinical trial among preterm infants with ROP reported 85.5% of treatment success with 0.4-mg dose of intravitreal aflibercept compared to 82.1% with laser photocoagulation at week 24.56 A Phase 3 randomized controlled multicenter clinical trial is conducted to assess the efficacy, safety, and tolerability of aflibercept compared to laser photocoagulation in patients with ROP (BUTTERFLEYE study: NCT04101721), although the findings are not published yet.57 Nonetheless, the use of aflibercept in ROP has been minimal compared to adult retinal pathologies.28
Safety of Anti-VEGF Therapy
Anti-VEGF drugs have been associated with ocular and systemic adverse effects.28,58 These adverse effects are intraocular inflammation, corneal opacification, endophthalmitis, cataract formation, ocular hemorrhage, and retinal detachment.45,58–60 The long-term ocular complications identified are abnormal vascular branching, shunt vessels, and refractive errors such as high myopia and astigmatism.28,61,62
Studies on systemic safety have reported lower motor skills and more possibilities of developing neurodevelopmental disability in patients receiving bevacizumab compared with laser therapy.63,64 Rare systemic complications such as hepatic dysfunction, nephropathy, and thromboembolic events have also been reported.28,58,65,66 Bevacizumab is detected in serum within one day of administration and can reduce serum VEGF levels for as long as 8 weeks.43,67,68
Ranibizumab has lesser potential toxicity than bevacizumab in infants with ROP.26,27,47 In RAINBOW study, the t1/2 of ranibizumab was about 5.6 days in intravitreal fluid and 0.3 days in systemic circulation.51 Contrary, bevacizumab has longer half-life of 21 days in systemic circulation of preterm infants.69 Further studies are warranted to explore the comparative safety and efficacy of bevacizumab, ranibizumab and, aflibercept.
Limitations of Anti-VEGF Therapy
The potential limitations with anti-VEGF therapy are the long-term follow-up time and lost to follow-up of infants residing in rural and outreach areas, which is a major concern in Asian countries.9,65 Since VEGF play an important role in retina, lungs, and brain development; thus a long-term follow-up is needed to assess any developmental issues in organ systems.18,45,65 Retinal examinations with long-term follow-ups are required since recurrence of ROP can occur up to 69 weeks of PMA after using anti-VEGF drugs.65 Regular follow-ups are required to assess the refractive errors, squint, systemic adverse effects (such as retinal ischemia, retinal tear, and delayed-onset retinal detachment), and recurrence of ROP.18,28,45,65 Compared to laser therapy, the recurrence of ROP is more prevalent with anti-VEGF uses, and the rate of recurrence varies with the type of anti-VEGF.45,70–73 The long-term systemic adverse effects of anti-VEGF agents limit their widespread use.28,45,70,71,74
There is an ambiguity related to the optimal dose of anti-VEGF in infants with ROP. Most of the doses used in infants are the half of the dose administered in adults for ocular neovascular diseases.48 However, studies suggest this dose to be relatively high for infants due to their low vitreous volume and body weight.45,48,75
The lower limit of bevacizumab- 0.031 mg, which was only 0.6% of the dose used in BEAT-ROP study was found effective for most ROP infants.76 Administration of this low-dose demonstrated better structural outcomes and reduced risk of neurodevelopmental or other organs disabilities.77
Similarly, a 0.2 mg of ranibizumab, one third of adult dose, is found effective in infants with ROP.45,69,78 The outcomes are supported by interim analysis of the RAINBOW extension study showing no new ocular structural abnormalities and possibly better vision-related quality of life.52
Recently a multicenter, dose de-escalation Phase 1 study, conducted by the Pediatric Eye Disease Investigator Group (PEDIG) reported, 0.004 mg could be the lowest effective dose of bevacizumab to minimize the systemic effects and promote normal retinal vascularization.79
Risk-Benefit Evaluation of Available Therapies for ROP Management
Cryotherapy was the first ablation therapy used to treat ROP, which was associated with ocular complications, including posterior retinal detachment, high myopia, retrolental tissue, and retinal folds with poor visual acuity.28,80 After cryotherapy, laser therapy has been the standard of care for type 1 high-risk pre-threshold ROP.28,45,81 The therapy is effective for ROP stages 1 to 3 though it demonstrates low success rates for AROP.18,45,51,74 Laser ablation has several advantages, including precise delivery, reduced ocular morbidity, regression of ROP within six weeks or less, and improved long-term outcomes.28,74 Although deemed effective, laser therapy may lead to potential ocular adverse effects such as permanent peripheral visual field loss, cataract formation, vitreous hemorrhage, iris synechiae, strabismus, high myopia, and induction of scarring.18,28,38,45,71,82
Now-a-days, intravitreal anti-VEGF agents for ROP have gained attention as a potential alternative. Compared to laser therapy, anti-VEGF treatment has a convenient and faster therapeutic procedure, with an injection administration time of 2 to 3 minutes/eye versus 30 to 40 minutes/eye for laser therapy.45,71,74 Furthermore, anti-VEGF administration does not require special equipment.45,71,74 Anti-VEGF agents can be used for infants who do not qualify for the laser procedure. The therapy can promote faster vascular regression, a lesser degree of peripheral visual field loss, a lesser risk of myopia, and does not induce any visible scarring compared with laser procedures.45,46,51,71,74
Anti-VEGF agents have shown better success rates in treating zone I, II ROP and AROP.28,51,71,82 A meta-analysis validates the efficacy of anti-VEGF agents in type-1 ROP and AROP over laser therapy.83 A recent large Asian population-based study reported a potential advantage of the anti-VEGF agents for the initial treatment of zone I ROP and A-ROP. Contrary to conventional laser therapy, the initial regression rate was 86% for the anti-VEGF group and 71% for laser ablation. Contrary, for zone II ROP, laser therapy and anti-VEGF agents showed similar efficacy.84
The combination of anti-VEGF and laser therapy is now in practice for Type 1 ROP and AROP.85 Although, post-combination treatment, few eyes required surgery (16 out of 87), while others demonstrated the presence of detached retina (4 out of 87).
The recurrence of ROP is observed as late as 50 weeks of PMA after IVB or IVR but earlier after laser. Longer follow-up is needed for infants treated with anti-VEGF, especially in cases with risk factors like low Apgar score, Zone I ROP, early PMA at initial treatment.
Selecting a Patient with ROP for Anti-VEGF Therapy
Based on the literature, anti-VEGF agents may be effective for treating patients with zone I, II ROP, and AROP where laser therapy is unsuccessful.28,51,71,82,83 Anti-VEGF agents can be considered a pre-operative measure in patients with retinal detachment and high vascularity to decrease intraoperative bleeding.82,83,86 The therapy may also be used in patients who do not qualify for laser therapy due to poorly dilating pupils and vitreous haze.74 Additionally, it is advised to AROP infants in high-risk cases of foveal development, retinal detachment, and recalcitrant disease after laser therapy.74 Social-economic aspects should be considered while selecting patients for anti-VEGF therapy to mitigate the risk of patients lost to follow-up.40
Few Clinical Scenarios for ROP Management
Scenario 1: Administration of Topical Anesthesia Prior to Anti-VEGF Injection in Infants with ROP
There is a shortfall of standardized techniques for the administration of anti-VEGF agents in ROP. A recent protocol on anti-VEGF injections recommends administering a topical anesthetic into the eye before anti-VEGF agents based on a neonatologist’s discretion.15,43 Studies have reported pain relief with topical anesthesia during anti-VEGF injections in the ROP.87,88
Scenario 2: Use of Topical Antibiotics as Prophylaxis or Post-Treatment to Anti-VEGF Injection
Neonates are vulnerable to infections; moreover, NICUs may be contaminated with pathologic bacteria; precautions are needed to prevent nosocomial infections. The protocol for anti-VEGF injections recommends antiseptic/antibiotic (betadine 5% or 10%) before and after intravitreal anti-VEGF injection.15,43,89 As per the guidelines of the Vitreo-retinal society of India (VRSI), routine use of topical antibiotics is recommended a day prior and 3 days post intravitreal anti-VEGF injections.15,89
Scenario 3: Intravitreal Anti-VEGF Injection in Both Eyes
The VRSI and AIOS guidelines for bevacizumab do not recommend simultaneous bilateral injections on the same day.39 Although injecting anti-VEGF on the same day in both eyes is logistically more convenient if ROP is bilaterally symmetrical in the eyes after taking informed consent.39 The delay in treating the next eye may affect the visual outcome. A recent survey demonstrated that injection of anti-VEGF in both eyes on the same day by 67.8% of the respondents.39
Scenario 4: Intravitreal Anti-VEGF Injection Along with Laser Therapy
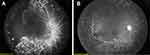
Anti-VEGF therapy and laser therapy are not recommended simultaneously. Laser therapy is recommended in cases of recurrence or with angiographic evidence of persistent leakage and neovascularization at the vascular-avascular junction after anti-VEGF.33 The Operational guidelines of India, 2018 recommend anti-VEGF as a “rescue” treatment in cases where laser treatment has failed or is impossible.15 Further, laser therapy may be required in the case of contraindications to anti-VEGF, such as conjunctivitis or external eye infections.90 Contraindications to using intravitreal anti-VEGF include ocular or periocular infections and severe intraocular inflammation.47 Figure 1 represents a case study where bevacizumab is followed by laser therapy.
Scenario 5: Follow-Up Criteria After Anti-VEGF Injections
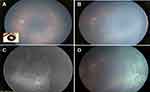
The updated protocol on ROP management recommends follow-up of infants 48 to 72 hours after anti-VEGF injections to assess complications such as endophthalmitis and recheck every 1 to 2 weeks.16 Fluorescein angiogram is recommended for all treated patients by 60 to 65 weeks PMA since complete retinal vascularization may not occur in many infants after anti-VEGF injections.43 Fluorescein angiography can be performed by limited number of devices in the hand-held format and hence makes it wide-spread utility limited in the current scenario. Reports have shown its utility by describing different patterns of regression, reactivation and response after anti-VEGF, aiding the clinician in the decision to retreat or follow.91–93 However, angiography is not readily available in most centers, and the specialist may rely on clinical examination and to treat the persistent peripheral avascular retina (PPAR), if any.25 The avascular retina might lead to recurrent ROP. Studies have also confirmed a higher incidence of the avascular retina caused by anti-VEGF therapy (18% of the eyes treated with bevacizumab). The single dose of anti-VEGF therapy might also lead to retinal detachment even after 2.5 to 3 years of dose administration, necessitating follow-ups after anti-VEGF therapy.94–96 Laser therapy is performed in those patients who have not experienced retinal vascularization following anti-VEGF therapy.15 Figure 2 elaborated on a case study post administering bevacizumab and witnessing a loss to follow-up.
Conclusion
There is a need to increase the ROP screening not only for preterm and low birth weight but also required in optimal gestational age infants with healthy birth weight. Although, current national guidelines consider laser therapy as the standard of care. However, anti-VEGF therapies have shown improved efficacy and thus emerged as a potential alternative to laser therapy. The adequate evidence on long-term safety is limited; therefore, anti-VEGF agents are restricted to posterior zone 1, zone I ROP, and AROP and also as rescue therapy following treatment failure with laser. More studies regarding the long-term safety, including systemic effects, dose, and schedule are thus warranted.
Acknowledgments
The authors would like to thank Dr. Shweta Varshney from IQVIA and Dr. Vineeth Salloju from Novartis Healthcare Private limited for writing/editing and reviewing process.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Chan-Ling T, Gole GA, Quinn GE, Adamson SJ, Darlow BA. Pathophysiology, screening and treatment of ROP: a multi-disciplinary perspective. Prog Retin Eye Res. 2018;62:77–119. doi:10.1016/j.preteyeres.2017.09.002
2. Smith LEH. Pathogenesis of retinopathy of prematurity. Growth Horm IGF Res. 2004;14(Suppl A):S140–S144. doi:10.1016/j.ghir.2004.03.030
3. Gilbert C. Retinopathy of prematurity: a global perspective of the epidemics, population of babies at risk and implications for control. Early Hum Dev. 2008;84(2):77–82. doi:10.1016/j.earlhumdev.2007.11.009
4. World Health Organization. Vision 2020: Global Initiative for the Elimination of Avoidable Blindness: Action Plan 2006–2011; 2007. Availbale from: https://apps.who.int/iris/handle/10665/43754.
5. Borroni C, Carlevaro C, Morzenti S, et al. Survey on retinopathy of prematurity (ROP) in Italy. Ital J Pediatr. 2013;39:43. doi:10.1186/1824-7288-39-43
6. Holmström G, El Azazi M, Jacobson L, Lennerstrand G. A population based, prospective study of the development of ROP in prematurely born children in the Stockholm area of Sweden. Br J Ophthalmol. 1993;77(7):417–423. doi:10.1136/bjo.77.7.417
7. Blencowe H, Lawn JE, Vazquez T, Fielder A, Gilbert C. Preterm-associated visual impairment and estimates of retinopathy of prematurity at regional and global levels for 2010. Pediatr Res. 2013;74(Suppl 1):35–49. doi:10.1038/pr.2013.205
8. Blencowe H, Moxon S, Gilbert C. Update on blindness due to retinopathy of prematurity globally and in India. Indian Pediatr. 2016;53(Suppl 2):S89–S92.
9. Vinekar A, Azad R, Dogra MR, Narendran V, Jalali S, Bende P. The Indian retinopathy of prematurity society: a baby step towards tackling the retinopathy of prematurity epidemic in India. Ann Eye Sci. 2017;2(27):2–6. doi:10.21037/aes.2017.04
10. Charan R, Dogra MR, Gupta A, Narang A. The incidence of retinopathy of prematurity in a neonatal care unit. Indian J Ophthalmol. 1995;43:123–126.
11. Gopal L, Sharma T, Ramachandran S, Shanmugasundaram R, Asha V. Retinopathy of prematurity: a study. Indian J Ophthalmol. 1995;43:59–61.
12. Ahuja AA, Reddy V, Adenuga OO, et al. Risk factors for retinopathy of prematurity in a district in south India: a prospective cohort study. Oman J Ophthalmol. 2018;11:33–37. doi:10.4103/ojo.OJO_97_2016
13. Vasavada D, Sengupta S, Prajapati VK, Patel S Incidence and risk factors of retinopathy of prematurity in western India - report from a regional institute of ophthalmology. Nepal J Ophthalmol. 2017;9:112–1120.
14. Maheshwari R, Kumar H, Paul VK, Singh M, Deorari AK, Tiwari HK. Incidence and risk factors of retinopathy of prematurity in a tertiary care newborn unit in New Delhi. Natl Med J India. 1996;9:211–214.
15. Public Health Foundation in India (PHFI). Project operational guidelines: prevention of blindness from retinopathy of prematurity in neonatal care units; 2019. Available from: https://phfi.org/wp-content/uploads/2019/05/2018-ROP-operational-guidelines.pdf.
16. Vinekar A, Mangalesh S, Jayadev C, Gilbert C, Dogra M, Shetty B. Impact of expansion of telemedicine screening for retinopathy of prematurity in India. Indian J Ophthalmol. 2017;65(5):390–395. doi:10.4103/ijo.IJO_211_17
17. Parappil H, Pai A, Mahmoud N, AlKhateeb M, Al Rifai H, El Shafei M. Management of retinopathy of prematurity in a neonatal unit: current approach. Wolters Kluwer Medknow Publ. 2019;8(4):203–211. doi:10.4103/jcn.JCN_102_18
18. National Neonatology Forum, India. Clinical practice guidelines: screening and management of retinopathy of prematurity. National Neonatology Forum; 2020. Availbale from: http://www.nnfi.org/assests/pdf/cpg-guidelines/ropp.pdf.
19. Hartnett ME, Penn JS. Mechanisms and management of retinopathy of prematurity. N Engl J Med. 2012;367(26):2515–2526. doi:10.1056/NEJMra1208129
20. Sankar MJ, Sankar J, Chandra P. Anti-vascular endothelial growth factor (VEGF) drugs for treatment of retinopathy of prematurity. Cochrane Database Syst Rev. 2018;1:CD009734. doi:10.1002/14651858.CD009734.pub3
21. Hardy RJ, Good WV, Dobson V, et al. Multicenter trial of early treatment for retinopathy of prematurity: study design. Control Clin Trials. 2004;25(3):311–325. doi:10.1016/j.cct.2004.03.003
22. Agarwal K, Balakrishnan D, Rani PK, Jalali S. Changing patterns of early childhood blinding conditions presenting to a tertiary eye center: the epidemic of retinopathy of prematurity in India. Indian J Ophthalmol. 2019;67(6):816–818. doi:10.4103/ijo.IJO_709_18
23. Patel SS, Shendurnikar N. Retinopathy of prematurity in India: incidence, risk factors, outcome and the applicability of current screening criteria. Int J Contemp Pediatr. 2019;6(6):2235–2241. doi:10.18203/2349-3291.ijcp20194698
24. National Health Mission. Resource document: revised guidelines for universal eye screening in newborns including ROP; 2022. Availbale from: https://nhm.gov.in/images/pdf/programmes/RBSK/Resource_Documents/Revised_ROP_Guidelines-Web_Optimized.pdf.
25. Chiang MF, Quinn GE, Fielder AR, et al. International classification of retinopathy of prematurity, third edition. Ophthalmology. 2021;128(10):e51–e68. doi:10.1016/j.ophtha.2021.05.031
26. Shukla R, Murthy GVS, Gilbert C, Vidyadhar B, Mukpalkar S. Operational guidelines for ROP in India: a summary. Indian J Ophthalmol. 2020;68(Suppl 1):S108–S114. doi:10.4103/ijo.IJO_1827_19
27. Fierson WM, Saunders RA, Good W; American Academy of Pediatrics Section on Ophthalmology, American Academy of Ophthalmology, American Association for Pediatric Ophthalmology and Strabismus, American Association of Certified Orthoptists. Screening examination of premature infants for retinopathy of prematurity. Pediatrics. 2013;131(1):189–195. doi:10.1542/peds.2012-2996
28. Enríquez AB, Avery RL, Baumal CR. Update on anti-vascular endothelial growth factor safety for retinopathy of prematurity. Asia Pac J Ophthalmol. 2020;9(4):358–368. doi:10.1097/APO.0000000000000302
29. Connolly BP, Ng EYJ, McNamara JA, Regillo CD, Vander JF, Tasman W. A comparison of laser photocoagulation with cryotherapy for threshold retinopathy of prematurity at 10 years: part 2. Refractive outcome. Ophthalmology. 2002;109(5):936–941. doi:10.1016/s0161-6420(01)01015-6
30. Cryotherapy for Retinopathy of Prematurity Cooperative Group. Multicenter trial of cryotherapy for retinopathy of prematurity. Preliminary results. Arch Ophthalmol. 1988;106(4):471–479. PMID: 2895630. doi:10.1001/archopht.1988.01060130517027
31. Early Treatment For Retinopathy Of Prematurity Cooperative Group. Revised indications for the treatment of retinopathy of prematurity: results of the early treatment for retinopathy of prematurity randomized trial. Arch Ophthalmol. 2003;121(12):1684–1694. PMID: 14662586. doi:10.1001/archopht.121.12.1684
32. Mintz-Hittner HA, Kennedy KA, Chuang AZ; BEAT-ROP Cooperative Group. Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity. N Engl J Med. 2011;364(7):603–615. doi:10.1056/NEJMoa1007374
33. Narnaware SH, Bawankule PK, Raje D. Aggressive Posterior Retinopathy of Prematurity (APROP): LASER as the primary modality of treatment. J Ophthalmic Vis Res. 2021;16(3):400–407. doi:10.18502/jovr.v16i3.9437
34. Singh SR, Katoch D, Handa S, et al. Safety and efficacy of 532 nm frequency-doubled Nd-YAG green laser photocoagulation for treatment of retinopathy of prematurity. Indian J Ophthalmol. 2019;67(6):860–865. doi:10.4103/ijo.IJO_325_19
35. Chhabra K, Kaur P, Singh K, Aggarwal A, Chalia D. Outcome of solid-state 532 nm green laser in high-risk retinopathy of prematurity at a tertiary care centre in India. Int Ophthalmol. 2018;38(1):287–291. doi:10.1007/s10792-017-0460-3
36. Kara C, Petriçli İS, Hekimoğlu E, Akil H, Beyazyildiz Ö. Treatment success of laser therapy for retinopathy of prematurity in referred and non-referred patients. Arq Bras Oftalmol. 2016;79(2):96–99. doi:10.5935/0004-2749.20160029
37. Vinekar A, Jayadev C, Mangalesh S, et al. Comparing the outcome of single versus multiple session laser photoablation of flat neovascularization in zone 1 aggressive posterior retinopathy of prematurity: a prospective randomized study. Retina. 2015;35(10):2130–2136. doi:10.1097/IAE.0000000000000604
38. Chawla D, Darlow BA. Anti-vascular endothelial growth factor preparations in the treatment of retinopathy of prematurity: balancing risks and benefits. Indian Pediatr. 2016;53(Suppl 2):S129–S136.
39. Gangwe A, Agrawal D, Vinekar A, Azad RV, Parchand SM, Agrawal D. Anti-vascular endothelial growth factor in the management of retinopathy of prematurity: a survey among the members of Indian retinopathy of prematurity society. Indian J Ophthalmol. 2021;69(8):2158–2163. doi:10.4103/ijo.IJO_200_21
40. Shanmugam PM. Changing paradigms of anti-VEGF in the Indian scenario. Indian J Ophthalmol. 2014;62(1):88–92. doi:10.4103/0301-4738.126189
41. Spandau U. What is the optimal dosage for intravitreal bevacizumab for retinopathy of prematurity? Acta Ophthalmol. 2013;91(2):e154. doi:10.1111/j.1755-3768.2012.02552.x
42. Wallace DK. Retinopathy of prematurity: anti-VEGF treatment for ROP: which drug and what dose? J AAPOS. 2016;20(6):476–478. doi:10.1016/j.jaapos.2016.08.013
43. Beck KD, Rahman EZ, Ells A, Mireskandari K, Berrocal AM, Harper CA. SAFER-ROP: updated protocol for anti-VEGF injections for retinopathy of prematurity. Ophthalmic Surg Lasers Imaging Retina. 2020;51(7):402–406. doi:10.3928/23258160-20200702-05
44. Sato T, Wada K, Arahori H, et al. Serum concentrations of bevacizumab (avastin) and vascular endothelial growth factor in infants with retinopathy of prematurity. Am J Ophthalmol. 2012;153(2):327–333.e1. doi:10.1016/j.ajo.2011.07.005
45. Tran KD, Cernichiaro-Espinosa LA, Berrocal AM. Management of retinopathy of prematurity--use of anti-VEGF therapy. Asia Pac J Ophthalmol. 2018;7(1):56–62. doi:10.22608/APO.2017436
46. VanderVeen DK, Cataltepe SU. Anti-vascular endothelial growth factor intravitreal therapy for retinopathy of prematurity. Semin Perinatol. 2019;43(6):375–380. doi:10.1053/j.semperi.2019.05.011
47. Novartis Pharmaceuticals UK Limited. Lucentis (Ranibizumab): anti-neovasculisation agent; 2022. Availbale from: https://www.novartis.com/sg-en/sites/novartis_sg/files/Lucentis-Feb2022.SIN-app180522-pdf.pdf.
48. Huang Q, Zhao P. Anti-vascular endothelial growth factor treatment for retinopathy of prematurity. Ann Eye Sci. 2017;2(7):47. doi:10.21037/aes.2017.02.06
49. Cheng HC, Lee SM, Hsieh YT, Lin PK. Efficacy of intravitreal injection of anti-vascular endothelial growth factor agents for stage 4 retinopathy of prematurity. Retina. 2015;35(4):660–666. doi:10.1097/IAE.0000000000000359
50. Stahl A, Krohne TU, Eter N, et al. Comparing alternative ranibizumab dosages for safety and efficacy in retinopathy of prematurity: a randomized clinical trial. JAMA Pediatr. 2018;172(3):278–286. doi:10.1001/jamapediatrics.2017.4838
51. Stahl A, Lepore D, Fielder A, et al. Ranibizumab versus laser therapy for the treatment of very low birthweight infants with retinopathy of prematurity (RAINBOW): an open-label randomised controlled trial. Lancet. 2019;394(10208):1551–1559. doi:10.1016/S0140-6736(19)31344-3
52. Marlow N, Stahl A, Lepore D, et al. 2-year outcomes of ranibizumab versus laser therapy for the treatment of very low birthweight infants with retinopathy of prematurity (RAINBOW extension study): prospective follow-up of an open label, randomised controlled trial. Lancet Child Adolesc Health. 2021;5(10):698–707. doi:10.1016/S2352-4642(21)00195-4
53. Zehetner C, Kralinger MT and Modi YS, et al. (2015). Systemic levels of vascular endothelial growth factor before and after intravitreal injection of aflibercept or ranibizumab in patients with age-related macular degeneration: a randomised, prospective trial. Acta Ophthalmol, 93(2), e154–e159. 10.1111/aos.12604
54. EYLEA® (aflibercept) Injection, for intravitreal use, Regeneron Pharmaceuticals, Inc; 2011. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/125387s061lbl.pdf.
55. Vedantham V. Intravitreal aflibercept injection in Indian eyes with retinopathy of prematurity. Indian J Ophthalmol. 2019;67(6):884–888. doi:10.4103/ijo.IJO_708_18
56. Stahl A, Sukgen EA, Wu WC, et al. Effect of intravitreal aflibercept vs laser photocoagulation on treatment success of retinopathy of prematurity: the FIREFLEYE randomized clinical trial. JAMA. 2022;328(4):348–359. doi:10.1001/jama.2022.10564
57. US National Library of Medicine. Study to assess the efficacy, safety, and tolerability of intravitreal aflibercept compared to laser photocoagulation in patients with retinopathy of prematurity (BUTTERFLEYE); 2019. Availbale from: https://clinicaltrials.gov/ct2/show/NCT04101721.
58. Falavarjani KG, Nguyen QD. Adverse events and complications associated with intravitreal injection of anti-VEGF agents: a review of literature. Eye. 2013;27(7):787–794. doi:10.1038/eye.2013.107
59. Eldweik L, Mantagos IS. Role of VEGF inhibition in the treatment of retinopathy of prematurity. Semin Ophthalmol. 2016;31(1–2):163–168. doi:10.3109/08820538.2015.1114847
60. Chandra P, Kumawat D, Tewari R, Azimeera S. Post-ranibizumab injection endophthalmitis in aggressive posterior retinopathy of prematurity. Indian J Ophthalmol. 2019;67(6):967–969. doi:10.4103/ijo.IJO_884_17
61. Lepore D, Quinn GE, Molle F, et al. Intravitreal bevacizumab versus laser treatment in type 1 retinopathy of prematurity: report on fluorescein angiographic findings. Ophthalmology. 2014;121(11):2212–2219. doi:10.1016/j.ophtha.2014.05.015
62. Mintz-Hittner HA, Geloneck MM. Review of effects of anti-VEGF treatment on refractive error. Eye Brain. 2016;8:135–140. doi:10.2147/EB.S99306
63. Lien R, Yu MH, Hsu KH, et al. Neurodevelopmental outcomes in infants with retinopathy of prematurity and bevacizumab treatment. PLoS One. 2016;11(1):e0148019. doi:10.1371/journal.pone.0148019
64. Morin J, Luu TM, Superstein R, et al. Neurodevelopmental outcomes following bevacizumab injections for retinopathy of prematurity. Pediatrics. 2016;137(4):e20153218. doi:10.1542/peds.2015-3218
65. Azad R, Gilbert C, Gangwe AB, et al. Retinopathy of prematurity: how to prevent the third epidemics in developing countries. Asia Pac J Ophthalmol. 2020;9(5):440–448. doi:10.1097/APO.0000000000000313
66. Jalali S, Kesarwani S, Hussain A. Outcomes of a protocol-based management for zone 1 retinopathy of prematurity: the Indian twin cities ROP screening program report number 2. Am J Ophthalmol. 2011;151(4):719–724.e2. doi:10.1016/j.ajo.2010.10.007
67. Wu WC, Lien R, Liao PJ, et al. Serum levels of vascular endothelial growth factor and related factors after intravitreous bevacizumab injection for retinopathy of prematurity. JAMA Ophthalmol. 2015;133(4):391–397. doi:10.1001/jamaophthalmol.2014.5373
68. Jalali S, Balakrishnan D, Zeynalova Z, Padhi TR, Rani PK. Serious adverse events and visual outcomes of rescue therapy using adjunct bevacizumab to laser and surgery for retinopathy of prematurity. The Indian twin cities retinopathy of prematurity screening database report number 5. Arch Dis Child Fetal Neonatal Ed. 2013;98(4):F327–F333. doi:10.1136/archdischild-2012-302365
69. Fidler M, Fleck BW, Stahl A, et al.; on behalf of the RAINBOW study group. Ranibizumab population pharmacokinetics and free VEGF pharmacodynamics in preterm infants with retinopathy of prematurity in the RAINBOW trial. Transl Vis Sci Tech. 2020;9(8):43. doi:10.1167/tvst.9.8.43
70. Stuart A. Current ROP therapies: how laser and anti-VEGF compare. Eye Net Mag. 2014. Availbale from: https://www.aao.org/eyenet/article/current-rop-therapies-how-laser-antivegf-compare. Accessed February 2, 2023.
71. VanderVeen DK, Melia M, Yang MB, Hutchinson AK, Wilson LB, Lambert SR. Anti-vascular endothelial growth factor therapy for primary treatment of type 1 retinopathy of prematurity: a report by the American academy of ophthalmology. Ophthalmology. 2017;124(5):619–633. doi:10.1016/j.ophtha.2016.12.025
72. Snyder LL, Garcia-Gonzalez JM, Shapiro MJ, Blair MP. Very late reactivation of retinopathy of prematurity after monotherapy with intravitreal bevacizumab. Ophthalmic Surg Lasers Imaging Retina. 2016;47(3):280–283. doi:10.3928/23258160-20160229-12
73. Hajrasouliha AR, Garcia-Gonzales JM, Shapiro MJ, Yoon H, Blair MP. Reactivation of retinopathy of prematurity three years after treatment with bevacizumab. Ophthalmic Surg Lasers Imaging Retina. 2017;48(3):255–259. doi:10.3928/23258160-20170301-10
74. Dudani AI, Dudani A, Dudani K. Commentary: antivascular endothelial growth factor and retinopathy of prematurity. Indian J Ophthalmol. 2019;67(6):969–970. doi:10.4103/ijo.IJO_1220_17
75. Tasman W. Retinopathy of prematurity: do we still have a problem?: The Charles L. Schepens lecture. Arch Ophthalmol. 2011;129(8):1083–1086. doi:10.1001/archophthalmol.2011.192
76. Wallace DK, Kraker RT, Freedman SF, et al. Assessment of lower doses of intravitreous bevacizumab for retinopathy of prematurity: a phase 1 dosing study. JAMA Ophthalmol. 2017;135(6):654–656. doi:10.1001/jamaophthalmol.2017.1055
77. Wallace DK, Dean TW, Hartnett ME, et al. A dosing study of bevacizumab for retinopathy of prematurity: late recurrences and additional treatments. Ophthalmology. 2018;125(12):1961–1966. doi:10.1016/j.ophtha.2018.05.001
78. Ells AL, Wesolosky JD, Ingram AD, Mitchell PC, Platt AS. Low-dose ranibizumab as primary treatment of posterior type I retinopathy of prematurity. Can J Ophthalmol. 2017;52(5):468–474. PMID: 28985806. doi:10.1016/j.jcjo.2017.02.012
79. Wallace DK, Kraker RT, Freedman SF, et al. Short-term outcomes after very low-dose intravitreous bevacizumab for retinopathy of prematurity. JAMA Ophthalmol. 2020;138(6):698–701. doi:10.1001/jamaophthalmol.2020.0334
80. Mills MD. Evaluating the Cryotherapy for Retinopathy of Prematurity Study (CRYO-ROP). Arch Ophthalmol. 2007;125(9):1276–1281. doi:10.1001/archopht.125.9.1276
81. Patel SN, Klufas MA. Evidence to date: ranibizumab and its potential in the treatment of retinopathy of prematurity. Eye Brain. 2019;11:25–35. doi:10.2147/EB.S189684
82. Tawse KL, Jeng-Miller KW, Baumal CR. Current practice patterns for treatment of retinopathy of prematurity. Ophthalmic Surg Lasers Imaging Retina. 2016;47(5):491–495. doi:10.3928/23258160-20160419-16
83. Wang SD, Zhang GM; Shenzhen Screening for Retinopathy of Prematurity Cooperative Group. Laser therapy versus intravitreal injection of anti-VEGF agents in monotherapy of ROP: a meta-analysis. Int J Ophthalmol. 2020;13(5):806–815. doi:10.18240/ijo.2020.05.17
84. Linghu D, Cheng Y, Zhu X, et al. Comparison of intravitreal anti-VEGF agents with laser photocoagulation for retinopathy of prematurity of 1627 eyes in China. Front Med. 2022;9:911095. PMID: 35712119; PMCID: PMC9193577. doi:10.3389/fmed.2022.911095
85. Sen P, Agarwal AAK, Bhende P, Ganesan S. Treatment outcomes of combination of anti-vascular endothelial growth factor injection and laser photocoagulation in type 1 ROP and APROP. Int Ophthalmol. 2022;42(1):95–101. doi:10.1007/s10792-021-02004-8
86. Gotz-Więckowska A, Chmielarz-Czarnocińska A, Pawlak M, Gadzinowski J, Mazela J. Ranibizumab after laser photocoagulation failure in retinopathy of prematurity (ROP) treatment. Sci Rep. 2017;7(1):11894. doi:10.1038/s41598-017-12264-z
87. Castellanos MAM, Schwartz S, Leal R, Chan RVP, Quiroz-Mercado H. Pain assessment in premature infants treated with intravitreal antiangiogenic therapy for retinopathy of prematurity under topical anesthesia. Graefes Arch Clin Exp Ophthalmol. 2013;251(2):491–494. doi:10.1007/s00417-012-2060-2
88. Tokgöz O, Sahin A, Tüfek A, et al. Inhalation anesthesia with sevoflurane during intravitreal bevacizumab injection in infants with retinopathy of prematurity. BioMed Res Int. 2013;2013:435387. doi:10.1155/2013/435387
89. Vitreo Retinal Society, India. Guidelines for intravitreal injection of avastin (bevacizumab); 2017. Availbale from: https://vrsi.in/wp-content/uploads/2018/02/Avastin_Guidlines_Book.pdf.
90. Kara C, Hekimoğlu E, Petriçli İS, Akıl H. Intravitreal bevacizumab as rescue therapy following treatment failure with laser photocoagulation in retinopathy of prematurity. J Curr Ophthalmol. 2018;30(1):80–84. doi:10.1016/j.joco.2017.08.007
91. Cheng Y, Liu TG, Li WY, Zhao MW, Liang JH. Fluorescein angiography of retinal vascular involution after intravitreal injection of ranibizumab for retinopathy of prematurity. Int J Ophthalmol. 2019;12(1):79–82. doi:10.18240/ijo.2019.01.12
92. Al Rasheed R, Adhi MI, Alowedi SA, Albdah B, Aldebasi T, Hazzazi MA. Long-term peripheral retinal vascular behavior in retinopathy of prematurity patients treated with ranibizumab intravitreal injection as monotherapy using fluorescein angiography. Int J Retina Vitr. 2022;8(1):53. doi:10.1186/s40942-022-00402-3
93. Sternfeld A, Rahmani S, Rossen JL, et al. Long-term retinal vasculature abnormalities following intravitreal bevacizumab for retinopathy of prematurity. Graefes Arch Clin Exp Ophthalmol. 2022;260:1915–1921. doi:10.1007/s00417-021-05499-0
94. Day S, Rainey AM, Harper CA III. Incomplete retinal vascularization after ranibizumab treatment of retinopathy of prematurity. Ophthalmic Surg Lasers Imaging Retina. 2017;48(1):75–78. doi:10.3928/23258160-20161219-11
95. Lepore D, Quinn GE, Molle F, et al. Follow-up to age 4 years of treatment of type 1 retinopathy of prematurity intravitreal bevacizumab injection versus laser: fluorescein angiographic findings. Ophthalmology. 2018;125(2):218–226. doi:10.1016/j.ophtha.2017.08.005
96. Isaac M, Tehrani N, Mireskandari K. Involution patterns of retinopathy of prematurity after treatment with intravitreal bevacizumab: implications for follow-up. Eye. 2016;30(3):333–341. doi:10.1038/eye.2015.289
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.